- *Corresponding Author:
- Victoria C. Khangembam
Division of Animal Biochemistry, ICAR-Indian Veterinary Research Institute, Bareilly-243 122, India
E-mail: drvictoriachanu@rediffmail.com
| Date of Submission | 06 July 2017 |
| Date of Revision | 31 March 2018 |
| Date of Acceptance | 03 October 2018 |
| Indian J Pharm Sci 2018;80(6):1069-1077 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Bioactive compounds present in Parkia javanica seeds are reported to have anticancer effect. The present study was taken up to evaluate the apoptosis inducing ability of methanol extract of Parkia javanica seeds in cancer cells. The extract was found to cause 50 % cell death in HeLa and MCF-7 cells at 0.54 and 0.74 mg/ml, respectively, which is lower than that of normal healthy cells. In haematoxylin-eosin and fluorescent staining of the extract-treated cancer cells, chromatin condensation and nuclear fragmentation were observed. Fluorescent-activated cell sorting analysis using Annexin V-fluorescein isothiocyanate/propidium iodide also revealed the presence of apoptotic and secondary necrotic cell populations in the extract-treated cancer cells. In gel electrophoresis, DNA fragmentation, a biochemical hallmark of apoptosis was also observed. Caspase-3, an enzyme activated both in extrinsic and intrinsic pathways of apoptosis was detected in extract-treated HeLa cells by immunocytochemistry. The results of the present study suggested that Parkia javanica seed has the ability to induce apoptosis in cancer cells and could be considered in future endeavours for development of safer natural anticancer agent.
Keywords
Parkia javanica, methanolic extract, apoptosis, cancer cells
In recent years, considerable efforts have been made to identify naturally occurring substances for development of alternative therapeutics against cancer. Phytochemicals like flavonoids from fruits and vegetables have shown to exert varied beneficial biological functions including antitumor activities [1]. Other plant-derived chemical compounds like terpenoids, anthraquinones and saponins are also reported to have anticancer effect [2-4]. Another good example of phytochemical used in treatment of cancer is alkaloids [5]. Such health beneficial bioactive compounds can also be sourced from plants, which are used in traditional medicine.
In the present work, Parkia javanica, a common vegetable which is reported to possess medicinal properties was evaluated for its anticancer activity against cancer cells. Various parts of this plant are edible right from the inflorescence and tender pods to mature seeds. The seeds are a good source of protein and minerals [6]. The matured seeds with or without the pod can be dried and stored ensuring its availability throughout the year. Traditional use of this plant includes treatment of diabetes, intestinal disorder, bleeding piles, diarrhoea and dysentery [7,8]. Such medicinal values of plant to protect against diseases may be associated with their antioxidant properties [9]. Previous in vitro antioxidant assay had shown that P. javanica seed extracts are rich in antioxidant activity [10]. Parkia species are known to have bioactive compounds such as cyclic polysulphides, which are used for treatment of kidney, ureter and urinary bladder infections due to their antibacterial activity [11]. Another compound, a cyclic sulphur containing amino acid, thioproline, which is responsible for the pungent smell in seeds had been identified in P. javanica [12]. This compound, thioproline produced anticarcinogenic effect against squamous cell carcinoma of rats [13,14]. Though the anticarcinogenic effect of phytochemicals present in P. javanica is long known, only few studies were reported on the anticancer activity of P. javanica seed against human cancer cells. Therefore, the present study was undertaken to evaluate the anticancer activity of P. javanica seed extract and its ability to induce apoptosis in cancer cells.
Materials and Methods
All the reagents used in the study were of analytical and cell culture grade and have been mentioned elsewhere in the text. The glassware used in the study were procured from Borosil, India. All the plastic wares including microfuge tubes, microtips were obtained from Tarson, India. Cover slips were obtained from Nunc, Denmark. Cell culture plate, tissue culture flask, cell scraper were from TPP (Switzerland) and 0.2 μm filter was purchased from Nalgene (USA). Cervical cancer cell line, HeLa and breast cancer cell line, MCF-7 were received as gifts from the Department of Pathology, All India Institute of Medical Sciences, New Delhi, India.
Preparation of plant extract
Dry P. javanica seeds were collected from Manipur, India and were identified in the Department of Botany under the identification number, 2011030657610A. The seeds were powdered and then extracted with methanol in a Soxhlet apparatus (Macro Scientific Works, Delhi). Evaporation of the solvent followed by freeze drying (Heto Power Dry LL3000 Freeze Dryer) yielded the crude dry extract (MPJ). The extract was dissolved in 1 % dimethyl sulfoxide (DMSO, in cell culture media), filtered and used for further tests.
Determination of IC50 of the extract in HeLa and MCF-7 cells
Cancer cells, HeLa and MCF-7 were suspended at a density of 1 00 000 cells/ml in Dulbecco's modified Eagle's medium (DMEM; Mediatech Inc, VA) and incubated for 24 h. The cells were then treated with various concentrations of MPJ ranging from 0.1 to 1 mg/ml. Negative control cells were treated with vehicle (1 % DMSO in DMEM). Cells treated with Fluracil (Biochem Pharmaceutical Industrial Ltd., Mumbai), a known anticancer drug was used as positive control for cell death. The viability of the cells was assessed by 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Amresco, USA) uptake method [15]. The assay was based on the reduction of MTT by the mitochondrial dehydrogenase of intact cells to a purple formazan product. The amount of formazan was determined by measuring the absorbance at 570 nm using ELISA plate reader (BioRad, Model 680). Results were expressed as the percent growth inhibition with respect to vehicle-treated cells and IC50 of MPJ was determined.
