- *Corresponding Author:
- Zichao Chen
School of Physical Education, Sichuan University, Chengdu, Sichuan, 610064, China
E-mail: sli37czz@163.com
| Date of Submission | 24 February 2017 |
| Date of Revision | 12 September 2017 |
| Date of Acceptance | 18 April 2018 |
| Indian J Pharm Sci 2018;80(3):510-515 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The main objective of this study was to investigate the antifatigue effects of 20(S)-ginsenoside Rg3 using forced swimming test in mice. Male Kunming mice were randomly divided into four groups; a control and three 20(S)-ginsenoside Rg3-treated groups. The control group was given distilled water whereas the treated groups were given different doses of 20(S)-ginsenoside Rg3 (10, 20 and 40 mg/kg). Treatments were administered by gavage once a day lasting for 28 days. After 28 days, forced swimming tests were performed and fatigue related biochemical parameters were examined. The results showed that 20(S)-ginsenoside Rg3 significantly (P<0.05) prolong exhaustive exercise time, significantly decrease (p<0.05) the blood lactic acid and serum urea nitrogen levels, significantly increase (p<0.05) the levels of glycogen, superoxide dismutase, glutathione peroxidase and catalase in liver and muscle, as well as significantly (p<0.05) reduce malondialdehyde levels in liver and muscle. These findings suggested that 20(S)-ginsenoside Rg3 has antifatigue effects, and its antifatigue mechanisms could be related to the fact that 20(S)-ginsenoside Rg3 can activate energy metabolism, eliminate the accumulation of metabolites, and protect exercise-induced oxidative stress.
Keywords
20(S)-ginsenoside Rg3, antifatigue, forced swimming test, biochemical parameters, mouse
Fatigue is best defined as the status that the living body's physiological processes cannot continue its function at a particular level or the organs cannot keep their predetermined exercise intensity, and it can be divided into mental and physical fatigue [1]. Physical fatigue, or peripheral fatigue, is mainly manifested as decreased exercise capability, while mental fatigue is mainly manifested as negative changes in well and behavior, such as decreased attention, reduced ability to concentrate, mental malaise, forgetfulness, somnolence and dizziness [2]. It is known that physical fatigue is one of the important reasons for the performance of the body and often leads to premature aging, cancer, depression and other potential physical illnesses [3]. At present, there are a series of theories about physical fatigue mechanisms, such as exhaustion theory, blockage theory, catastrophe theory, protective suppression theory and free radical theory [4]. In recent years, free radical theory has attracted more and more attention, and antioxidants play an important role in delaying the degree of fatigue through the oxidation of inter or intra cellular oxidizable substrates [5]. Since the clinical drugs used to treat fatigue are not very effective, potential substitutes from natural ingredients are worth investigating. In fact, it has been reported that a variety of natural antioxidant components, such as flavonoids, polysaccharides, polypeptides, and saponins, are effective in preventing or delaying fatigue [6,7].
Ginseng, the dried root of Panax ginseng C. A. Mayer (Araliaceae), is one of the most widely used herbal medicines in many countries and regions of East Asia and Southeast Asia, and it is a tonic, restorative and antiaging agent in the Traditional Chinese Medicine [8]. Ginsenosides, a group of triterpenoids dammarane structure of saponins, are the most important bioactive ingredients in ginseng, which are considered to be responsible for the nutraceutical actions of ginseng [9]. Nearly 60 saponin compounds have been obtained from ginseng, including dammarane-type protopanoxadiol saponin, dammarane-type protopanaxatriol saponin, oleanane-type saponin, and other types of saponins [10]. The pharmacological effects of these ginsenosides are different, some of which even show the opposite effects [11]. Among these, ginsenoside Rg3 accounts for about 4.7 % of all saponins, which has been widely paid attention to due to its extensive pharmacological activities, such as antioxidant, antistress, anticancer, antidiabetic, antiinflammation, antinociceptive and antiangiogenic activities [12,13]. According to the different spatial positions of the groups attached to the C-20 position, ginsenoside Rg3 has two kinds of epimers, i.e. 20(R)-Rg3 and 20(S)-Rg3, and compared with the R form, the S form exhibits significantly higher antioxidant capacity [14]. In fact, strong antioxidants have been shown to reduce the severity of fatigue because they can relieve some peroxides by removing reactive oxygen species produced during exercise [15]. In addition, researchers have found that 20(R)-Rg3 had antifatigue effect on mice after intranasal administration, which could increase the storage of glycogen and reduce the accumulation of metabolites [16]. However, there are few studies on the antifatigue activity of 20(S)-Rg3. Therefore, the current study was designed to evaluate the antifatigue effects of 20(S)-Rg3 using forced swimming test in mice. The antifatigue mechanisms were also clarified by measuring related biochemical parameters, including blood lactic acid (BLA), serum urea nitrogen (SUN), tissue glycogen, serum antioxidant enzymes and malondialdehyde (MDA).
