- Corresponding Author:
- Nilima S. Rajurkar
Department of Chemistry, University of Pune, Pune-411 007, India
E-mail: nsraj@chem.unipune.ac.in
| Date of Submission | 19 July 2010 |
| Date of Revision | 28 March 2011 |
| Date of Acceptance | 1 April 2011 |
| Indian J Pharm Sci, 2011, 73 (2): 146-151 |
Abstract
The powder samples and methanol extract of 11 medicinal plants were subjected to analysis of proximate composition and measurement of antioxidant activity. Different parameters studied include phenolic contents, moisture, ash, crude fiber, fats and waxes. The assays employed were ferric reducing antioxidant power, trolox equivalent antioxidant capacity and scavenging effect on the 1,1-diphenyl-2-picrylhydrazyl free radical. Results obtained indicate that the antioxidant potential varied significantly from plant to plant. The total phenolic contents were determined spectrophotometrically using Folin-Ciocalteu reagent. Significant correlation is observed between ferric reducing antioxidant power and phenolic contents (R2= 0.96). These findings show that the polyphenolic constituents in the extracts are responsible for free radical scavenging capacity.
It is well accepted that reactive oxygen species (ROS), such as superoxide anion (O2 -.), hydrogen peroxide (H2O2) and hydroxyl radical (HO˙) formed in vivo are highly reactive chemical species and can be generated endogenously as well as exogenously. Excess production of ROS leads to oxidative stress, which can cause number of diseases. In such conditions dietary intake of antioxidant compounds are needed in assisting the body to neutralize the free radicals to remove the harmful effects of oxidative stress. Fruits, vegetables, grains and medicinal plants are known to contain number of phenolic compounds with strong antioxidant activity. These compounds are found to be well correlated with antioxidant potential [1].
There is an increasing trend to replace synthetic antioxidants, which are of safety concern [1,2], with the natural antioxidants available from plant extracts or isolated products of plant origin. Present study was undertaken to determine phytochemical content and ROS scavenging inhibitory activity of 11 medicinal plants from Western Ghats of India in order to evaluate their potential as a natural antioxidative source. These plants are used traditionally for diseases such as bronchitis, diabetes, heart diseases and asthma for several years. The antioxidant activities were determined by in vitro assays to compare their antioxidant effects. These include inhibition of DPPH (1,1-diphenyl-2-picrylhydrazyl), Trolox equivalent antioxidant capacity (TEAC) using ABTS (2,2’-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid) as an oxidant and FRAP (Ferric reducing antioxidant power). Total phenolic and flavonoid contents were also determined.
Materials and Methods
All the reagents and chemicals used in the experiments were of analytical grade. The chemicals DPPH, TPTZ (2,4,6-tripyridyl-S-triazine), ABTS, 2-thiobarbituric acid, ascorbic acid and Folin-Ciocalteu reagent, were obtained from Sigma Chemical Co., USA. 2,2’-Azobis(2-amidinopropane) dihydrochloride (AAPH) and Trolox (6-hydroxy-2,5,7,8-tetramethyl chromane 2-carboxylic acid) were obtained from Aldrich Chemicals Co., U.S.A. All other chemicals used were obtained from the local suppliers.
Eleven medicinal plants were collected from the Western Ghats of Maharashtra (India) and identified from Botanical Survey of India (Table 1). The samples were cleaned using tap water to remove the soil and then rinsed with deionised water. The plant samples were dried for 10-15 days in the shade at room temperature. These samples were then ground to a fine powder and were stored in polythene bottles at 4° till the time of experiments.
