- *Corresponding Author:
- D. Philomina
School of Biotechnology and Chemical Engineering, Vellore Institute of Technology, Deemed University, Vellore-632 014, India.
E-mail: dphilomina@gmail.com
| Date of Submission | 15 October 2005 |
| Date of Revision | 06 March 2006 |
| Date of Acceptance | 23 December 2006 |
| Indian J. Pharm. Sci., 2006, 68 (6): 806-809 |
Abstract
Three different methods for extraction of colchicine have been studied, and it was found that extraction with petroleum ether and chloroform was the best and most reliable method. The amount of colchicine in six different species of Gloriosa , viz., Gloriosa superba, Gloriosa rothchildiana, Gloriosa planti, Gloriosa lutea, Gloriosa casuariana and Gloriosa vuchuria , has been determined using high performance liquid chromatography. Of the six different species of Gloriosa considered for this study, Gloriosa planti exhibited the highest level of colchicines, followed by Gloriosa lutea, Gloriosa casuariana and Gloriosa superba . The relatively high colchicine content in the above-mentioned species of Gloriosa , these could be recommended as substitute plants for Colchicum for the alkaloid colchicine.
Colchicine is the drug of choice to relieve acute attacks of gout and familial Mediterranean fever [1]. At present there is renewed interest in the use of colchicine as a possible cure for cancer-related diseases [2]. Colchicine itself is too toxic for human use as an antitumour drug and hence use has been made of its derivatives, viz., Demecolchicine, trimethyl colchicine acid methyl ester, 2- demethyl and 3-demethylthio colchicine, which are less toxic and have been evaluated as anti-leukaemia agents. Data collected on 3-demethylcolchicine shows this compound to be a broad spectrum antitumour agent of some promise. Colchicine and its analogues have been used clinically for the treatment of certain forms of leukaemia and solid tumour. Owing to its potent affinity for tubulin, colchicine is used in biological and breeding studies to produce polyploids and in tubulin-binding assays as a positive control [3-4].
Among the Indian medicinal plants, the corms of Colchicum luteum and the seeds of Iphigenia contain alkaloid, chiefly colchicine, to the extent of about 0.25% and 0.9% respectively [5]. These plants are not available in sufficient quantities to warrant any commercial utilization. Gloriosa superba is another plant containing colchicine [6]. A mixture of alkaloids consisting mainly of colchicine from dried tubers of Gloriosa superba has been isolated [7]. Hence Gloriosa L. is known to be a substitute plant of tropics to Colchicum autumnale for the alkaloid colchicine.
Several analytical methods for the determination of colchicine in pharmaceutical preparations, in biological fluids and in plant extracts have been described [8-11]. The aim of the present study is to develop an accurate and reliable method for the extraction and the quantification of colchicine and to identify the species of Gloriosa with high colchicine content. Three different methods of extraction of colchicine have been studied and the concentration quantified using high performance liquid chromatography (HPLC) in six different species of Gloriosa.
Tubers of six different species of Gloriosa, viz., Gloriosa superba, Gloriosa rothchildiana, Gloriosa planti, Gloriosa lutea, Gloriosa casuariana and Gloriosa vuchuria, were grown in vivo in nursery at the host institute. One-year old corms from each species were collected and sliced into small pieces for freeze drying at -20°. After 7 d, the freeze dried plant material was ground to fine powder and then used for extraction of colchicine.
In the first method for extraction (Method 1)1, 0.5 g of powdered plant material was extracted twice with 25 ml of petroleum ether with frequent shaking for 1 h, followed each time by filtration. The solid residues were air dried and then extracted with 10 ml of dichloromethane at room temperature for 30 min with frequent shaking. Then 10% solution of ammonia (0.5 ml) was added to the mixture with vigorous shaking for 10 min; the mixture was left undisturbed for 30 min and then filtered. The residue was washed twice with 10 ml of dichloromethane and then combined with the filtrate. The organic phase was evaporated to dryness and then dissolved in 1 ml of 70% ethanol to yield the test sample.