Cytotoxicity assay of MPJ
For cytotoxicity assay of MPJ on normal healthy cells, lymphocytes were isolated by density gradient centrifugation according to the method of Baker and Knoblock [16] from a healthy donor. Lymphocytes were counted using the trypan blue exclusion method and diluted to the appropriate concentration (106/ml) with Roswell Park Memorial Institute medium containing 10 % fetal bovine serum and 1 % penicillin (10 000 IU) and streptomycin (100 μg/ml). One hundred microlitres of the cell suspension was dispensed into each well of a 96-well plate. The cells were treated with different concentrations of MPJ. The cytotoxic effect of MPJ on healthy lymphocytes was determined by MTT assay.
Haematoxylin and Eosin staining of cells
HeLa and MCF-7 cells were prepared for morphological examination [17]. Cells were grown on cover slips at a density of 1 00 000 cells/ml in six well plate and allowed to attach for 24 h and then treated with the IC50 of MPJ. The cells were then incubated for 24, 48 and 72 h. The cover slips were then removed from the wells and processed for haematoxylin and eosin staining [18]. The cover slips were then mounted on a clean glass slide and observed under microscope (Nikon Eclipse E600) fitted with camera (Olympus, DP71).
Fluorescent staining of nuclei
HeLa and MCF-7 cells (100 000 cells/ml) were seeded in six well plates containing cover slips and incubated for 24 h. The growth media was removed and the cells were then treated with MPJ and incubated for 24 h. Media was removed from the well, washed once with serum free media and 1 ml of phosphate-buffered saline (PBS) containing 0.2 μg of Hoechst S769121 (Invitrogen) was added and kept for 5 min in dark at room temperature. The cover slip was then mounted on a clean glass slide with aqueous mountant (Vector Laboratories, Inc. Burlingame, CA) and observed under microscope.
Assessment of cell populations by fluorescenceactivated cell sorting (FACS)
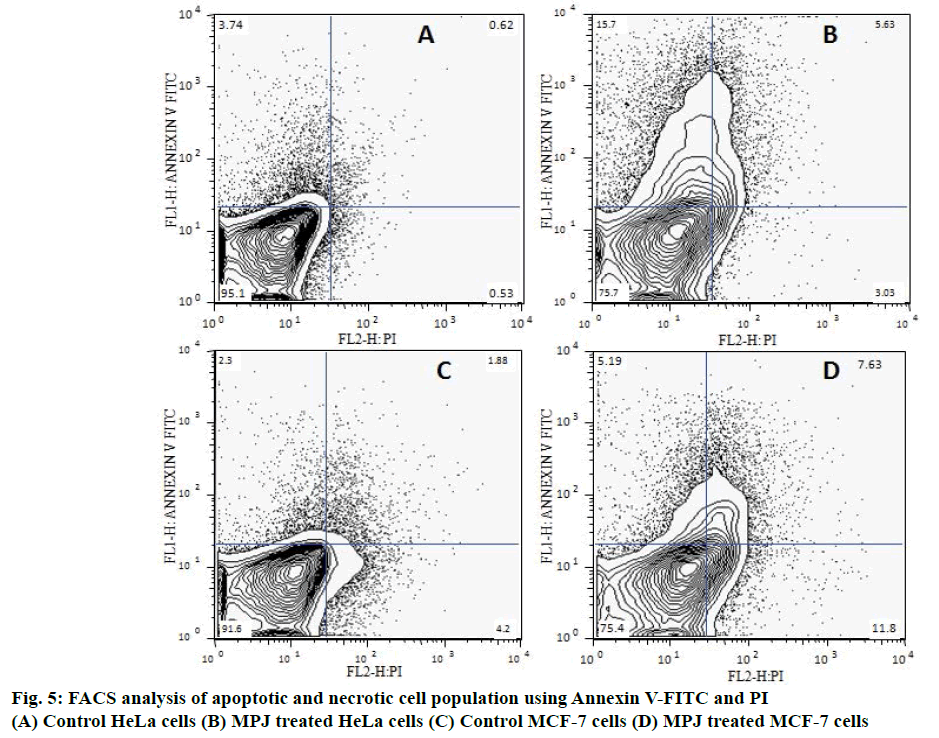
The apoptotic and necrotic cell population in the treated cancer cells was analysed by FACS (BD FACS Calibur, USA) using Annexin V-FITC (Calbiochem, EMD chemicals Inc. CA) and propidium iodide (PI; Sigma Chemicals Co., St. Louis, USA) as per the protocols of the kit. After exposing to the extract for 12 h, cells were harvested and the cell suspension was adjusted to a concentration to 1 00 000 cells/ml. The harvested cells were washed with PBS and suspended in 100 μl binding buffer (1X). Cells were incubated with 5 μl Annexin V-FITC and 5 μl PI for 15 min at room temperature in dark and 400 μl binding buffer (1X) was added to each microtubes. The FITC and PI fluorescence were measured through FL-1 filter (530 nm) and FL-2 filter (585 nm), respectively and more than 50 000 events were acquired. Annexin V/PI dot plots was positioned into quadrants to distinguish live cells (Annexin V–/PI–), early apoptotic/primary apoptotic cells (Annexin V+/PI–), late apoptotic/ secondary apoptotic cells (Annexin V+/PI+) and Annexin V–/PI+ necrotic cells [19].
