- *Corresponding Author:
- S. Munawar
V L College of Pharmacy, Raichur, Karnataka, India-584103
E-mail: msmvlcp4@gmail.com
| Date of Received | 22 December 2020 |
| Date of Revision | 21 January 2021 |
| Date of Acceptance | 14 April 2021 |
| Indian J Pharm Sci 2021;83(2):384-388 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study was aimed to evaluate the anti-ferlility activity of Caesalpinia pulcherrima Linn in rats. The plant Caesalpinia pulcherrima is a shrub or small tree. The plant is normally considered to be an ornamental shrub which is planted in gardens and parks for its beautiful red or yellow flowers. The phytochemical screening of the plant has shown the presence of various chemical constituents like alkaloids, flavonoids, steroids and triterpenes. The bark extracts of Caesalpinia pulcherrima have exhibited significant estrogenic activity. Both the aqueous and alcoholic extracts have produced reduction in ovarian and increase in uterine weight in a dose dependent manner. Also there is a significant increase in antiimplantation and resorption in a dose dependent manner. The activity shown can be attributed to the presence of phytochemical constituents.
Keywords
Caesalpinia pulcherrima, anti-fertility activity, saponins, steroids
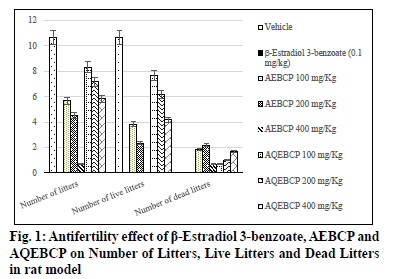
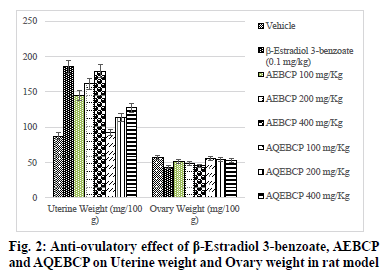
In the present scenario most emerging problem with the underdeveloped and developing countries is population growth. It itself leads to various other problems like lack of employment opportunities, poverty, illiteracy, low living standards and many more. Providing with birth control measures and educating with the same can avoid the population growth to some extent. Thus it can indirectly help for the growth a country in a positive manner. At present there are several methods to control fertility but these methods can end with complications which make them to use at a lesser extent[1]. So the present study was aimed to explore the natural potential of herbs as anti-fertility agent. The plant C. pulcherrima is a shrub or small tree[2]. The plant contains a flavonoid, myricitroside. The leaves, flowers and fruit contain tannins, gums, resin and benzoic acid. Presence of cyanidin, diglucoside is also reported from the flowers and hydrocyanic acid from the leaves. The root contains caesalpin type diterpenoids along with sitosterol[3]. The bark of the C. pulcherrima was collected from Raichur, Karnataka, India and was authenticated and confirmed by Dr. V. Hemant Kumar, Botanist V.L. College of Pharmacy, Raichur, Karnataka. The alcoholic (AEBCP) extract of dried bark powder was obtained through soxhalation and the aqueous extract (AQEBCP) was prepared by maceration process. The obtained filtrate was concentrated on the water bath[4-6]. Albino rats (Wistar strain) of either sex weighing between 150-200 g and Albino mice of either sex (16-20 g) were procured from National Centre for Laboratory Animal Sciences, C/O Sri Venkateswara Enterprises, Bengaluru for experimental purpose. All animal studies were performed in accordance to the Guidelines of Committee for the Purpose of Control And Supervision of Experiments on Animals (CPCSEA) (Registration Number 557/02/c/CPCSEA) and Institutional Animal Ethical Committee (IAEC) of V.L. College of Pharmacy, Raichur (Karnataka) and all the procedures were followed as per rules and regulations. The oral acute toxicity of AQEBCP and AEBCP was determined by following procedure of OECD Guidelines No.425 method of CPCSEA, from the observed LD50 doses 1/20th, 1/10th and 1/5th doses were selected for the present study considered as low, medium and high doses respectively. The treatment protocol for different groups of rats is given below. Group 1-Normal control (distilled water); Group-2-Standard β-Estradiol 3-benzoate in olive oil (0.1 mg/Kg daily for 20 d); Group-3-AEBCP Low dose (100 mg/Kg) for 20 d p.o; Group-4-AEBCP Medium dose (200 mg/Kg) for 20 d p.o; Group-5-AEBCP High dose (400 mg/Kg) for 20 d p.o; Group-6-AQEBCP