In the second method (Method 2) [12], 12 g of plant material was extracted for 6 h in a Soxhlet extractor with methanol. The extract was diluted with distilled water and then partitioned against petroleum ether, and finally the aqueous phase was extracted with chloroform. The chloroform extract was then evaporated and the residue redissolved in methanol and filtered through 0.45 μm filter. The filtrate thus obtained constituted the test sample.
In the third method (Method 3) [13], 20 g of freeze dried material was extracted using 200 ml methanol in a cold room (10°) overnight and the homogenate was centrifuged at 1252 × g for 5 min. The methanolic extract was evaporated to dryness and then the residues redissolved in 50 ml water. The aqueous extract was then centrifuged at 7826 × g for 5 min. The supernatant was first partitioned twice against petroleum ether and then discarded, and then once in diethyl ether, discarding the supernatant each time. The residue was washed five times with equal volumes of chloroform, which was retained and evaporated to dryness. The chloroform residue was redissolved in 95% HPLC grade methanol and then filtered through a 0.45 μm millipore filter to yield the test sample.
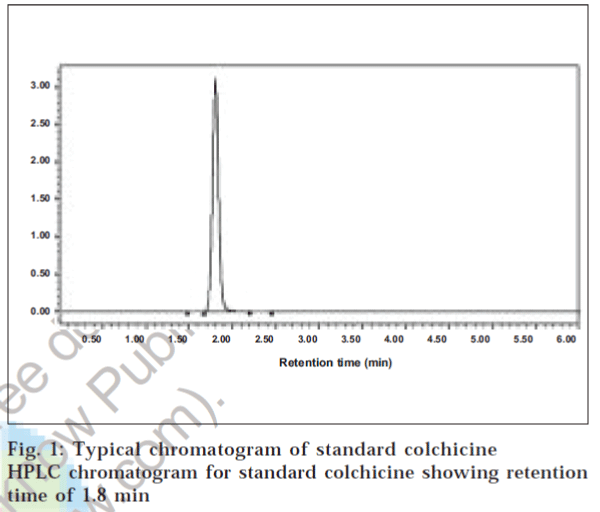
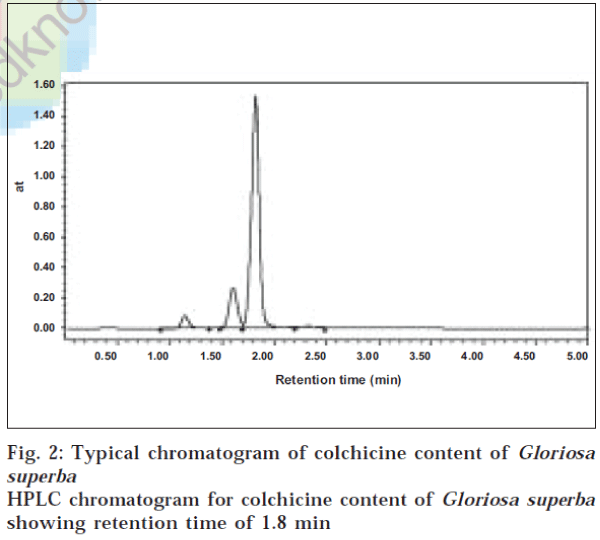
Identification of colchicine was done by comparing the retention time of the sample with that of the standard obtained from Sigma, USA. A Waters HPLC system equipped with a binary pump 1525 and porous silica with 5 μm diameter C18 4.6 × 150 mm column was used for separation. The mobile phase consisted of acetonitrile: 3% acetic acid (60:40), at a flow rate of 1 ml/min. The peaks eluted were detected at 245 nm and identified with authentic standards. The HPLC method was used to estimate the colchicine content in the six different species of Gloriosa. Colchicine extracted by three different methods was eluted at 1.8 min, which was ensured in samples by comparison with the standard containing 10 mg/ml colchicine as control.