Materials and Methods
All experimental procedures involving animals were carried out in accordance with the Regulations on the Administration of Laboratory Animals of China (Order No. 2 of 1988 of National Science and Technology Commission of the People's Republic of China, promulgated on October 31, 1988). Male Kunming mice (18-22 g) were obtained from Sichuan Research Animal Center (Chengdu, China). The animals were fed under controlled environmental conditions of temperature (24±1°), humidity (55±5 %) and a 12 h light/dark cycle, and maintained on standard pellet diet and tap water ad libitum. This study was formally approved by the Animal Research Ethics Committee at Sichuan University (Chengdu, China).
20(S)-Rg3 (purity >98 %) was bought from Hubei Ju Sheng Technology Co., Ltd. (Tianmen, China). The assay kits for BLA and tissue glycogen were bought from Hongshun Sheng Technology Co., Ltd. (Shanghai, China). The assay kit for SUN was bought from Yanyu Chemicals Co., Ltd. (Shanghai, China). The assay kits for superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT) and MDA were bought from Jianchen Institute of Biology (Nanjing, China). All other chemicals and reagents were of analytical grade and were bought through commercial channels.
Experimental design
After an adaptation period of one week, 40 mice were randomly divided into four groups, each consisting of eight (n=10) animals. The first group designated as control (C) group got physiological saline (1.0 ml), while the other three groups got 10, 20 and 40 mg/kg of 20(S)-Rg3, respectively and were accordingly designated as low-dose 20(S)-Rg3 (Rg3-L) group, moderate-dose 20(S)-Rg3 (Rg3-M) group and highdose 20(S)-Rg3 (Rg3-H) group. Treatments were administered by gavage once a day lasting for 28 d. 20(S)-Rg3 solutions were prepared through dissolving it in 1.0 ml physiological saline. The dose of 20(S)-Rg3 was based on a preliminary experiment.
After 28 d, forced swimming tests were performed as described previously by Xu and Zhang [17] and made slight adjustment. Briefly, after the last treatment, the mice rested for 30 min and then were placed in an acrylic plastic pool (90×60×60 cm), whose depth was 30 cm, and the water temperature were kept at 25±1°. Exhaustion was defined when the mice failed to ascend to the water surface for breathing within 7 s [18]. The exhaustive swimming time was used as an indicator of exercise endurance and antifatigue effects.
Biochemical analysis
As soon as the forced swimming tests were finished, the mice were anaesthetized with ether and killed by decapitation. The blood samples were collected for the BLA determination, and serum was separated by centrifugation (1064×g, 15 min) at 4° for the determination of SUN. After the blood was collected, the liver and gastrocnemius muscle were immediately removed and homogenized in a cold 10 % KCl solution (10 ml/g tissue) using a homogenizer. The suspension was centrifuged (1064×g, 10 min) at 4° and clear supernatant was used for the determination of glycogen, SOD, GPx, CAT and MDA. The above biochemical parameters were determined using commercial kits according to the manufacturer’s recommended protocol.
