- *Corresponding Author:
- Grace E. Ukpo
Department of Pharmaceutical Chemistry, Faculty of Pharmacy
E-mail: graceukpo@yahoo.com
| Date of Submission | 5 October 2011 |
| Date of Revision | 31 August 2012 |
| Date of Acceptance | 6 September 2012 |
| Indian J Pharm Sci 2012, 74 (5): 454-457 |
Abstract
The aim of the present study was to investigate the toxicological effects of moxifloxacin in mice to determine the toxicological implications. Forty mice of both sexes were divided into four groups of 10 mice each, designated as A, B, C and D. Group A served as the control and received 2 ml of distilled water, while Groups B, C and D were orally administered 12.5, 25 and 50 mg/kg body weight of moxifloxacin once daily for 7 days, respectively. The weights of the mice were recorded before and throughout the duration of drug administration. Blood samples were collected for serum analysis. Total blood protein, cholesterol, triglyceride, creatinine, activities of aspartate transaminase, alanine transaminase and alkaline phosphatase, levels of high density lipoproteinâ??cholesterol and low density lipoproteinâ??cholesterol were assayed. There were significant (P≤0.05) differences in the concentrations of serum creatinine, urea, aspartate transaminase, alanine transaminase and alkaline phosphatase, levels of high density lipoproteinâ??cholesterol, low density lipoproteinâ??cholesterol, cholesterol and triglyceride of mice administered moxifloxacin. Serum level of total bilirubin in low dose treated animals was not significantly different from that of the control group animals, but there were significant dose dependent decrease in the animals treated with 25 mg/kg as well as 50 mg/kg. Data of the study indicate there was a dose dependent reduction in the protein metabolites, lipid profile and liver enzyme activities of mice administered moxifloxacin.The aim of the present study was to investigate the toxicological effects of moxifloxacin in mice to determine the toxicological implications. Forty mice of both sexes were divided into four groups of 10 mice each, designated as A, B, C and D. Group A served as the control and received 2 ml of distilled water, while Groups B, C and D were orally administered 12.5, 25 and 50 mg/kg body weight of moxifloxacin once daily for 7 days, respectively. The weights of the mice were recorded before and throughout the duration of drug administration. Blood samples were collected for serum analysis. Total blood protein, cholesterol, triglyceride, creatinine, activities of aspartate transaminase, alanine transaminase and alkaline phosphatase, levels of high density lipoproteinâ??cholesterol and low density lipoproteinâ??cholesterol were assayed. There were significant (P≤0.05) differences in the concentrations of serum creatinine, urea, aspartate transaminase, alanine transaminase and alkaline phosphatase, levels of high density lipoproteinâ??cholesterol, low density lipoproteinâ??cholesterol, cholesterol and triglyceride of mice administered moxifloxacin. Serum level of total bilirubin in low dose treated animals was not significantly different from that of the control group animals, but there were significant dose dependent decrease in the animals treated with 25 mg/kg as well as 50 mg/kg. Data of the study indicate there was a dose dependent reduction in the protein metabolites, lipid profile and liver enzyme activities of mice administered moxifloxacin.
Keywords
Blood chemistry, liver enzymes, mice, moxifloxacin
Moxifloxacin, a chiral 8?methoxy fluoroquinolone derivative is a synthetic broad spectrum antibacterial agent [1?3] which is widely used in the management of tuberculosis [4?8]. It is found in high concentration in body tissues, fluids and bone [9,10]. It exerts its antibacterial activity by penetrating the bacterial cell inhibiting the DNA gyrase and topoisomerase II and IV which are required by bacterial DNA relocation, transcription, repair and recombination [3].
Moxifloxacin is absorbed after oral administration, undergoes limited metabolism and its glucuronide conjugate is excreted exclusively in the urine while the sulphate conjugate is eliminated in the faeces. It is moderately protein bound and is not metabolized by cytochrome P450 system [11,12]. Plasma elimination half?life ranges from 8.2 to 15.1 h in healthy volunteers [11,13?15].
Adverse effects are mild to moderate in severity and required no treatment. The common adverse effects are gastrointestinal upset and neurotoxic side effects [1,13,16]. Quinolones bind to DNA, but the doses of quinolones do not have genotoxic effects and do not present mutagenic hazard to human [17]. In the present study, the histological and toxicological effects of moxifloxacin in mice are investigated to determine the toxicological implications.