DNA fragmentation analysis by gel electrophoresis
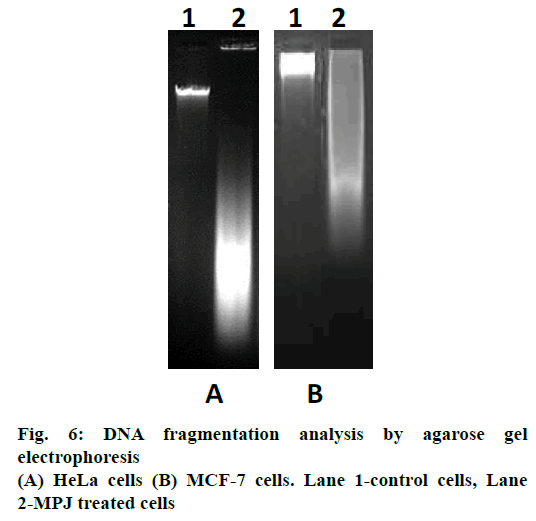
DNA fragmentation in the MPJ-treated cancer cells was assessed by agarose gel electrophoresis [20]. Following treatment with MPJ for 24 h, the cells (both adherent and non-adherent) were washed with cold PBS and pelleted by centrifugation. Cell pellets were then lysed with 50 μl of lysis buffer (1 % Triton X-100 in 20 mM ethylenediaminetetraacetic acid, 50 mM TrisHCl, pH 7.5) for 20 s and centrifuged at 1600×g for 5 min. The supernatant was removed and extraction from the pellet was repeated with the same amount of lysis buffer. Supernatants containing DNA fragments was brought to 1 % sodium dodecyl sulfate and treated with 5 μg/μl RNase A for 2 h at 56° followed by protein digestion with proteinase K (2.5 μg/μl) for 2 h at 37°. DNA was precipitated with 0.5 volume of 10 M ammonium acetate and 2.5 volume of cold absolute ethanol. The resulting DNA was collected by centrifugation, washed with cold 75 % v/v ethanol. The DNA pellet was dissolved in 30 μl Tris-EDTA buffer. The extracted DNA was subjected to electrophoresis through a 1.5 % agarose gel containing ethidium bromide. DNA bands were visualized under ultraviolet illumination (Alpha Imager Gel Documentation System, Alpha Inno Tech Corporation, USA).
Immunocytochemistry for detection of activated caspase-3 in HeLa cells
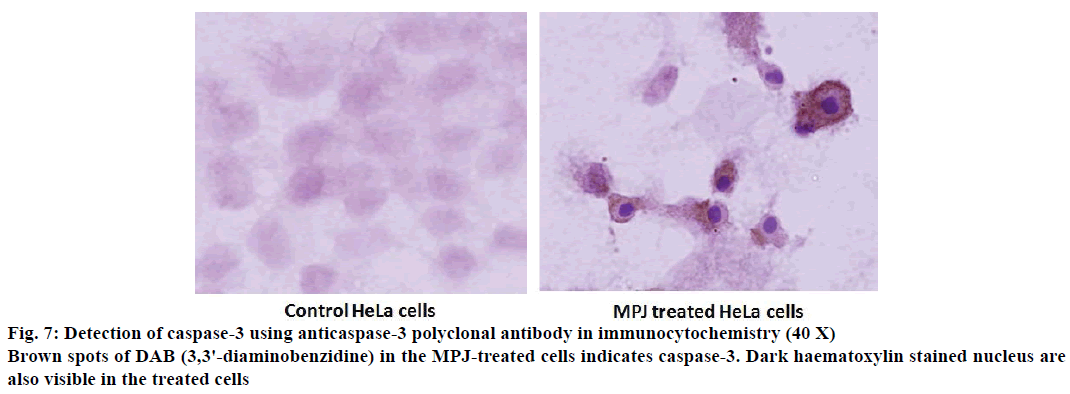
Immunocytochemistry for detection of activated caspase-3 in HeLa cells was done using caspase-3 polyclonal antibody (Cayman Chemical Company). The cells were grown on cover slips and treated with MPJ for 12 h. The cells were washed with PBS and then fixed by adding 4 % paraformaldehyde (PFA) followed by incubation for 15 min. The cells were then washed three times with Tween-20 in PBS (PBT, 0.05 %) and incubated for 30 min with 1 % bovine serum albumin (BSA). After washing with PBT thrice, the cells were incubated with 0.2 ml caspase-3 polyclonal antibody diluted in 0.1 % BSA (1:1000) for 60 min at room temperature. Washing was done again with PBT three times for 5 min each followed by incubation with secondary antibody (PK-8800, Vectastain Universal Quick Kit) for 40 min at room temperature. After another round of washing to remove the unbound secondary antibody, the cells were incubated with three drops of Streptavidin-HRP for 40 min at room temperature followed by washing again. The cells were then incubated with chromogenic substrate 3,3' diaminobenzidine and haematoxylin (used as counterstain) for 5 min at room temperature, washed with PBT and fixed again with 0.5 ml of 4 % PFA. The coverslip was mounted on glass slide using aqueous mountant and observed under microscope.