| V. Number | Name | Family | Common name | Parts | Medicinal uses | |
|---|---|---|---|---|---|---|
| analyzed | ||||||
| ACANHS3 | Acacia nilotica(L.) Willd.ex Del. ssp. | Mimosaceae | Babhul | Bark | Ulcers | |
| Indica (Benth) Brenan | ||||||
| CENAHS2 | Centellaasiatica(L.) Urb. | Apiaceae | Mandukparni | Leaf | Tuberculosis | |
| CYPRHS4 | CyperusrotundusL. ssp. rotundus | Cyperaceae | Nagarmotha | Root | Fever, antibiotic, analgesic | |
| GLYGHS11 | GlycyrrhizaglabraL. | Fabaceae | Jeshthamadh | Root | Cough, sore throats | |
| GYMSHS1 | Gymnemasylvestre(Retz.) R. Br. and S. | Asclepiadaceae | Bedakipala | Leaf | Diabetics, urinary tract infections | |
| PTEMHS10 | PterocarpusmarsupiumRoxb. | Fabaceae | Vijaysar | Stem | Diabetes, ulcer | |
| RAUSHS8 | Rauvolfia serpentine (L.) Benth. Ex Kurz. | Apocynaceae | Sarpagandha | Root | Hypertension | |
| TERCHS6 | TerminaliacuneataRoth (T. arjunaRoxb) | Combretaceae | Arjunsal | Bark | Heart diseases, gastric ulcers | |
| TINCHS7 | Tinosporacordifolia(Wild.) Miers | Menispermaceae | Gulwel | Root | Chronic fevers pains | |
| TRITHS5 | TribulusterrestrisL. | Zygophyllaceae | Gokhru | Fruit | Cough, asthma, heart diseases | |
| VITENHS9 | VitexnegundoL. | Verbenaceae | Nirgudi | Leaf | Chronic bronchitis | |
Table 1: Medicinal plants analysed in the present study
The plant extracts were prepared in methanol by adding 100 ml of methanol to 1 g of plant powder. The infusions were stirred on the magnetic stirrer at room temperature for 5 h. This was then centrifuged at 6000 rpm at 4° for 10 min and the supernatant was stored at -4° for further analysis. Moisture content, total ash, acid insoluble ashes were determined using standard methods. All the spectrophotometric measurements of the following assays were performed by UV/Vis spectrophotometer (Model No.UV-1650PC, Shimadzu, Japan).
Phytochemical determination
Powdered plant samples (5 g) were extracted with a mixture of methanol and water (150 ml) in the volume ratio 4:1 using Soxhlet for 12 h. The extract was cooled and filtered through Whatman filter paper No. 41. The residue obtained was extracted with 125 ml of ethyl acetate and the percentage of crude fibres was calculated from the residue. The amount of fats and waxes was determined by evaporating the ethyl acetate.
The filtrate (methanol and water) was reduced to approximately 1/10th of its original volume and acidified with 2M H2SO4. This filtrate was extracted with 75 ml (3×25 ml) chloroform in a separating funnel. The chloroform layer was separated and evaporated to dryness on a water bath maintained at 45°. This contains phenolics and terpenoids. The aqueous layer obtained after the separation was adjusted to pH 10 with 2M NaOH. It was further extracted with 60 ml chloroform and methanol (3:1) followed by extraction with 40 ml chloroform in a separating funnel.
The separated aqueous basic layer was evaporated to dryness on a water bath. The residue so obtained consists of quaternary alkaloids and N-oxides. The organic layer (chloroform and methanol) was transferred to a beaker and solvent is evaporated to dryness. The residue so obtained was the basic extract consisting of alkaloids.
DPPH Radical Scavenging assay
Total free radical scavenging capacity of the extracts from different plant samples were estimated according to the previously reported method [3] with slight modification using the stable DPPH radical, which has an absorption maximum at 515 nm. A solution of the radical is prepared by dissolving 2.4 mg DPPH in 100 ml methanol. A test solution (5 μl) was added to 3.995 ml of methanolic DPPH. The mixture was shaken vigorously and kept at room temperature for 30 min in the dark. Absorbance of the reaction mixture was measured at 515 nm spectrophotometrically. Absorbance of the DPPH radical without antioxidant, i.e. blank was also measured. All the determinations were performed in triplicate. The capability to scavenge the DPPH radical was calculated using the following equation [4]. DPPH Scavenged (%)= ((AB–AA)/AB)×100…..(1), where, AB is absorbance of blank at t= 0 min; AA is absorbance of the antioxidant at t= 30 min. A calibration curve was plotted with % DPPH scavenged versus concentration of standard antioxidant (Trolox).
ABTS radical scavenging assay
Free radical scavenging activity of plant samples was determined by ABTS radical cation decolorization assay [5]. ABTS·+ cation radical was produced by the reaction between 7 mM ABTS in water and 2.45 mM potassium persulfate (1:1), stored in the dark at room temperature for 12-16 h before use. ABTS·+ solution was then diluted with methanol to obtain an absorbance of 0.700 at 734 nm. After the addition of 5 μl of plant extract to 3.995 ml of diluted ABTS·+ solution, the absorbance was measured at 30 min after the initial mixing. An appropriate solvent blank was run in each assay. All the measurements were carried out at least three times. Percent inhibition of absorbance at 734 nm was calculated using the formula, ABTS·+ scavenging effect (%) = ((AB–AA)/ AB)×100 (2), where, AB is absorbance of ABTS radical + methanol; AA is absorbance of ABTS radical + sample extract/standard. Trolox was used as standard substance.