Drugs used were obtained from the Lagos University Teaching Hospital Pharmacy. The moxifloxacin was manufactured by Swiss Pharma Nigeria Ltd., Lagos, Nigeria. Vehicle used to dissolve the drugs was distilled water.
Forty Wistar mice of both sexes weighing 17.5?19.0 g were used for the study. The animals were maintained and used in accordance with the Institute of Laboratory Animal Research Guidelines for care and use of animals in experimental studies [18]. They were obtained from the animal breeding centre of the Nigerian Institute of Medical Research, Yaba, Lagos. They were housed in the College of Medicine, University of Lagos animal house under optimum conditions such as well?ventilated room with temperature controlled at 25±2°. They were maintained on standard feed obtained from Pfizer and provided with water ad libitum.
After 2 weeks acclimatization, the mice were divided into four groups of 10 mice each designated as A, B, C and D. Group A served as the control and received 2 ml of distilled water while Groups B, C and D received moxifloxacin of 12.5, 25 and 50 mg/kg of body weight once daily at 7.30 am for 7 days by oral gavage. The weights of the mice were recorded before and throughout the duration of drug administration. Blood samples were collected, centrifuged at 5000 rpm for 10 min. The serum obtained was stored at −20° until required for biochemical assays.
The serum samples were assayed for alkaline phosphatase (ALP) using the phenolphthalein method [19], alanine transaminase (ALT) and aspartate transaminase (AST) were determined by the procedure of Rietman and Frankel [20]. Total protein was measured by Lowry method [21]. Urea was assayed using urease Bertthelot method [22], total cholesterol using enzymatic endpoint method [23,24] and creatinine assay was done by alkaline picrate method [25]. Total or direct bilirubin in plasma was determined using Jandrassik and Grof technique as described by Tolman [26].
Data were expressed as mean±standard error of mean. Comparison between control and treated groups of mice were performed with a one way analysis of variance. Statistical significance was set at P≤0.05.
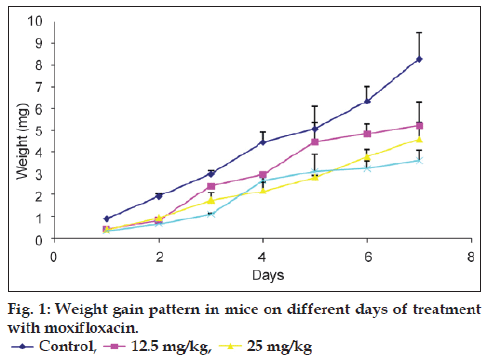
The administered doses of moxifloxacin did not result in death of any of the mice. All the animals were observed to gain weight at the end of drug administration but not as much as the control group (fig. 1). As dosage of the drug administered to the mice increased, a reduction in the rate of weight gain was observed. There were no significant differences (P≥0.05) in the weight gain in the animals for all the dose levels. The mean difference in the body weight for treated group was significantly different from the control.
The ALP activities increased from 10.6±71.20 to 18.3±30.88 in the group which received 12.5 mg/kg body weight, 57.0±1.16 for the group that received 25 mg/kg body weight and 72.0±1.00 IU/L for the group that received 50 mg/kg body weight, respectively. Moxifloxacin caused significant increase (P<0.05) on the serum activities of the hepatic enzymes, AST, ALT and the other biochemical parameters investigated namely, creatinine, cholesterol, triglyceride, high density lipoprotein?cholesterol (HDL?C), low density lipoprotein?cholesterol (LDL?C) and urea. Serum level of total bilirubin in control animals was not significantly different from the serum level obtained in the mice that received 12.5 mg/kg of moxifloxacin. However, there was significant dose dependent decrease in the serum levels of this parameter in the animals treated with 25 and 50 mg/ kg, respectively (Table 1).