Low dose (100 mg/Kg) for 20 d p.o; Group-7-AQEBCP Medium dose (200 mg/Kg) for 20 d p.o; Group-8-AQEBCP High dose (400 mg/ Kg) for 20 d p.o. Adult female (Wistar) rats weighing between 150-160 g were mated with adult males and vaginal smears were observed under a microscope. D 1 of pregnancy was designated as the day on which spermatozoa was observed in the vaginal smear. Pregnant rats in the group III- V were killed on d 9 of pregnancy and percent anti-implantation was determined. Rats in the groups VI-VIII were killed on d 20 of pregnancy. Percent abortion was determined by dividing the percent of resorption by the number of implantation sites. Other groups of pregnant rats were allowed to labor in which the labor time, litter size, number of dead and viable fetuses were recorded. Determination of viable fetuses was based on gross appearance of live fetuses at the time of labor[7]. Percentage of resorption index=no of animals aborted×100/No of implantations. Percentage of animals aborted=No of implants-No of litters. Group-1- Normal control (distilled water); Group-2-Standard β-Estradiol 3-benzoate in olive oil (0.1 mg/Kg daily for 15 d); Group-3-Low dose (100 mg/Kg) to AEBCP for 15 d p.o; Group-4-Medium dose (200 mg/Kg) to AEBCP for 15 d p.o; Group-5-High dose (400 mg/Kg) of AEBCP for 15 d p.o; Group-6-Low dose (100 mg/ Kg) of AQEBCP for 15 d p.o; Group-7-Medium dose (200 mg/Kg) of AQEBCP for 15 d p.o; Group-8-High dose (400 mg/Kg) of AQEBCP for 15 d p.o. Female rats were divided into 8 groups (n=6), vaginal smear from each rat were examined daily for 15 d and those rats exhibiting three regular cycles were considered for experiment and treated with vehicle, standard and extracts at low, medium and high doses p.o. daily once for 15 d in the estrus phase. The 15 d treatment should cover three regular estrus cycles. Vaginal smear from each animal were observed every morning between 9-10 AM. On the 16th d, 24 h after the treatment of last dose, the rats from each group will be anesthetized and sacrificed. Ovaries and uteri were dissected out, free from extra deposition and are weighed[8]. All the data is analyzed using graph Pad Prism by following one way Analysis of variance (ANOVA) and are considered significant at p<0.05. In vehicle treated control group number of litters is noted as (10.67±0.6667) standard β-Estradiol 3-benzoate treated group is noted with (0.0±0.0) litters. A significant and dose dependent reduction in number of litters is recorded with AEBCP (5.667±0.5578, 4.500±1.522, 0.6667±0.4216) and AQEBCP (8.333±0.5578, 7.167±0.6009, 5.833±0.7923) treated groups at three different dose levels. AEBCP exhibited relatively better effect than AQEBCP, higher dose of AEBCP treated group is noted with (0.0) number of litters. In control group (10.67±0.6667) it is noted that all litters are alive. No litters are formed after treatment with standard β-Estradiol 3-benzoate (0.0±0.0). Hence there is no chance for live litters. A significant reduction in number of live litters is noted with AEBCP (3.833±0.5426, 2.333±0.9189, 0.0±0.0) and AQEBCP (7.667±0.4216, 6.167±0.4773, 4.167±0.4773) treated rats. AEBCP exhibited relatively better antifertility activity than AQEBCP further higher dose of AEBCP is noted with (0.0) of live litters. In normal control no dead litters are noted and the group treated with standard β-Estradiol 3-benzoate also recorded with no dead litters as this group is already noted with (0.0±0.0) number of litters. A significant increase in number of dead litters is noted with AEBCP (1.833±0.7491, 2.167±0.3073, 0.6667±0.3333) and AQEBCP (0.6667±0.4216, 1.000±0.4216, 1.667±0.2582) treated groups at three different dose levels. However AEBCP exhibited relatively better antifertility activity than AQEBCP (fig. 1). In normal control no anti-implantation is noted where in standard drug β-Estradiol 3-benzoate treated group (100 %) antiimplantation effect is noted. A dose dependent and significant anti implantation effect is noted with AEBCP (36.16 %, 37.5 %, 43.16 %) and AQEBCP (19.5 %, 25.08 %, 37.58 %) treated groups at three different dose levels as mentioned earlier. AEBCP exhibited relatively better anti-implantation effect than AQEBCP. In normal control group (00.0 %) and in standard drug β-Estradiol 3-benzoate treated group (100 %) resorption is noted. Both the AEBCP (26.10 %, 40 %, 90.32 %) and AQEBCP (13.76 %, 20.35 %, 22.16 %) exhibited a significant and dose dependent % resorption effect and AEBCP exhibited relatively better effect than AQEBCP (Table 1). In normal control group average uterine weight is noted as (87.40±3.397 mg/100 g). A significant increase in uterine weight is noted with standard drug β-Estradiol 3-benzoate treated group (185.6±5.484 mg/100 g) when compared to vehicle treated control group. A significant and dose dependent increase in uterine weights are noted with AEBCP (145.1±5.063 mg/100 g, 161.3 ±7.446 mg/100 g, 179.5±2.808 mg/100 g) and AQEBCP (92.74±1.591 mg/100 g, 113.7±3.377 mg/100 g, 127.8±0.981 mg/100 g) treated rats at three different dose levels AEBCP exhibited relatively better anti-ovulatory effect in uterine weight than AQEBCP (Table 2). In normal control group average ovary weight is noted as (56.92±0.6964 mg/100 g). When compared to the normal control, standard drug β-Estradiol 3-benzoate treated group the ovary weight is significantly decreased (43.16±0.7647 mg/100 g). Where in the groups treated with three different doses of AEBCP (51.70±0.8267 mg/100 g, 48.67±1.017 mg/100 g, 45.31±0.5816 mg/100 g) and AQEBCP (56.26±0.7319 mg/100 g, 54.09±1.220 mg/100 g, 52.87±1.048 mg/100 g) a significant reduction in ovary weight is recorded except with low dose of AQBCP treated group. Adose dependent effect is noted with both the extracts (fig. 2). The development of a country is dependent on various factors. Population problem plays a major force and has a negative impact over it as the country needs to face drastic effects on particularly with employment, education, shelter, health care and sanitation & environment[9]. Abnormal increase in population increases the scarcity of food, water, energy and supply of raw materials[10]. One of the best ways to avoid population related problems is birth control[11]. Population explosion affect economic & health impact in the developing countries. Hence a global search for antifertility agents is continued throughout the World to tackle the problem[10]. Majority of research workers opine that traditional system of medicine will definitely provide a reliable contraceptive agent from the plant origin; World Health Organization (WHO) also encouraged the research workers to come out with a most valuable antifertility agent from the plants. Many years of scientific publications also proved antiproductive activity in a good number of plant extracts and their phytoconstituents with varied chemical structures/nature[12]. Contraceptive agents either from natural origin (plant based products) or synthetic (chemical) substances are intended to inhibit either production of sperm or its motility in males and in the opposite sex not allowing formation of ovum and produce definite changes in endometrium render it non receptive to fertilized ovum. Among three popular methods to control population i.e. Abortion, Sterilization and Contraception, the last one is the most popularizing one[10]. Sterilization methods based on traditional system using herbal medicines are used to prevent contraception. Drugs from the synthetic origin used for contraception with both oral and parenteral route of administration leave the individuals with a great number of adverse effects including increased trend of blood transaminases, cholesterol with other unwanted effects like headache, fatigue, depression, indigestion, weight gain, hypermenorrhea and intermenorrheal bleeding. Further these medications disturb protein, lipid and carbohydrates, enzymes and vitamins metabolism. Because of these risk factors associated with drugs necessitate exploring contraceptive