Results and Discussion
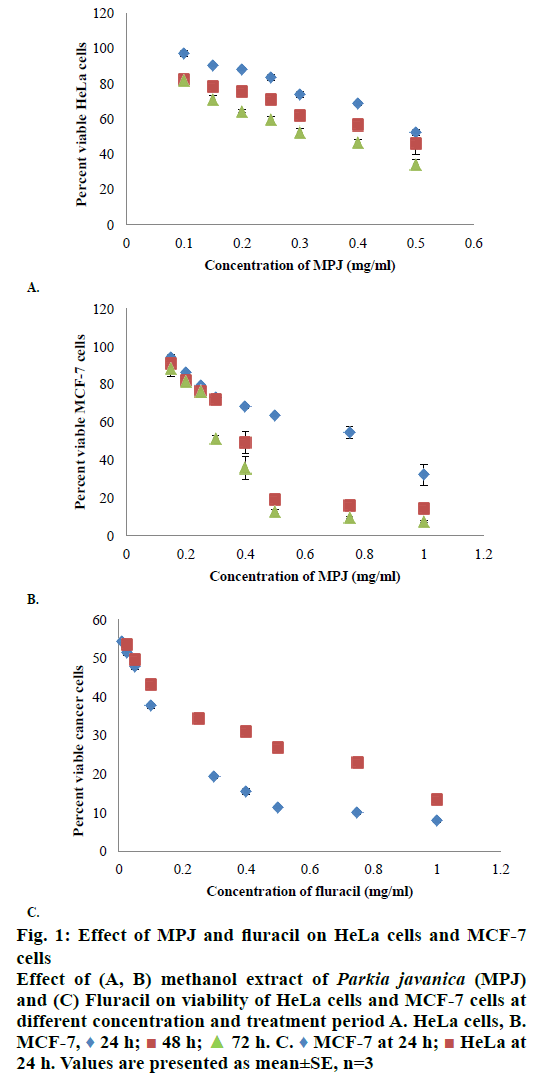
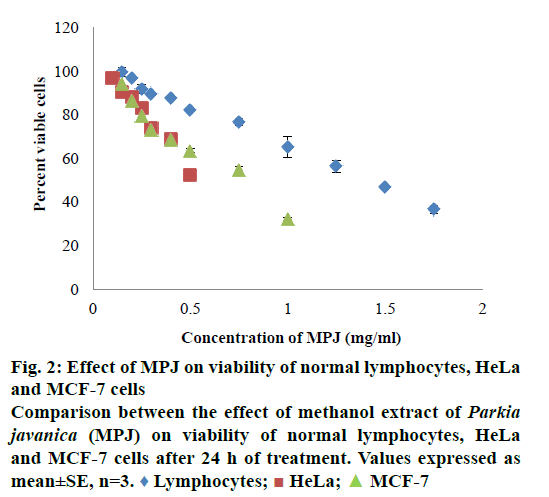
In in vitro evaluation, viability of both HeLa and MCF-7 cells treated with MPJ was found to be reduced with increase in treatment period. IC50 of MPJ on HeLa and MCF-7 cells at 24 h were found to be 0.54 and 0.74 mg/ml, respectively (Figure 1A and B). IC50 of Fluracil at 24 h was found to be 46 μg/ml and 25.7 μg/ml for HeLa and MCF-7 cells, respectively (Figure 1C). At 72 h of treatment with Fluracil, almost all HeLa and MCF-7 cells were found to be dead. In lymphocytes, MPJ was found to cause 50 % cell death at a concentration of 1.41 mg/ml (Figure 2). This concentration is much higher as compared to IC50 of MPJ in HeLa and MCF-7 cells. In the present study, further assays were carried out by treating the cancer cells with the IC50 of MPJ.
Figure 1: Effect of MPJ and fluracil on HeLa cells and MCF-7
cells
Effect of (A, B) methanol extract of Parkia javanica (MPJ)
and (C) Fluracil on viability of HeLa cells and MCF-7 cells at
different concentration and treatment period A. HeLa cells, B.
MCF-7,  24 h;
24 h;  48 h;
48 h; 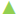 72 h. C.