Ferric reducing antioxidant power
The antioxidant capacity of the medicinal plants was estimated spectrophotometrically following the procedure of Benzie and Strain [6]. The method is based on the reduction of Fe3+ TPTZ complex (colorless complex) to Fe2+-tripyridyltriazine (blue colored complex) formed by the action of electron donating antioxidants at low pH. This reaction is monitored by measuring the change in absorbance at 593 nm. The Ferric reducing antioxidant power (FRAP) reagent was prepared by mixing 300 mM acetate buffer, 10 ml TPTZ in 40 mM HCl and 20 mM FeCl3.6H2O in the proportion of 10:1:1 at 37°. Freshly prepared working FRAP reagent was pipetted using 1-5 ml variable micropipette (3.995 ml) and mixed with 5 μl of the appropriately diluted plant sample and mixed thoroughly. An intense blue color complex was formed when ferric tripyridyl triazine (Fe3+ TPTZ) complex was reduced to ferrous (Fe2+) form and the absorbance at 593 nm was recorded against a reagent blank (3.995 ml FRAP reagent+5 μl distilled water) after 30 min incubation at 37°. All the determinations were performed in triplicates. The calibration curve was prepared by plotting the absorbance at 593 nm versus different concentrations of FeSO4. The concentrations of FeSO4 were in turn plotted against concentration of standard antioxidant trolox. The FRAP values were obtained by comparing the absorbance change in the test mixture with those obtained from increasing concentrations of Fe3+ and expressed as mg of Trolox equivalent per gram of sample.
Determination of total phenolic content
The total phenolic contents in medicinal plants were determined spectrophotometrically according to Folin-Ciocalteu method [7]. Gallic acid was used to set up the standard curve. The content of phenolic compounds of the samples was expressed as gallic acid equivalents (GAE) in mg per gram dry weight. All the samples were analyzed in triplicates.
Determination of total flavonoid content
The AlCl3 method [8] was used for quantification of the total flavonoid content of the methanolic plant extracts. 20 μl of the sample extract was added to a solution of 2% AlCl3.6H2O. The mixture was vigorously shaken and diluted with double distilled water to make the total volume 10 ml. The absorbance of the reaction mixture was measured after 10 min incubation at 440 nm. Flavonoid contents were expressed as quercetin equivalents in mg per gram dry material. All the determinations were performed in triplicates. Correlation coefficients (R2) to determine the relationship between two variables (between different RSA tests and content of total phenolic and flavonoid compounds) were calculated using MS Excel software.
Results and Discussion
In this study, 11 medicinal plants were analysed for their proximate composition and antioxidant activity. Table 1 gives a list of medicinal plants analysed along with parts used and medicinal uses. The proximate compositions of different medicinal plants are presented in Tables 2 and 3. The moisture content of the plant samples was found to range between 3.89 to 8.30%. The ash content showed a wide range from 4.8% to 25.1%. The acid insoluble ash content ranged from 2.4 g/100g in Cyperus rotundus to 21.5 g/100 g in Acacia nilotica. These parameters indicate the amount of organic and inorganic materials in plant samples. The crude fibre contents detected in plant samples ranges from 68.58–84.98%. Almost all the samples are good source of fibre. The fat content extractable from ethyl acetate of medicinal plants ranged from 0.06 g/100 g, which is lowest for Acacia nilotica to 1.2 g/100 g and is highest for Gymnema sylvestre. The phenolics and terpenoids content varied considerably from 1.16 g/100g (Rauwolfia serpentine) to 5.24 g/100 g (Acacia nilotica). It is observed from Table 2 that alkaloids in all samples were found less than Q alkaloids and N oxides.