Statistical analysis
All data are expressed as means±SD. Statistical analysis was performed using SPSS 16.0 software for one-way analysis of variance and then Duncan multi-range post hoc tests. P<0.05 was found to be statistically significant.
Results and Discussion
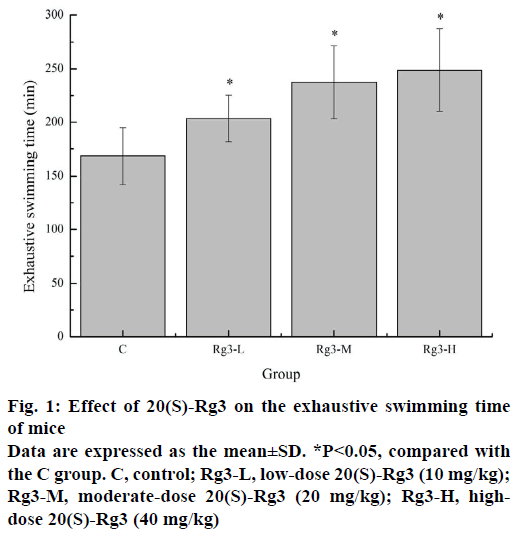
The forced swimming test is a commonly used experimental animal model to evaluate the potential antifatigue effect of the drug, because it is very effective in evaluating the exercise endurance of animals and has a highly repeatable result [19]. The exhaustive swimming time is directly related to the fatigue degree. As shown in Figure 1, compared with the C group (168.39±26.37 min), the exhaustive exercise times of the Rg3-L, Rg3-M and Rg3-H groups (203.56±21.89, 237.39±34.25, and 248.76±38.71 min) were significantly longer (p<0.05). These results revealed, 20(S)-Rg3 has obvious antifatigue effects.
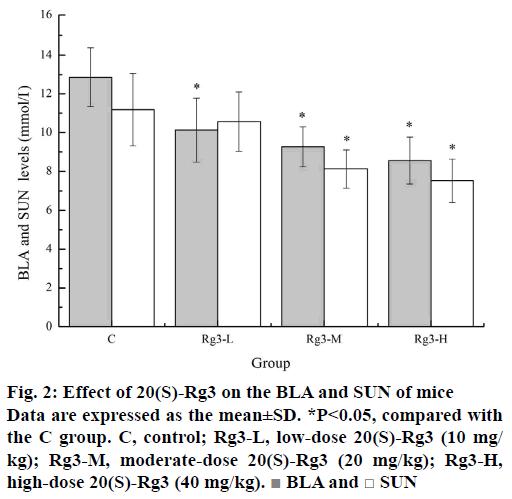
Lactic acid is the glycolysis product of carbohydrate under anaerobic conditions. Under normal circumstances, the production and removal of BLA is in a dynamic balance. Intensive exercise can be a critical point where BLA production increases beyond its removal rate, which can lead to various biochemical and physiological side effects of glycolysis, phosphofructokinase and muscle contraction caused by calcium release [20]. It has been confirmed that the increase in BLA and the resulting lactic acidosis in skeletal muscle during exercise are the main causes of muscle fatigue [21]. Urea nitrogen is a metabolite of protein and amino acids. SUN represents normal kidney function. However, there are many factors, such as protein decomposition, dehydration, stress, and fatigue, can lead to changes in SUN levels [3]. After intense exercise, when the body cannot get enough energy through sugar and fat catabolism, the SUN level will increase due to strong protein catabolism. Thus, the increased SUN usually reflects the decomposition of the protein, which will compromise the muscle's contraction strength and eventually lead to fatigue. As can be seen from Figure 2, the BLA levels of the Rg3-L, Rg3-M and Rg3-H groups (10.13±1.65, 9.27±1.03, and 8.56±1.21 mmol/ml) were significantly lower (p<0.05) than that of the C group (12.86±1.52 mmol/ml). Similarly, the SUN levels of the Rg3-M and Rg3-H groups (8.13±0.98, and 7.52±1.11 mmol/ml) were also significantly lower (p<0.05) than that of the C group (11.19±1.87 mmol/ml). These results indicated that 20(S)-Rg3 can effectively delay or reduce the production of BLA and reduce the energy of protein catabolism, which is an important reason for its delayed fatigue effect.