72 h. C.  MCF-7 at 24 h;
MCF-7 at 24 h;  HeLa at
24 h. Values are presented as mean±SE, n=3
HeLa at
24 h. Values are presented as mean±SE, n=3
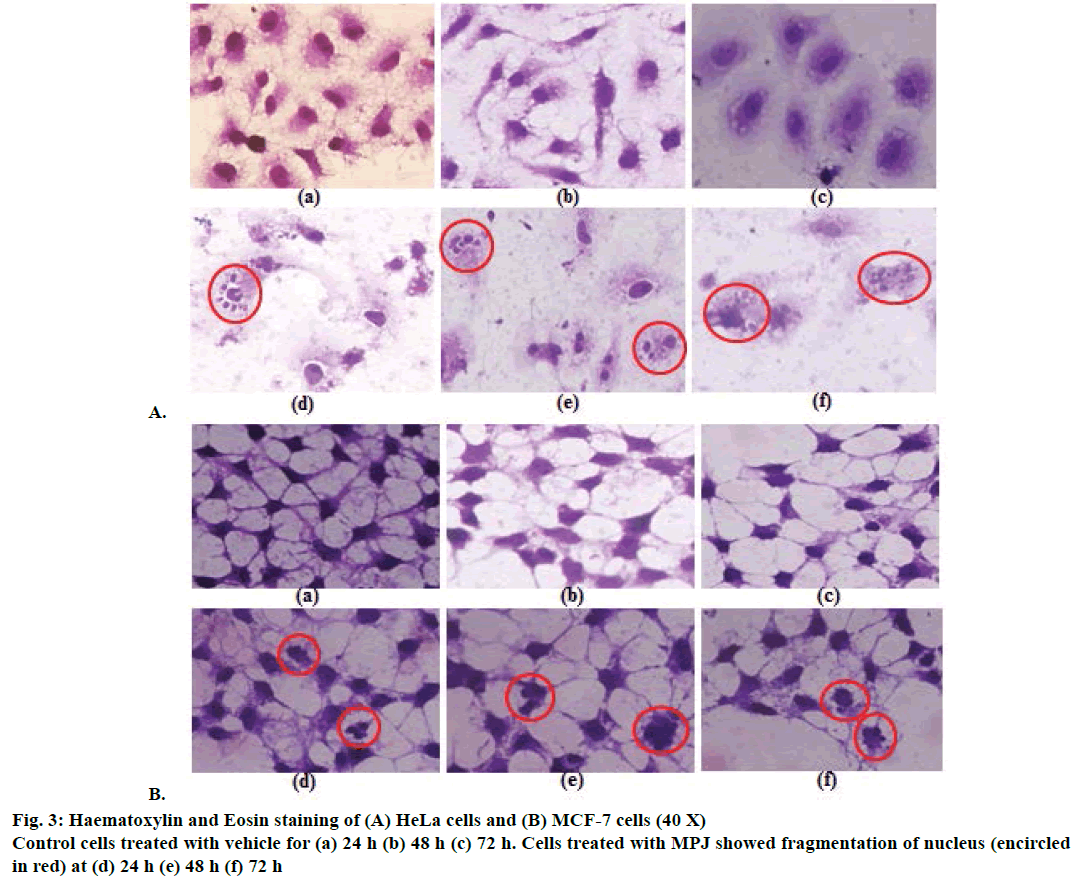
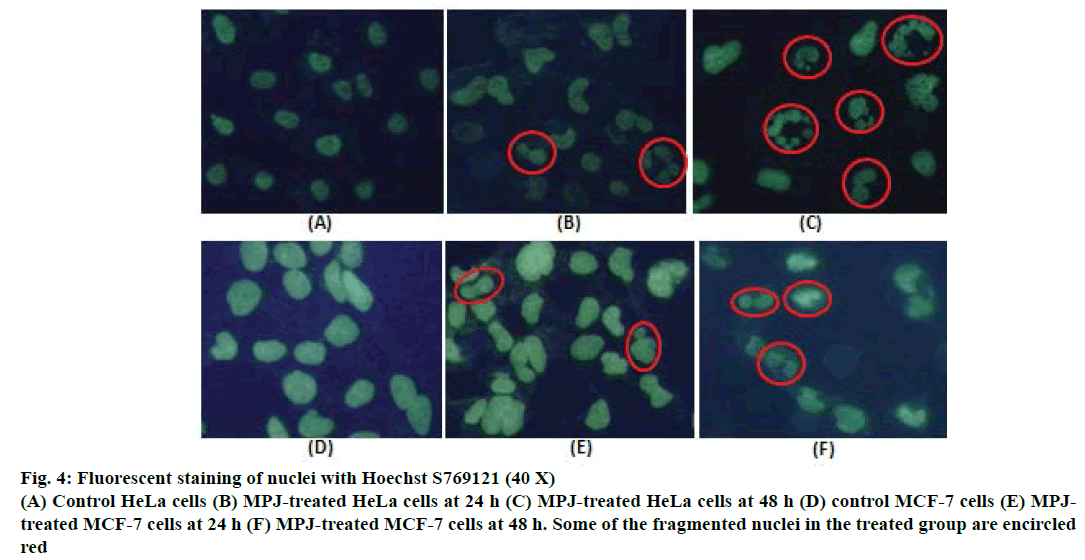
After treatment of both HeLa and MCF-7 cells with MPJ for 24, 48 and 72 h, morphological changes were observed. Clear nuclear fragmentation could be seen in HeLa cells from 24 h onwards (Figure 3A). MCF-7 cells also showed nuclear fragmentation at 24 h of treatment however the changes were not as prominent as that of HeLa cells (Figure 3B). Cytoplasmic vacuolation was observed in both control (treated only with vehicle) and MPJ-treated HeLa cells at 72 h. In fluorescent staining of nuclei with Hoechst S769121, control cells showed round or oval greenish-yellow nucleus whereas MPJtreated HeLa cells showed many fragmented nuclei (Figure 4). Fragmentation of nucleus was also observed in MPJ-treated MCF-7 cells but was not as clear as observed in HeLa cells.