| Medicinal plants | Phenolics and terpenoids | Fibers | Fats and waxes | Alkaloids | Q alkaloids and N,N-oxides |
|---|---|---|---|---|---|
| Cyperusrotundus | 1.36±0.12 | 84.98±5.38 | 0.11±0.02 | 0.30±0.02 | 12.88±0.66 |
| Pterocarpusmarsupium | 2.16±0.16 | 79.88±6.02 | 0.28±0.02 | 0.14±0.01 | 15.98±1.23 |
| Terminaliaarjuna | 3.64±0.23 | 69.22±2.60 | 0.08±0.01 | 0.54±0.04 | 27.84±1.76 |
| Tribulasterrestris | 1.74±0.12 | 82.26±4.02 | 0.44±0.03 | 0.34±0.02 | 19.70±1.14 |
| Tinosporacordifolia | 1.98±0.09 | 76.98±3.95 | 0.16±0.01 | 0.56±0.03 | 16.88±0.88 |
| Glycyrrhizaglabra | 4.94±0.43 | 69.32±2.45 | 0.42±0.02 | 0.44±0.03 | 26.64±1.82 |
| Rauwolfia serpentine | 1.16±0.08 | 77.18±3.95 | 0.12±0.01 | 2.06±0.11 | 16.40±0.77 |
| Gymnemasylvestre | 1.24±0.10 | 69.40±2.70 | 1.2±0.09 | 0.50±0.03 | 23.58±1.65 |
| Vitexnegundo | 2.72±0.18 | 78.88±6.67 | 0.24±0.02 | 0.18±0.014 | 18.48±0.97 |
| Centellaasiatica | 3.72±0.24 | 75.08±5.26 | 0.20±0.01 | 1.08±0.05 | 18.86±1.25 |
| Acacia nilotica | 5.24±0.41 | 68.58±2.39 | 0.06±0.01 | 0.48±0.04 | 25.34±1.82 |
Values are given in g/100 g of sample.
Table 2: Proximate compositions of different medicinal plants
| Medicinal plants | Moisture content | Ash | Acid Insoluble ash |
|---|---|---|---|
| Cyperusrotundus | 5.56±0.31 | 9.50±0.69 | 2.4±0.12 |
| Pterocarpusmarsupium | 7.78±0.39 | 12.4±0.68 | 12.0±0.44 |
| Terminaliaarjuna | 8.30±0.54 | 23.20±0.61 | 17.1±0.74 |
| Tribulasterrestris | 3.89±0.32 | 11.70±0.59 | 8.4±0.58 |
| Tinosporacordifolia | 5.55±0.39 | 10.8±0.74 | 4.9±0.30 |
| Glycyrrhizaglabra | 7.21±0.34 | 8.0±0.26 | 4.8±0.35 |
| Rauwolfiaserpentina | 6.66±0.38 | 4.8±0.23 | 2.6±0.09 |
| Gymnemasylvestre | 4.99±0.24 | 16.4±0.98 | 6.8±0.41 |
| Vitexnegundo | 6.10±0.54 | 8.2±0.46 | 8.0±0.24 |
| Centellaasiatica | 6.66±0.36 | 12.90±0.43 | 8.1±0.29 |
| Acacia nilotica | 6.11±0.29 | 25.1±0.69 | 21.5±0.97 |
Values are given in g/100 g of the sample.
Table 3: Moisture, ash content and acid insoluble ash of different medicinal plants
In the present investigation, the commonly accepted assays viz DPPH, FRAP and ABTS were used for the evaluation of antioxidant activity of plant extracts. The total phenolic contents and flavonoid contents were also determined. The results of these analyses are given in Table 4 and are an average of three independent measurements.
| Medicinal plants (Botanical names) | TEAC (mg/gdw)a | (Total phenolic)b | (Total flavonoid)c | ||
|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | |||
| Cyperusrotundus | 0.81±0.06 | 0.65±0.05 | 0.88±0.05 | 0.58±0.04 | 0.90±0.07 |
| Pterocarpusmarsupium | 0.94±0.07 | 1.11±0.08 | 0.90±0.05 | 1.52±0.07 | 3.67±0.22 |
| Terminaliaarjuna | 1.44±0.10 | 6.99±0.31 | 10.9±0.39 | 7.93±0.38 | 3.12±0.23 |
| Tribulasterrestris | 0.21±0.02 | 1.43±0.09 | 0.43±0.03 | 0.43±0.04 | 3.87±0.17 |
| Tinosporacordifolia | 0.20±0.02 | 1.82±0.07 | 0.36±0.02 | 0.23±0.04 | 1.50±0.09 |
| Glycyrrhizaglabra | 1.40±0.04 | 3.34±0.14 | 1.73±0.13 | 1.62±0.12 | 6.03±0.42 |
| Rauwolfia serpentine | 0.45±0.03 | 1.78±0.09 | 0.63±0.04 | 0.52±0.04 | 1.30±0.06 |
| Gymnemasylvestre | 0.76±0.06 | 1.83±0.08 | 1.00±0.07 | 1.14±0.07 | 5.55±0.35 |
| Vitexnegundo | 1.47±0.07 | 2.53±0.12 | 2.69±0.11 | 3.07±0.15 | 14.13±0.73 |
| Centellaasiatica | 1.44±0.12 | 1.86±0.11 | 0.73±0.05 | 0.75±0.06 | 7.49±0.62 |
| Acacia nilotica | 1.50±0.09 | 7.37±0.29 | 18.28±0.41 | 15.88±0.54 | 9.88±0.58 |
aTEAC, Trolox equivalent antioxidant capacity (mg, Trolox equivalents/gdw). Absorbance was converted to equivalent activity of Trolox per g of dry weight based on a standard curve. bTotal phenolic content expressed as mg of gallic acid equivalent (GAE)/g of dry weight (dw). cTotal flavonoid content expressed as mg of quercetin equivalents/g of dry weight (dw).