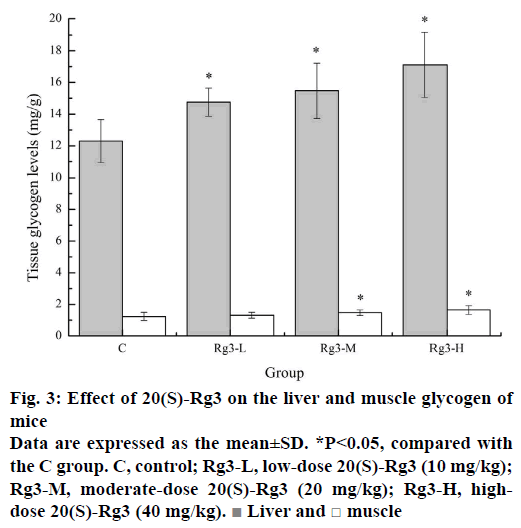
Energy storage and replenishment are most closely related to fatigue and exercise performance. Energy for exercise is derived initially from the breakdown of glycogen. Strenuous exercise will deplete muscle glycogen, and later, energy will be produced from circulating glucose released by the liver. Depletion of liver glycogen may lead to hypoglycaemia impairing nervous function in over exercise fatigue condition [22]. Therefore, glycogen is one of the sensitive indicators that reflects the fatigue degree. Figure 3 showed the effects of different doses of 20(S)-Rg3 on glycogen in liver and muscle. The liver glycogen levels of the Rg3-L, Rg3-M and Rg3-H groups (14.75±0.89, 15.47±1.75, and 17.11±2.06 mg/g) were significantly higher (p<0.05) than that of the C group (12.29±1.36 mg/g). Similarly, the muscle glycogen levels of the Rg3-M and Rg3-H groups (1.47±0.17, and 1.64±0.28 mg/g) were significantly higher (p<0.05) than that of the C group (1.23±0.26 mg/g). These results indicated that 20(S)-Rg3 can alleviate the consumption of glycogen or increase the glycogen reserve, which plays a role in the recovery of fatigue.
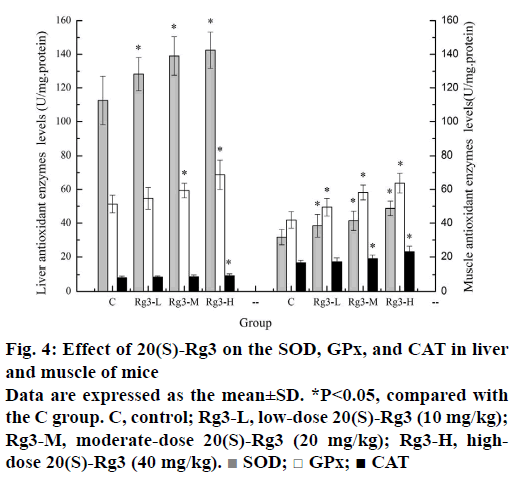
Strenuous exercise increases the oxygen utilization and ROS production rate may exceed the ability of the body's antioxidant defense system to remove them, resulting in increased oxidative stress, further causing the oxidation of lipids, proteins and nucleic acids, which may ultimately damage biomembranes and muscular structures, thereby inducing muscle damage and fatigue [23]. Enzyme antioxidant defense systems, including SOD, CAT and GPx, are important for scavenging free radicals and their metabolites. SOD catalyses the conversion of superoxide radical to H2O2, which is the major antioxidant enzyme to remove superoxide radicals. GPx is a selenium enzyme that catalyses the reduction of hydroperoxides at the expense of reduced glutathione. In addition, GPx is considered to be an effective antilipid peroxidation protective enzyme. CAT is predominantly located in the peroxisomes and is responsible for reducing H2O2 produced by long chain fatty acid metabolism in the peroxisomes [24]. Since body's antioxidant defense system is weak in fatigue state, improved antioxidant enzymes activity can help fight fatigue. Figure 4 showed the effects of different doses of 20(S)-Rg3 on antioxidant enzymes in liver and muscle. The SOD levels in liver of the Rg3-L, Rg3-M and Rg3-H groups (128.21±10.86, 138.99±11.45, and 142.43±12.78 U/ mg.protein) were significantly higher (p<0.05) than that of the C group (112.61±14.41U/mg.protein), The SOD levels in muscle