| Biochemical parameters | Control | Treated groups (dose in mg/kg body weight) | ||
|---|---|---|---|---|
| 12.5 | 25 | 50 | ||
| Creatinine (µmol/l) | 15.47 ± 0.145 | 31.77 ± 0.491? | 35.03 ± 1.45? | 41.47 ± 0.745? |
| Cholesterol (mg/dl) | 33.67 ± 0.882 | 69.3 ± 0.666? | 111.7 ± 2.33? | 121.7 ± 1.20? |
| HDL-C (mg/dl) | 20.00 ± 0.577 | 37.67 ± 0.882? | 52.33 ± 1.45? | 71.33 ± 1.76? |
| LDL-C (mg/dl) | 7.667 ± 0.33 | 14.00 ± 1.16? | 26.33 ± 1.45? | 43.33 ± 0.88? |
| Triglyceride (mg/dl) | 34.67 ± 0.882 | 70.00 ± 1.16? | 186.0 ± 1.73? | 192.3 ± 0.882? |
| Total bilirubin (mg/dl) | 1.833 ± 0.009 | 1.583 ± 0.058? | 0.663 ± 0.13? | 0.273 ± 0.028? |
| Urea (mg/dl) | 21.77 ± 0.99 | 39.90 ± 1.48? | 52.30 ± 1.60? | 66.17 ± 0.88? |
| ALP (U/l) | 10.67 ± 1.20 | 18.33 ± 0.88? | 57.00 ± 1.16? | 72.00 ± 1.00? |
| AST (U/l) | 42.37 ± 1.22 | 90.83 ± 1.55? | 128.1 ± 1.54? | 155.0 ± 1.94? |
| ALT (U/l) | 19.13 ± 0.318 | 41.27 ± 1.13? | 52.4 ± 1.13? | 70.5 ± 0.458? |
Table 1: Biochemical Parameters In Mice After Oral Administration Of Moxifloxacin
The results obtained show the effect of moxifloxacin on the activities of the liver enzymes, protein metabolites and lipids were dependent on the dose administered. Serum levels of hepatic enzymes, AST and ALT and ALP were significantly elevated. The liver is known to play a major role in the metabolic processes of most substances. Thus any disturbance in the liver can affect the level of the biochemical parameters present in the liver. The activities of these enzymes are usually elevated during damage to liver cells and tissues [27]. In the present study, the increase in AST and ALT could be due to damage to the liver or skeletal muscle, as they are useful indices for identifying inflammation and necrosis of the liver [28]. ALT has its highest concentration in the liver, with kidney and skeletal muscles having lesser activity of these enzymes. It is an indicator of acute liver damage. The liver plays a role in metabolic process of many drugs. Thus any disturbance in the liver can affect the level of biochemical parameters present in the liver cells. ALP is a known marker enzyme for plasma membrane and endoplasmic reticulum of the tissue [29]. It is an indicator of acute liver damage [30]. Thus increases in these enzymes in the study indicate inflammation or damage of liver cells. Bilirubin is a useful index of the excretory function of the liver, in addition to its being a useful tool in the assessment of haemolytic anaemia. In this study, there were no significant changes in the bilirubin levels of the mice treated with 25 mg/ kg body weight of moxifloxacin compared to the control while increase in dose led to lower levels of bilirubin. Damaged liver cells cannot conjugate bilirubin or remove unconjugated bilirubin from the blood [28]. This results in the increase in total bilirubin observed in the study. Consequently, it may be stated that the excretory function of the liver in mice is not affected significantly, as a result of the administration of oral doses of moxifloxacin.
There was significant increase in the serum creatinine, cholesterol, HDL?C, LDL?C, triglyceride and urea levels, while a decrease was observed for bilirubin as the dose administered to the mice is increased. This is due to damaging tissues. The increase in serum cholesterol observed might be due to prolonged administration of the drug for 7 days. It shows the drug is releasing more cholesterol into the blood thus increasing the serum level of free cholesterol. Increase in the total protein level in the liver leads to increase in the amount of urea formation. AST and ALT, which are localized within the cells in the liver, kidney, heart and muscle are used in assessing and monitoring liver [31]. Thus increase in their values could result in organ dysfunction [32]. These results give an insight into the effect of moxifloxacin on the organs of the mice despite the claim of safety of the drug reported in literature.
References
- Dalhoff A, Petersen U, Endermann R. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone. Chemotherapy 1996;42:410-25.
- Woodcock JM, Andrews JM, Boswell FJ, Brenwald NP, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother 1997;41:101-6.
- Pestova E, Millichap JJ, Noskin GA, Peterson LR. Intracellular targets of moxifloxacin: A comparison with other fluoroquinolones. J AntimicrobChemother 2000;45:583-90.
- Valway SE, Sanchez MP, Shinnick TF, Orme I, Agerton T, Hoy D, et al. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N Engl J Med 1998;338:633-9.
- Lu T, Drlica K. In vitro activity of C-8-methoxy fluoroquinolones against mycobacteria when combined with anti-tuberculosis agents. J AntimicrobChemother 2003;52:1025-8.
- Rodríguez JC, Ruiz M, López M, Royo G. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int J Antimicrob Agents 2002;20:464-7.