Figure 4: Fluorescent staining of nuclei with Hoechst S769121 (40 X)
(A) Control HeLa cells (B) MPJ-treated HeLa cells at 24 h (C) MPJ-treated HeLa cells at 48 h (D) control MCF-7 cells (E) MPJtreated
MCF-7 cells at 24 h (F) MPJ-treated MCF-7 cells at 48 h. Some of the fragmented nuclei in the treated group are encircled
red
Apoptotic and necrotic cell populations in both control and MPJ-treated cancer cells were analysed by FACS using Annexin V-FITC and PI (Figure 5). Positioning of quadrants on Annexin V/PI dot plots showed that the control HeLa cells was 95 % Annexin V–/PI– and 3.74 % positive for Annexin V-FITC. MPJ-treated HeLa cells showed 15.7 % Annexin V+/PI– and 5.63 % Annexin V+/PI+ with 3.03 % Annexin V–/ PI+. Control MCF-7 cells were 91.6 % Annexin V–/ PI– with around 4 % Annexin V–/PI+. Treated MCF-7 cells showed 75.4 % Annexin V–/PI–, 5.19 % Annexin V+/PI–, 7.65 % Annexin V+/PI+ and 6.38 % Annexin V–/PI+ cell populations.
In gel electrophoresis, DNA isolated from control cells did not exhibit ladder formation. DNA isolated from MPJ-treated HeLa cells showed smearing away from the well while MCF-7 cells showed smearing near the well (Figure 6).
Caspase-3, a key enzyme of apoptosis was detected in the MPJ-treated HeLa cells using polyclonal caspase-3 antibody by immunocytochemistry (Figure 7). Brown spots were observed in the cytosol of the treated cells indicating the presence of caspase-3. Nucleus of treated HeLa cells took deep hematoxylin stain and showed typical condensed chromatin pulled away from the nuclear envelope.
Apoptosis is a popular target of many cancer treatment strategies [21]. Non-surgical approach for treatment of cancers targets either rectification of a defective apoptotic pathway or activation of apoptotic mechanism to induce apoptosis in malignant cells [22]. Several medicines are available in the market for treatment of cancer however toxicity of the established drugs is a major issue [23]. With increase concern about side effects of chemotherapy, many researches are nowadays turning towards plants or plant-derived natural products leading to identification of various plants with anticancer property. In the present study, apoptosis inducing ability of P. javanica seed extract was evaluated in cancer cells. Very recently, the cytotoxicity of aqueous methanol extract of P. javanica fruit in sarcoma-180 (S-180), A549, AGS, and MDAMB435S cells had been reported [24]. In that study, green fruits of P. javanica available only seasonally were used. Moreover, the whole fruit is not edible and a layer of the skin needs to be removed before consumption as vegetable. So, the present study was taken up to evaluate the anticancer property of dry seeds of P. javanica, which are edible as well as available throughout the year.
As a preliminary study, phytochemical screening of aqueous, ethanol and methanol extract of P. javanica seeds was carried out [25]. Based on the results of phytochemical analysis, methanol extract of P. javanica seeds was selected for evaluation of anticancer activity. MPJ was found to cause death of HeLa and MCF-7 cells at a non-toxic concentration to normal cells. IC50 of MPJ on normal healthy lymphocytes was around 2.6 and 1.9 times higher than that of HeLa and MCF-7 cells, respectively. It indicates that the extract has comparatively higher specificity towards cancer cells. A drug with high specificity and potency to kill cancer cells without fatal effect on normal cells would be ideal [26]. Commercially available anticancer drug Fluracil was found to inhibit 50 % of HeLa and MCF-7 cells at 46 μg/ml and 25.7 μg/ml, respectively. 5-Fluorouracil (5-FU) causing 50 % cell death in HeLa at 36 μg/ml when treated for 48 h and MCF-7 at 31.2 μg/ml when used as 5-FU encapsulated CS/Au nanocomposite had been reported [27,28]. As compared to Fluracil, the extract produced similar effects on the cancer cells at a higher dose. Requirement of higher dose of the extract to cause 50 % death in cancer cells may be due to the antagonistic relationship among phytochemicals present in the crude extract. It had been reported that co-occurrence of alkaloids and saponins significantly reduced antioxidant activity and these compounds should be kept exclusive to each other in drug formulations [29].
In haematoxylin and eosin staining, morphological changes particularly in the nucleus of MPJ-treated cells were observed. There was chromatin condensation starting from periphery of the nuclear membrane leading to nuclear condensation. These are common feature of apoptosis during late stages and the condensed nucleus finally break up inside an intact cell membrane [30]. Fragmentation of nucleus was clearly visible in the MPJ-treated HeLa cells. In fluorescent staining with Hoechst S769121, similar picture of nuclear fragmentation was observed in MPJ-treated cells while control cells showed rounded intact nuclei. Nuclear morphological changes in apoptosis such as convoluted nuclei, and clumps of chromatin may occur before or simultaneously with other hallmarks of apoptosis [31]. Cells undergoing apoptosis in vitro ultimately go through secondary necrosis [32]. To discern the presence of apoptotic and necrotic cell populations, both control as well as MPJ-treated cells were stained with Annexin V-FITC/PI and analysed by FACS. Annexin V binds to phosphatidylserine, which is exposed on the external leaflet of plasma membrane during early stages of apoptosis and FITC gives fluorescence for detection [33,34]. PI was used as a counter stain to detect necrotic cells because it is a membrane impermeant nucleic acid stain generally excluded by viable cells and a commonly used fluorescent dye in multicolour fluorescent techniques [35,36]. When Annexin V-FITC/ PI dots were positioned into quadrants, different cell populations consisting of live (Annexin V-FITC–/PI–), early apoptotic (Annexin V-FITC+/PI–), late apoptotic (Annexin V-FITC+/PI+) and necrotic cells (Annexin V-FITC–/PI+) were observed. HeLa cells showed higher percent of early apoptotic than late apoptotic and necrotic cells. MCF-7 cells showed higher percent of necrotic cell populations followed by late apoptotic and early apoptotic cells, which suggest that the cells were undergoing secondary necrosis.