of the Rg3-L, Rg3-M and Rg3-H groups (38.47±4.61, 41.39±5.72, and 48.74±5.36 U/ mg.protein) were significantly higher (p<0.05) than that of the C group (31.74±4.53 U/mg.protein), The GPx levels in liver of the Rg3-M and Rg3-H groups (59.34±4.87, and 68.69±8.74 U/mg.protein) were significantly higher (p<0.05) than that of the C group (51.34±5.21 U/mg.protein). The GPx levels in muscle of the Rg3-L, Rg3-M and Rg3-H groups (49.44±5.05, 58.21±4.36, and 63.75±5.84 U/mg.protein) were significantly higher (p<0.05) than that of the C group (41.89±4.95 U/mg.protein). The CAT levels in liver of the Rg3-H group (9.37±1.13 U/mg.protein) were significantly higher (p<0.05) than that of the C group (8.13±0.96 U/mg.protein). The CAT levels in muscle of the Rg3-M and Rg3-H groups (18.94±2.14, and 23.13±3.18 U/mg.protein) were significantly higher (p<0.05) than that of the C group (16.51±1.52 U/ mg.protein). These results indicated that 20(S)-Rg3 can reduce oxidative stress and delay fatigue by increasing antioxidant enzymes activity, and it may be because that exogenous antioxidants of 20(S)-Rg3 can directly or indirectly work with the endogenous antioxidant synergies to form a cooperative network of cellular antioxidants to protect the fatigue.
Figure 4: Effect of 20(S)-Rg3 on the SOD, GPx, and CAT in liver and muscle of mice
Data are expressed as the mean±SD. *P<0.05, compared with the C group. C, control; Rg3-L, low-dose 20(S)-Rg3 (10 mg/kg); Rg3-M, moderate-dose 20(S)-Rg3 (20 mg/kg); Rg3-H, highdose 20(S)-Rg3 (40 mg/kg).  SOD; □ GPx; ■ CAT
SOD; □ GPx; ■ CAT
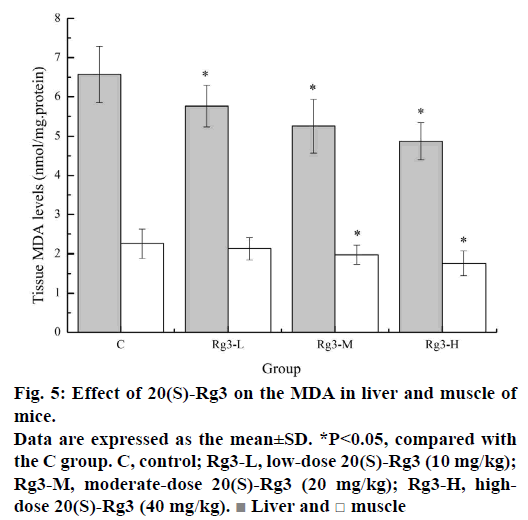
Lipid peroxidation is known to play an important role in the pathophysiology of fatigue. Strenuous exercise-induced ROS can attack polyunsaturated fatty acids, leading to lipid peroxidation. Early studies have shown that lipid peroxidation in liver and muscle tissue is increased during strenuous exercise [25]. MDA is one of the most important secondary products produced during the oxidation of polyunsaturated fatty acids, and its content is positively correlated with lipid peroxidation. MDA is commonly used as an indicator of the degree of lipid peroxidation [26]. As shown in Figure 5, the MDA levels in liver of the Rg3-L, Rg3-M and Rg3-H groups (5.76±0.53, 5.25±0.68, and 4.87±0.47 nmol/mg.protein) were significantly lower (p<0.05) than that of the C group (6.57±0.72 nmol/mg.protein). The MDA levels in muscle of the Rg3-M and Rg3-H groups (1.98±0.24, and 1.76±0.31 nmol/mg.protein) were significantly lower (p<0.05) than that of the C group (2.26±0.37 nmol/mg.protein). These results indicated that Rg3 can reduce lipid peroxidation to ameliorate fatigue.