- Gosling R, Leonard O, Bongard E, Esther G, Morris RW. The bactericidal activity of moxifloxacin in patients with pulmonary tuberculosis. J AntimicrobChemother 2007;37:161-7.
- de Almeida MV, Saraiva MF, de Souza MV, da Costa CF, Vicente FR, Lourenço MC. Synthesis and antitubercular activity of lipophilic moxifloxacin and gatifloxacin derivatives. Bioorg Med Chem Lett. 2007;17:5661-4.
- Malincarne L, Ghebregzabher M, Moretti MV, Egidi AM, Canovari B, Tavolieri G, et al. Penetration of moxifloxacin into bone in patients undergoing total knee arthroplasty. J Antimicrob Chemother 2006;57:950-4.
- Landersdorfer CB, Kinzig M, Hennig FF, Bulitta JB, Holzgrabe U, Drusano GL, et al. Penetration of moxifloxacin into bone evaluated by Monte Carlo simulation. Antimicrob Agents Chemother 2009;53:2074-81.
- Stass H, Kubitza D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother 1999;43:83-90.
- Balfour JA, Wiseman LR. Moxifloxacin. Drugs 1999;57:363-73.
- Wise R, Andrews JM, Marshall G, Hartman G. Pharmacokinetics and inflammatory-fluid penetration of moxifloxacin following oral or intravenous administration. Antimicrob Agents Chemother 1999;43:1508-10.
- Stass H, Dalhoff A, Kubitza D, Schühly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother 1998;42:2060-5.
- Vishwanathan K, Bartlett MG, Stewart JT. Determination of moxifloxacin in human plasma by liquid chromatography electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal 2002;30:961-8.
- Lubasch A, Keller I, Borner K, Koeppe P, Lode H. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob Agents Chemother 2000;44:2600-3.
- Scholar EM. Fluoroquinolones: Past, present and future of a novel group of antibacterial agents. Am J Pharm Educ 2003;66:164-72.
- Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Research (ILAR) Commission on life Science, National Research Council; 1996. Available from: http://www.nap.edu/ openbook.php?record_id=5140andpage=1. [Last accessed 2010 Apr 22].
- Babson AL, Greeley SJ, Coleman CM, Phillips GE. Phenolphthalein monophosphate as a substrate for serum alkaline phosphatase.Clin Chem 1966;12:482-90.
- Rietman S, Frankel S. A colorimetric method for aspartate and alanine aminotransferases in serum. Am J Chem Path 1957;28:56-8.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75.
- Weatherbum MW. Phenylhypochlorite reaction for determination of ammonia. Anal Chem 1967;39:971.
- Trinder P. Enzymatic determination of total serum cholesterol. Ann Clin Biochem 1969;6:24-7.
- Roeschlau P, Bernt E, Gruber WJ. Enzymatic determination of total cholesterol in serum.Clin Chem Clin Biochem 1974;12:226
- Tietz NW, Pruden EL, Siggard-Anderson O. Electrolytes, blood gasses and acid base balance. In: Tietz NW, editor. Textbook of Clinical Chemistry. Philadelphia: Saunders Publishers; 1986, p. 1188-96.
- Tolman KG, Rej R. Liver function. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia: W.B. Saunders; 1999. p. 1125-77.
- Traynor J, Mactier R, Geddes CC, Fox JG. How to measure renal function in clinical practice. BMJ 2006;333:733-7.
- Nerbert WT. Fundamental of clinical chemistry. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia: W.B. Saunders; 1983. p. 1056-92.
- Tilkian SM, Conover MB, Tilkian AG. Clinical Implications of Laboratory Tests. St. Louis: C. V. Mosby Company; 1979, p. 3-44; 117-132; 154-159.
- Shah M, Patel P, Phadke M, Menon S, Francis M, Sane RT. Evaluation of the effect of aqueous extract from powder of root, stem, leaves and whole plant of Phyllantus delbillis against CCl4 induced rat liver dysfunction. Indian Drug 2002;39:333-7.
- Wada H, Snell EE. Enzymatic transamination of pyridoxamine. II. Crystalline pyridoxamine-pyruvate transaminase. J Biol Chem 1962;237:133-7.
- Wells RM, McIntyre RH, Morgan AK, Davie PS. Physiological stress responses in big gamefish after capture: Observations on plasma chemistry and blood factors. Comp Biochem Physiol A Comp Physiol 1986;84:565-71.