Table 4: Antioxidant capacity, total phenolic and flavonoid content values of different medicinal plants
An examination of Table 4 reveals that the total antioxidant activity, measured by DPPH method, ranged from 0.20 to 1.50 mg trolox equivalent per g dry weight (mg, TEAC/g dw). The results from the antioxidant assay showed that extract of all plants can scavenge the radical to a certain extent.
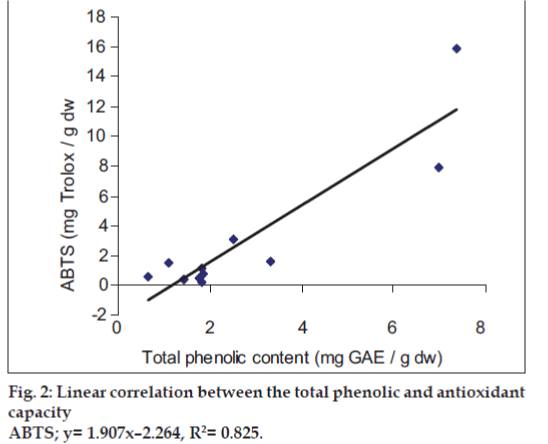
The relative antioxidant ability to scavenge the radical ABTS+ has been compared with the standard Trolox. ABTS radical cation was produced in the stable form using potassium persulphate. After getting the stable absorbance, the antioxidant plant extract is added to the reaction medium and the antioxidant power was measured by studying decolorization. The TEAC values ranged from 0.65 to 7.37 mg, TEAC/ g dw. The lowest value was observed for Cyperus rotundus (0.65 mg, TEAC/g dw) and highest for Acacia nilotica (7.37 mg, TEAC/g dw). It is to be noted that the TEAC values for all plant species obtained by ABTS assay were higher than those obtained by DPPH assay.
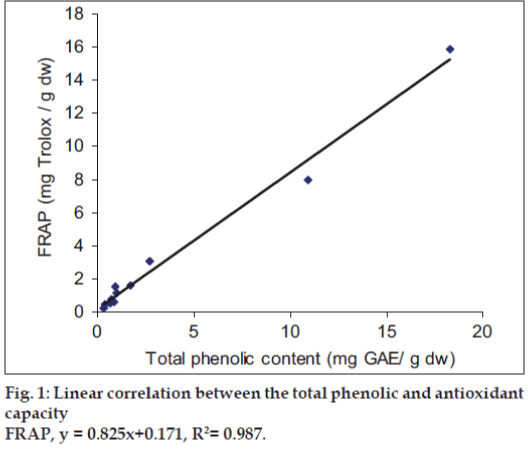
FRAP assay measures the reducing potential of an antioxidant reacting with a ferric tripyridyltriazine (Fe3+-TPTZ) complex and producing a colored ferrous tripyridyltriazine (Fe2+-TPTZ). The free radical chain breaking takes place through donating a hydrogen atom. At low pH of about 3.6, reduction of Fe3+-TPTZ complex to blue colored Fe2+-TPTZ takes place, which has absorbance at 593 nm. FRAP values of the studied plants varied from 0.36 mg, Trolox equivalent/g dw of sample (Tinospora cordifolia) to 18.28 mg, Trolox equivalent/g dw of sample (Acacia nilotica). The results obtained are highly reproducible and related linearly with the molar concentration of the antioxidants present. This is in accordance with the results reported by Benzie et al [9], and Jeong et al [10].