It can be concluded from the present study that 20(S)-Rg3 has antifatigue effects by enhancing the exhaustive exercise time of mice, and its antifatigue mechanisms involves the following factors: first, 20(S)-Rg3 eliminates the accumulation of metabolites by reducing BLA and SUN levels; second, 20(S)-Rg3 activates energy metabolism by increasing the glycogen levels in liver and muscle; third, 20(S)-Rg3 protects exercise-induced oxidative stress by increasing the levels of SOD, GPx, and CAT, as well as decreasing the MDA levels in liver and muscle of mice. Further research is needed to elucidate its antifatigue molecular mechanisms and antifatigue-associated genes, such as glucose transport protein 4 and AMP-activated protein kinase. The findings of this study provide convincing evidences that 20(S)-Rg3 can be an ergogenic and antifatigue agent for the athletes in exercise.
Financial support and sponsorship
The work was supported by the Cross-Disciplinary Research Projects of Sichuan University of China (No. SKQY201618, and No.SKQY201617).
Conflict of interest
Authors declare that there are no conflicts of interest.
References
- Ni W, Gao T, Wang H, Du Y, Li J, Li C, et al. Anti-fatigue activity of polysaccharides from the fruits of four Tibetan plateau indigenous medicinal plants. J Ethnopharmacol 2013;150(2):529-35.
- Panossian A, Wikman G. Evidence-based efficacy of adaptogens in fatigue and molecular mechanisms related to their stress-protective activity. Curr Clin Pharmacol 2009;4(3):198-219.
- Han L, Meng J, Yang Y, Ye Y, Suo YE. Antioxidant and antifatigue activities of seed oil from the berries of three indigenous plants in Tibetan Plateau. J Food Nutr Res 2015;3(7):445-57.
- Chen QP, Wei P. Icariin supplementation protects mice from exercise-induced oxidant stress in liver. Food Sci Biotechnol 2013;22(5):1-5.
- Kumar GP, Anand T, Singsit D, Khanum F, Anilakumar KR. Evaluation of antioxidant and antifatigue properties of Trigonella foenum-graecum L. in rats subjected to weight loaded forced swim test. Pharma J 2013;5(2):66e71.
- Zhao XN, Liang JL, Chen HB, Liang YE, Guo HZ, Su ZR, et al. Anti-Fatigue and antioxidant activity of the polysaccharides isolated from Millettiae speciosae Champ. Leguminosae. Nutrients 2015;7(10):8657-69.
- Wu C, Chen R, Wang XS, Shen B, Yue W, Wu Q. Antioxidant and anti-fatigue activities of phenolic extract from the seed coat of Euryale ferox Salisb. and identification of three phenolic compounds by LC-ESI-MS/MS. Molecules 2013;18(9):11003-21.
- Wang H, Peng D, Xie J. Ginseng leaf-stem: bioactive constituents and pharmacological functions. Chin Med 2009;4:20.