A biochemical hallmark of apoptosis is cleavage of chromosomal DNA into oligonucleosomal size fragments [37]. DNA isolated from MPJ-treated HeLa cells showed DNA fragments smearing away from the wells in agarose gel electrophoresis. However, DNA isolated from MPJ-treated MCF-7 cells showed smearing near the wells, which was indicative of formation of high molecular weight DNA, which precedes fragmentation into oligonucleosomal length fragments during apoptosis [38,31]. It had been documented that irregular size genomic DNA fragments of necrotic cells can also be observed as smear in gel electrophoresis [39]. In apoptosis, there is activation of caspases (a family of endoproteases), which result in either inactivation or activation of substrates generating a series of signalling molecules that participate in ordered processes of cell death [40]. In our study, activated caspase-3, the main effector caspase of apoptosis was detected in MPJ-treated HeLa cells by immunocytochemistry. Additionally, MPJ-treated cells showed deep haematoxylin stained condensed nuclei, which is a typical feature of apoptotic cell with condensed chromatin pulled away from the nuclear envelope [31]. Since MCF-7 doesn't express caspase-3 due to the functional deletion in the CASP-3 gene [41], immunocytochemistry for detection of caspase-3 was not done in this cell line.
In the present study, the features of apoptosis observed in HeLa and MCF-7 cells were different. Fragmentation of nucleus was found to be more in MPJ-treated HeLa cells as compared to MCF-7 cells. The difference may be due to the activation of caspase-3 in HeLa cells as this enzyme causes DNA fragmentation and other structural changes associated with apoptosis [41], HeLa cells might have undergone caspase-3 pathway while MCF-7 cells might have adopted other pathways of apoptosis. From the study, it was found that MPJinduced apoptosis in the cancer cells and causes cell death at a lower concentration as compared to that of normal cells. Future work on P. javanica may be focussed on the identification of active compounds in the seeds responsible for the specificity and potency of killing the cancer cells. Further, it would be worth finding out whether the inclusion of P. javanica seeds in diet lead to chemoprevention.
Acknowledgements
The authors are grateful to the Director, IVRI for providing necessary facilities and ICAR, New Delhi for financial assistance to this project. The authors thank Dr A. K. Dinda, Professor, Department of Pathology, All India Institute of Medical Sciences, New Delhi, India for providing the cell lines at gratis and Dr D. K. Saxena, Reader, Department of Botany for identifying the plant material.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry 2000;55:481-504.
- Ajikumar PK, Tyo K, Carlsen S, Mucha O, Phon TH, Stephanopoulos G. Terpenoids: Opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm 2008;5(2):167-90.
- Tin MM, Cho CH, Chan K, James AE, Ko JK. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumour xenograft. Carcinogenesis 2007;28:1347-55.
- Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anticancer properties of anthraquinones from rhubarb. Med Res Rev 2007;27:609-30.
- Kittakoop P, Mahidol C, Ruchirawat S. Alkaloids as important scaffolds in therapeutic drugs for the treatments of cancer, tuberculosis, and smoking cessation. Curr Topics Med Chem 2014;14(2):239-52.
- Salam JS. Parkia roxburghii, G. Don.: a potential oil yielding plant in Manipur. Agric Sci Digest 1996;16(3-4):189-91.
- Khan MH, Yadava PS. Antidiabetic plants used Thoubal district of Manipur, Northeast India. Indian J Tradit Know 2010;9(3):510-4.
- Khumbongmayum AD, Khan ML, Tripathi RS. Ethnomedicinal plants in the sacred groves of Manipur. Indian J Tradit Know 2005;4(1):21-32.
- Jivad N, Rabiei Z. A review study on medicinal plants used in the treatment of learning and memory impairments. Asian Pac J Trop Biomed 2014;4(10):780-89.
- Chanu KV, Ali MA, Kataria M. Antioxidant activities of two medicinal vegetables: Parkia javanica and Phlogacanthus thyrsiflorus. Int J Pharm Pharm Sci 2012;4(1):102-6.
- Gmelin R, Susilo R, Fenwick GR. Cyclic polysulphides from Parkia speciosa. Phytochemistry 1981;20(11):2521-3.
- Suvachittanont W, Kurashima Y, Esumi H, Tsuda M. Formation of thiazolidine-4-carboxyiic acid (thioproline), an effective nitrite-trapping agent in human body, in Parkia speciosa seeds and other edible leguminous seeds in Thailand. Food Chem 1996;55(4):359-63.