It can be seen from Table 4 that the total phenolic content of the samples ranges from 0.23 to 15.88 mg Gallic acid equivalent/g dw. Among the studied plants, Acacia nilotica shows the highest amount of phenolic content (15.88 mg, GAE/g dw) while the lowest content was observed in Tinospora cordifolia (0.23 mg, GAE/g dw). The considerable difference between the results of phenolic content are may be due to environmental related factors like maturity period, climate, location, temperature, fertility, diseases, part tested and pest exposure [11]. In addition, rainfalls are also reported to affect the phenolic content [12]. The presence of different phenolic compounds may be the cause in the variation of total phenolics in the plant extracts. It is observed that almost all the plant samples are rich in flavonoids. The total flavonoid content is found to vary between 0.90 (Cyperus rotundus) to 14.13 mg quercetin equivalent/g dw (Vitex negundo).
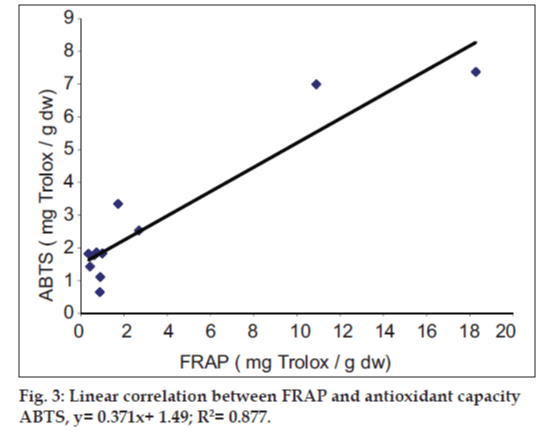
It was observed that the phenolic contents correlates well with its FRAP and ABTS assays. The correlation coefficients of TPC with FRAP and ABTS assays are 0.987 and 0.825, respectively, confirming that phenolic compounds are likely to contribute to radical scavenging activity of these plant extracts (figs.1 and 2). The significant correlation is also seen between ABTS assay and FRAP values (Correlation coefficient, R2=0.877, fig.3). Our results are in agreement with those reported by Zheng et al. [13] who found a strong correlation between total phenolic content and FRAP assay.
It was observed that the phenolic content in the extracts showed a much higher correlation with reducing power than with the radical scavenging activity. It could be estimated that the phenolic compounds present in the extracts act as an antioxidants directly through the mechanism of the reduction of oxidized intermediate in the chain reaction.
The present study provides the useful information about proximate composition, antioxidant properties and polyphenolic contents of some Indian medicinal plants, which are used for the therapeutic purposes. The low moisture content indicates good quality of plant material and its prolonged shelf life. It is interesting that the total phenolic content and flavonoid content did not correlate well with the results from the DPPH· test. The findings of this study support the fact that some medicinal plants commonly consumed in India are promising sources of potential antioxidants.
Acknowledgements
One of the authors, S. M. Hande would like to acknowledge the financial assistance provided by UGC, New Delhi.
References
- Grice HC. Safety evaluation of butylatedhydroxytoluene (BHT) in the liver, lung and gastrointestinal tract. Food ChemToxicol 1986;24:1127-30.
- Tripathi R, Mohan H, Kamat JP. Modulation of oxidative damage by natural products. Food Chem 2007;100:81-90.
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebens- WissTechnol 1995;28:25-30.
- Yen GC, Duh PD. Scavenging effect of methanolic extracts of peanut hulls on free radical and active oxygen species. J Agric Food Chem 1994;42:629-32.
- Re R, Pellegrini N, Proteggente A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free RadicBiol Med 1999;26:1231-7.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as measurement of “antioxidant power” The FRAP assay. Anal Biochem 1996;239:70-6.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol 1999;299:152-78.
- Ordon-Ez AA, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechiumedule (Jacq.) Swart extracts. Food Chem 2006;97:452-8.
- Benzie IF, Wai Y, Strain JJ. Antioxidant (reducing) efficiency of ascorbate in plasma is not affected by concentration. J NutrBiochem 1999;10:146-50.
- Jeong SM, Kim SY, Kim DR, Jo SC, Nam K, Ahn DU. Effects of heat treatment on the antioxidant activity of extracts from citrus peels. J Agric Food Chem 2004;52:3389-93.
- Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem 2005;53:7749-59.
- Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 2001;49:5165-70.
- Zheng W, Wang SY. Effect of the plant growth temperature on antioxidant capacity in strawberry. J Agric Food Chem 2001;49:4977-82.