- Yu SH, Huang HY, Korivi M, Hsu MF, Huang CY, Hou CW, et al. Oral Rg-1 supplementation strengthens antioxidant defense system against exercise-induced oxidative stress in rat skeletal muscles. J Int Soc Sports Nutr 2012;9(1):23.
- Luo H, Sun C, Sun Y, Wu Q, Li Y, Song J, et al. Analysis of the transcriptome of Panax notoginseng root uncovers putative triterpene saponin-biosynthetic genes and genetic markers. BMC Genomics 2011; S5:1-5.
- Korivi M, Hou CW, Huang CY, Lee SD, Hsu MF, Yu SH, et al. Ginsenoside-Rg1 protects the liver against exhaustive exercise-induced oxidative stress in rats. Evid Based Complement Alternat Med 2012; 932165:1-7.
- Kim YJ, Park SM, Jung HS, Lee EJ, Kim TK, Kim TN, et al. Ginsenoside Rg3 prevents INS-1 cell death from intermittent high glucose stress. Islets 2016;8(3):57-64.
- Ahn EJ, Choi GJ, Kang H, Baek CW, Jung YH. Antinociceptive effects of ginsenoside Rg3 in a rat model of incisional pain. Eur Surg Res 2016;57(3-4):211.
- Yue PY, Wong DY, Wu PK, Leung PY, Mak NK, Yeung HW, et al. The angiosuppressive effects of 20(R)-ginsenoside Rg3. Biochem Pharmacol 2006;72(4):437-45.
- Sun S, Niu H, Yang T, Lin Q, Luo F, Ma M. Antioxidant and anti-fatigue activities of egg white peptides prepared by pepsin digestion. J Sci Food Agric 2014;94(15):3195-200.
- Tang W, Zhang Y, Gao J, Ding X, Gao S. The anti-fatigue effect of 20(R)-ginsenoside Rg3 in mice by intranasally administration. Biol Pharm Bull 2008;31(11):2024-27.
- Xu YX, Zhang JJ. Evaluation of anti-fatigue activity of total saponins of Radix notoginseng. Indian J Med Res 2013;137(1):151-55.
- Cai RL, Yang MH, Shi Y, Chen J, Li YC, Qi Y. Antifatigue activity of phenylethanoid-rich extract from Cistanche deserticola. Phytother Res 2010;24(2):313-15.
- Chen CS, Wang ML, Liu RH, Chen SP, Lu TM, Tsai WY, E, et al. Antifatigue effect of aqueous extract of Anisomeles indica (L) Kuntze in mice. Trop J Pharm Res 2016;15(3):489-95.
- Wu RE, Huang WC, Liao CC, Chang YK, Kan NW, Huang CC. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013;18(4):4689-702.
- Xu C, Lv J, Lo YM, Cui SW, Hu X, Fan M. Effects of oat β-glucan on endurance exercise and its anti-fatigue properties in trained rats. Carbohydr Polym 2013;92(2):1159-65.
- Moon PD, Kim KY, Rew KH, Kim HM, Jeong HJ. Antifatigue effects of porcine placenta and its amino acids in a behavioral test on mice. Can J Physiol Pharmacol 2014;92(11):937-44.
- Liu Y, Liu C. Antifatigue and increasing exercise performance of Actinidia arguta crude alkaloids in mice. J Food Drug Anal 2016;24(4):738-45.
- Zheng X, Long W, Liu G, Zhang X, Yang X. Effect of seabuckthorn (Hippophae rhamnoides ssp. sinensis) leaf extract on the swimming endurance and exhaustive exercise-induced oxidative stress of rats. J Sci Food Agric 2012;92(4):736-42.
- Zhang C, Zheng X. Effects of polysaccharides from Pseudostellaria heterophylla on exercise endurance capacity and oxidative stress in forced swimming rats. Sci Res Essays 2011;6(11):2360-65.
- Guo R, Qi B. Protective effect of polysaccharides from Cortex Eucommiae on exhaustive exercise-induced oxidative stress in mice. J Biomed Res 2015;26(2):375-79.