drugs from indigenous medicinal plant origin[13]. Among the plants recorded in Indian medicinal literature, rural populations many use by these for one or other medicinal purposes. Further contraceptive effect with these plants is already recorded in ancient texts. These antifertility agents which are obtained from indigenous plants provide with larger benefits as the cost of these would be within the range of common man, especially in developing countries. Earlier it was reported that plants with estrogenic activity produce antifertility activity directly by influencing pituitary gland through peripheral modulation of leutinizing hormone (LH) and follicle stimulating hormone (FSH) by decreasing their secretion and also block ovulation. In addition, the plants may have abortifacient or antiprogestational effects[14]. It was reported that estrogenic activity causes a significant increase in uterine weight, diameter of the uterus, thickness of endometrium, height of the endometrial epithelium and vaginal epithelial cornification in immature rats[14]. Antifertility activity effect may be due to uterine failure to form deciduoma in the endometrium, which is very essential for blastocyst implantation[15]. For implantation of zygote exact equilibrium of estrogen and progesterone is essential and any disturbance in the level of these hormones may cause antifertility effect. It was reported that decrease in wet weight of ovary after treatment with standard/both the extracts indicate an inhibition of ovulation through suppression of follicle stimulating hormone. Several factors like Hormonal influence, alterations at implantation site, changes in endometrium of the uterus can be accounted for antifertility activity. Further it was suggested that loss of implantation may be due antizygotic, blasto cytotoxic or anti-implantation activities. It was earlier reported that an increase in the number of dead fetus as well as reduced survival ratio is an indication of the abortifacient activity The implantation index, resorption index and pre implantation loss are used to evaluate the number of blastocytes implanted in the uterus and also the underdeveloped ones. Further increase in resorption index indicates failure in the development of embryo after implantation[13]. It was reported that substances with antifertility activity produce these effects by inhibiting ovulation and steroidogenesis at ovarian level[16]. The antifertility activity of contraceptive steroids could be due to their estrogenic nature. These cause rapid passage of ova through oviducts and expulsion of the same from uterus and also due to degeneration of fertilized ova while transported uterus too early. This correlates with reduction in number of implants. Further uterine environment rather than the above mentioned plays an important role and a prolonged maintenance of diestrus phase due to the treatment also responsible for antifertility activity in this there is no change. Several phytochemicals like steroids, triterpinoids, flavonoids, myricitoside and caesalpin type diterpenoids along with sitosterol, alkaloids, terpenoids, phenolics, saponins and glycosides are already reported with their antifertility activity. Some of the phytoconstituents mentioned above are present (AEBCP: alkaloids, flavonoids, steroids, triterpenes, tannins, glycosides, saponins, carbohydrates, proteins and amino acids) (AQEBCP: alkaloids, flavonoids, steroids, triterpenes, tannins, glycosides, saponins, carbohydrates, proteins and amino acids) in both alcohol and aqueous extracts. Hence these can be accounted for the observed antifertility activity in the present study.
| Groups | Treatment | Number of litters | Number of live litters | Number of dead litters | % Anti implantation | % Resorption |
|---|---|---|---|---|---|---|
| I | Vehicle (distilled water) |
10.67 ±0.6667 |
10.67 ±0.6667 |
0.0 ±0.0 |
00 | 00 |
| II | β-Estradiol 3-benzoate 0.1 mg/Kg |
0.0 ±0.0*** |
0.0 ±0.0*** |
0.0 ±0.0*** |
100 | 100 |
| III | AEBCP 100 mg/Kg | 5.667 ±0.5578*** |