- Tahira T, Tsuda M, Wakabayashi K, Nagao M, Sugimura T. Kinectics of nitrosation of thioproline, the precursor of a major nitroso compound in human urine, and its role as a nitrite scavenger. Gan 1984;75(10):889-94.
- Tahira T, Ohgaki H, Wakabayashi K, Nagao M, Sugimura T. The inhibitory effect of thioproline on carcinogenesis induced by N-benzylmethylamine and nitrite. Food Chem Toxicol 1988;26(6):511-6.
- Hansen MB, Neilsen SE, Berg K. Re-examination and further development of a precise and rapid dye methods measuring cell growth/cell kill. J Immunol Methods 1989;119:203-10.
- Baker PE, Knoblock KF. Bovine costimulator. I. Production kinetics, partial purification, and quantification in serum-free Iscove's medium. Vet Immunol Immunopathol 1982;3(4):365-379.
- Kirana C, Record IR, McIntosh GH, Jones GP. Screening for Antitumor Activity of 11 Species of Indonesian Zingiberaceae Using Human MCF-7 and HT-29 cancer cells. Pharm Biol 2003;41(4):271-6.
- Lillie RD. Histopathologic Technique and Practical Histochemistry. 3rd ed. New York: Mc. Graw Hill; 1965. p. 525-58.
- Vermes L, Haanen C, Steffens-Nakkens H, Reutellingsperger C. A novel assay for apoptosis; flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995;184:39-51.
- Herrmann M, Lorenz HM, Voll R, Grunke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res 1994;22:5506-7.
- Wong RSY. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 2011;30:87.
- Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and Molecular Targeting Therapy in Cancer. Biomed Res Int 2014;150845.
- Prakash O, Kumar A, Kumar P, Ajeet. Anticancer Potential of Plants and Natural Products: A Review. Am J Pharmacol Sci 2013;1(6):104-15.
- Patra K, Jana S, Sarkar A, Karmakar S, Jana J, Gupta M, et al. Parkia javanica extract induces apoptosis in S-180 cells via the intrinsic pathway of apoptosis. Nutr Cancer 2016;68(4):689-707.
- Chanu KV, Devi LG, Srivastava SK, Thakuria D, Kataria M, Telang AG. Phytochemical analysis and evaluation of anticancer activity of Parkia javanica seeds. Pharma Innov J 2018;7(5):305-11.
- Das AK. Anticancer Effect of Antimalarial Artemisinin Compounds. Ann Med Health Sci Res 2015;5(2):93-102.
- Khanna VG, Kannabiran K. Anticancer-cytotoxic activity of saponins isolated from the leaves of Gymnema sylvestre and Eclipta prostrate on HeLa cells. Int J of Green Pharm 2009;3:227-9.
- Nivethaa EAK, Dhanavel S, Narayanan V, Vasu CA, Stephen A. An in vitro cytotoxicity study of 5-fluorouracil encapsulated chitosan/gold nanocomposites towards MCF-7 cells. RSC Advances 2015;5:1024-32.
- Milugo TK, Omosa LK, Ochanda JO, Owuor BO, Wamunyokoli FA, Oyugi JO, et al. Antagonistic effect of alkaloids and saponins on bioactivity in the quinine tree (Rauvolfia caffrasond): further evidence to support biotechnology in traditional medicinal plants. BMC Complement Altern Med 2013;13:285.
- Ziegler U, Groscurth P. Morphological Features of Cell Death. News Physiol Sci 2004;19(3):124-8.
- Johnson VL, Ko SC, Holmstrm TH, Eriksson JE, Chow SC. Effector caspases are dispensable for the early nuclear morphological changes during chemical-induced apoptosis. J Cell Sci 2000;113:2941-53.
- Elmore S. Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol 2007;35(4):495-516.
- Prasad S, Pandey MK, Yadav VR, Aggarwal BB. Gambogic acid inhibits stat3 phosphorylation through activation of protein tyrosine phosphatase shp-1: Potential role in proliferation and apoptosis. Cancer Prev Res 2011;4:1084-94.
- Chen J, Zhou M, Zhang Q, Xu J, Ouyang J. Anticancer Effect and Apoptosis Induction of Gambogic Acid in Human Leukemia Cell Line K562 in vitro. Med Sci Monit 2015;21:1604-10.
- Fraker PJ, King LE, Lill-Elghanian D, Telford WG. Quantification of apoptotic events in pure and heterogeneous populations of cells using the flow cytometer. Methods Cell Biol 1995;46:57-76.
- Moore A, Donahue CJ, Bauer KD, Mather JP. Simultaneous measurement of cell cycle and apoptotic cell death. Methods Cell Biol 1998;57:265-78.
- Zhang JH, Xu, M. DNA fragmentation in apoptosis. Cell Res 2000;10:205-11.
- Chan FL, Choi HL, Chen ZY, Chan PS, Huang Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett 2000;160:219-28.
- Suman S, Pandey A, Chandna S. An improved non-enzymatic “DNA ladder assay” for more sensitive and early detection of apoptosis. Cytotechnology 2012;64(1):9-14.
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 2015;7(4):a026716.
- Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 1998;273:9357-60.