The concentration levels of colchicine determined in six different species of Gloriosa by three extraction methods are represented in Table 1, and the chromatogram for standard and colchicine extracted by the first method is depicted in figs. 1 and 2 respectively. The concentration of colchicine determined by extraction with petroleum ether and dichloromethane revealed that of the six species considered for study, Gloriosa planti exhibited the highest value of colchicine (0.342 mg/g), followed by Gloriosa lutea, Gloriosa casuariana and Gloriosa superba (0.294, 0.246, 0.211 mg/g respectively).
| Species | Method 1 Colchicine (mg/g) | Method 2 Colchicine (mg/g) | Method 3 Colchicine (mg/g) |
|---|---|---|---|
| Gloriosasuperba | 0.211 ± 0.001 | 0.206 ± 0.035 | 0.137 ± 0.029 |
| Gloriosarocthcildiana | 0.162 ± 0.001 | 0.151 ± 0.039 | 0.136 ± 0.017 |
| Gloriosaplanti | 0.342 ± 0.047 | 0.254 ± 0.037 | 0.141 ± 0.016 |
| Gloriosalutea | 0.294 ± 0.019 | 0.280 ± 0.017 | 0.166 ± 0.014 |
| Gloriosacasuariana | 0.246 ± 0.032 | 0.217 ± 0.041 | 0.142 ± 0.034 |
| Gloriosavuchuria | 0.150 ± 0.030 | 0.132 ± 0.027 | 0.145 ± 0.017 |
Table 1: Colchicine content in different species of gloriosa
Extraction of colchicine using petroleum ether and dichloromethane was equally effective as compared to Soxhlet extraction. The sample required for estimation and the quantity of solvents consumed for extraction are comparatively less for the first two methods when compared with the third method of extraction, and the first two methods consume less time. Nevertheless, extraction with petroleum ether and dichloromethane can be suggested as the most efficient and reliable method for extraction of colchicine.
Colchicine levels in Gloriosa superba corms to the level of around 0.9% (DM) have been reported earlier [13]. Gloriosa species apparently would be a better source of commercial colchicine than Colchicum, where the level of colchicine reported was around 0.2% [14]. Earlier study of Gloriosa superba revealed that colchicine levels are the highest during the initial growth of plant, and these levels decline during maturation [15]. Comparisons with the previous reports are difficult due to the fact that alkaloid content also varies with locality and season. However, the relatively high colchicine content in Gloriosa planti, Gloriosa lutea and Gloriosa casuariana in this study encourages the cultivation of these species under suitable conditions.
Acknowledgements
The authors are grateful to the Management of Vellore Institute of Technology, Vellore, for providing support to carry out this work and the Technology Business Incubator at this Institute for the chromatographic analysis.
References
- Alali, F., Tawaha, K. and Qasaymch, R.M., Phytochem. Anal., 2004, 15, 27.
- Evans, D.A., Tanis, S.P. and Hart, D.J., J. Amer. Chem. Soc., 1981, 103, 5813.
- Trease, S.E. and Evans, D., In; Pharmacognosy, 12th Edn.,BalliereTindali, London, 1983, 593.
- Poutaraud, A. and Champay, N., Rev. Suisse. Agric., 1995, 27, 93.
- Kapadia, V.H., Dev, S., Rao, R.S. and Ansari, M.Y., Phytochemistry, 1972, 11, 1193.
- Sarin, Y.K., Jamwal, P.S., Gupta, P.K. and Atal, C.K., Curr. Sci., 1974, 43, 87.
- Clewer, H.W.V., Green, S.S. and Tuttin, F., J. Chem. Soc. (London), 1915, 107, 835.
- Sutlupinar, N., Husek, A., Potesilova, H., Dvorackova, S., Hanus, V., Sedmera, P. and Simanek, V., Planta Med., 1988, 54, 243.
- Poulev, A., Deus-Neumann, B., Bombardelli, E. and Zenk, M., Planta Med., 1994, 60, 77.
- Ondra, P., Valka, I., Vicar, J., Sutlupinar, N. and Simanek, V., J. Chromatogr., 1995 704, 351.
- Fayyad, M., Alali, F., Alkofahi, A. and Tell, A., Nat. Prod. Lett., 2002, 16, 395.
- Hayashi, T., Yoshida, K. and Sano, K., Phytochemistry 1988, 27, 1371.
- Finnie, J.F. and Van Staden, J., J. Plant Physiol., 1991, 138, 691.
- Bellet, P. and Gaignault, J.C., Ann. Pharm. Fr., 1985 43, 345.
- Thakur, R.S., Potesilova, H. and Santavy, F., Planta Med., 1975, 28, 201.