3.833 ±0.5426*** |
1.833 ±0.7491*** |
36.16 | 26.10 |
| IV | AEBCP 200 mg/Kg | 4.500 ±1.522*** |
2.333 ±0.9189*** |
2.167 ±0.3073*** |
37.5 | 40 |
| V | AEBCP 400 mg/Kg | 0.6667 ±0.4216*** |
0.0 ±0.0*** |
0.6667 ±0.3333*** |
43.16 | 90.32 |
| VI | AQEBCP 100 mg/Kg | 8.333 ±0.5578 ns |
7.667 ±0.4216 * |
0.6667 ±0.4216 ns |
19.5 | 13.76 |
| VII | AQEBCP 200 mg/Kg | 7.167 ±0.6009* |
6.167 ±0.4773** |
1.000 ±0.4216* |
25.08 | 20.35 |
| VIII | AQEBCP 400 mg/Kg | 5.833 ±0.7923*** |
4.167 ±0.4773*** |
1.667 ±0.2582*** |
37.58 | 22.16 |
n=6, Significant at *p<0.05, **p<0.01, ***p<0.001 and ns=non-significant AQEBCP-Aqueous extract of bark of C .pulcherrima, AEBCP-Alcoholic extract of bark of C .pulcherrima
Table 1: Antifertility Effect of Β-Estradiol 3-Benzoate, Aebcp And Aqebcp on Number, Live and Dead Litters in Rat Model
| Groups | Treatment | Uterine Weight (mg/100 g) |
Ovary Weight (mg/100 g) |
|---|---|---|---|
| I | Vehicle (distilled water) |
87.40±3.397 | 56.92±0.6964 |
| II | β-Estradiol 3-benzoate 0.1 mg/Kg |
185.6±5.484*** | 43.16±0.7647*** |
| III | AEBCP 100 mg/Kg | 145.1±5.063*** | 51.70±0.8267*** |
| IV | AEBCP 200 mg/Kg | 161.3 ±7.446*** | 48.67±1.017*** |
| V | AEBCP 400 mg/Kg | 179.5±2.808 *** | 45.31±0.5816*** |
| VI | AQEBCP 100 mg/Kg | 92.74±1.591 ns | 56.26±0.7319 ns |
| VII | AQEBCP 200 mg/Kg | 113.7±3.377*** | 54.09±1.220* |
| VIII | AQEBCP 400 mg/Kg | 127.8±0.9818*** | 52.87±1.048* |
n=6, Significant at *p<0.05, **p<0.01, ***p<0.001 and ns=non-significant AQEBCP-Aqueous extract of bark of C .pulcherrima, AEBCP- Alcoholic extract of bark of C .pulcherrima
Table 2: Anti-ovulatory Effect of Β-Estradiol 3-Benzoate, Aebcp And Aqebcp on Uterine and Ovary Weights in Rat Model
Conflict of interests:
The authors declared no conflicts of interest.
References
- Shikha CB. Overview and Evaluation of Antifertility Models. Inter J Chem Tech Res 2017;10(4):151-9.
- Kirtikar KR, Basu BD. Indian medicinal plants, 2nd ed. Periodical experts book agency, Delhi 1991;4:848.
- Werdelin L, Nilsonne A. The evolution of the scrotum and testicular descent in mammals: A phylogenetic view. J Theor Biol 1999;196(1):61-72.
- Mehtha RM. Pharmaceutics-1. 1st ed. Vallabha Prakashan, Delhi 1994;138-40.
- Khandelwal K. Practical pharmacognosy. Pragati Books Pvt. Ltd; 2008.
- Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. 39th ed. Nirali Prakashan, Pune 2007;607-11.
- Piyachaturawat P, Glinsukon T, Chanjarunee A. Antifertility effect of Citrus hystrix DC. J Ethnopharmacol 1985;13(1):105-10.
- Thakare VN, Kothavade PS, Dhote VV, Deshpande AD. Antifertility activity of ethanolic extract of Allium cepa Linn in rats. Int J Pharmtech Res 2009;1(1):73-8.
- Ghulam MS, Ajab KM, Mushtaq A, Zafar M, Ahmed KA. Observations on antifertility and abortifacient herbal drugs. Afr J Biotech 2009;8(9):1959-64.
- Unny R, Chauhan AK, Joshi YC, Dobhal MP, Gupta RS. A review on potentiality of medicinal plants as the source of new contraceptive principles. Phytomedicine 2003;10(2-3):233-60.
- Singh A, Singh SK. Evaluation of antifertility potential of Brahmi in male mouse. Contraception 2009;79(1):71-9.
- Nath D, Sethi N, Singh RK, Jain AK. Commonly used Indian abortifacient plants with special reference to their teratologic effects in rats. J Ethnopharmacol 1992;36(2):147-54.
- Yakubu MT, Bukoye BB. Abortifacient potentials of the aqueous extract of Bambusa vulgaris leaves in pregnant Dutch rabbits. Contraception 2009;80(3):308-13.
- Hiremath SP, Rudresh K, Badami S, Patil SB, Patil SR. Post-coital antifertility activity of Acalypha indica L. J Ethnopharmacol 1999;67(3):253-8.
- Yakubu MT, Adeshina AO, Oladiji AT, Akanji MA, Oloyede O, Jimoh GA, et al. Abortifacient potential of aqueous extract of Senna alata leaves in rats. Int J Reprod Contracept 2010;21(3):163-77.
- Ganguly M, Devi N, Mahanta R, Borthakur MK. Effect of Mimosa pudica root extract on vaginal estrous and serum hormones for screening of antifertility activity in albino mice. Contraception 2007;76(6):482-5.