- *Corresponding Author:
- K. P. R. Chowdary
Industrial Pharmacy Division, University College of Pharmaceutical Sciences, Andhra University, Visakhapatnam-530 003, India
E-mail: profkprc@rediffmail.com
| Date of Submission | 20 May 2005 |
| Date of Revision | 21 October 2005 |
| Date of Acceptance | 18 July 2006 |
| Indian J Pharm Sci, 2006, 68 (4): 461-464 |
Abstract
Olibanum resin was evaluated as microencapsulating agent and to prepare resin-coated microcapsules. Resin-coated microcapsules of nifedipine were prepared by an industrially feasible emulsification-solvent evaporation method and the microcapsules were investigated. The resin-coated microcapsules are spherical, discrete, free-flowing and multinucleate monolithic type. Microencapsulation efficiency was in the range 98-105%. Nifedipine release from the resin-coated microcapsules was slow over 24 h and depended on core: coat ratio, wall thickness and size of the microcapsules. Drug release was by non-fickian diffusion mechanism. Good linear relationships were observed between wall thickness of the microcapsules and release rate and T50 values. Resin-coated microcapsules of nifedipine exhibited good controlled release characteristics and were found suitable for once a day oral controlled release products.
Controlled release drug delivery systems are aimed at controlling the rate of drug delivery, sustaining the duration of the activity and targeting the delivery of the drug to a tissue. Drug release from these systems should be at a desired rate, predictable and reproducible. Microencapsulation and microcapsules are widely accepted for controlled release. Polymers and release retarding materials used as a coat play a vital role in controlling the drug release from the microcapsules. Microencapsulation by various polymers and their applications are described in standard text books [1,2]. Though a variety of polymeric materials are available to serve as release retarding core materials, there is a continued need to develop new, safe and effective release retarding coat materials for microencapsulation.
Olibanum is a gum resin obtained from Boswellia serrata, Roxburgh and other species of Boswellia. Olibanum consists [3] chiefly an acid resin (56-60%), gum (30-36%) and volatile oil (3-8%). The resin contains [4] mainly a resin acid (boswellic acid) and a resene (olibanoresene) in equal proportions. Ether soluble resin extracted from olibanum exhibited excellent release retarding properties in matrix tablets for controlled release due to its hydrophobic water repellant properties (unpublished data, P. Mohapatra). Preliminary studies indicated that the resin has good film forming property when dried from chloroform solution. In the present work the resin extracted from the olibanum was evaluated as coating material in microencapsulation. Studies were carried out on microencapsulation of nifedipine by the resin and evaluation of the resin-coated microcapsules of nifedipine for controlled drug release. Nifedipine is used in the treatment of angina pectoris and in the management of hypertension. It has a short biological half-life of 2-3 h and is eliminated rapidly and its antihypertensive effect lasts only for a few hours [5]. Hence to prolong its duration of action and to improve patient compliance sustained release products are needed for nifedipine. Sustained release products of nifedipine also avoid vasodilatation related adverse effects such as flushing and palpitation. Nifedipine Extended Release tablets are official in USP XXIV [6].
Materials and Methods
Nifedipine was a gift sample from M/s Cipla Ltd., Mumbai. Chloroform GR (Merck), diethyl ether (Qualigens), methanol (Qualigens), sodium carboxymethylcellulose (sodium CMC having a viscosity of 1500-3000 cps of a 1% w/v solution at 25°) and sodium lauryl sulphate (Loba Chemie) were used.
The resin used as coat material was extracted from olibanum in the laboratory as follows: Powdered olibanum (10 g) was extracted repeatedly with 4×50 ml quantities of solvent ether. The ether extracts were collected in a porcelain dish and concentrated to dryness at 40°. The dry mass was powdered and the size was reduced to 200 mesh.
Preparation of microcapsules
An emulsification-solvent evaporation method was tried to prepare resin-coated microcapsules. Resin (0.2 g) was dissolved in chloroform (5 ml) to form a homogeneous solution. Core material, nifedipine (0.8 g) was added to the polymer (resin) solution (5 ml) and mixed thoroughly. The resulting mixture was then added in a thin stream to 200 ml of an aqueous mucilage of sodium CMC (0.5% w/ v) contained in a 450 ml beaker while stirring at 1000 rpm to emulsify the added dispersion as fine droplets. A Remi medium duty stirrer with speed meter (Model RQT 124) was used for stirring. The solvent, chloroform was then removed by continuous stirring at room temperature (28°) for 3 h to produce spherical microcapsules. The microcapsules were collected by vacuum filtration and washed repeatedly with water. The product was then air dried to obtain discrete microcapsules. Different proportions of core:coat namely 9:1 (MC1) and 8:2 (MC2) were used to prepare microcapsules with varying coat thickness.
Estimation of nifedipine
Nifedipine content of the microcapsules was estimated by UV spectrophotometric method based on the measurement of absorbance at 238 nm in phosphate buffer of pH 6.8. The method was validated for linearity, accuracy and precision. The method obeyed Beer’s law in the concentration range 1-10 μg/ml. When a standard drug solution was assayed repeatedly (n = 6), the mean error (accuracy) and relative standard deviation (precision) were found to be 0.6 and 0.8 per cent, respectively.
Characterization of microcapsules
For the size distribution analysis, different fractions in a batch were separated by sieving using a range of standard sieves. The amounts retained on different sieves were weighed. Encapsulation efficiency was calculated using the equation, encapsulation efficiency = (estimated percent drug content/ theoretical percent drug content)×100. Theoretical mean wall thickness of the microcapsules was determined by the method of Luu et al. [7] using the equation, h= r (1-p) d1/3[pd2+(1-p) d1] where h is the wall thickness, r is the mean radius of the microcapsules, d1 is the density of the core material, d2 is the density of the coat material and p is the proportion of the medicament in the microcapsules. The photomicrograph of the microcapsules was taken using a trinocular compound microscope.
Drug release study
Release of nifedipine from the resin-coated microcapsules of size 20/30, 30/50 and 50/80 was studied in phosphate buffer of pH 6.8 containing 1% SLS (900 ml) using an eight station dissolution rate test apparatus (model Disso2000, M/s Lab India) with a paddle stirrer at 50 rpm and 37±0.50 as prescribed for nifedipine extended release tablets in USP XXIV. A sample of microcapsules equivalent to 20 mg of nifedipine were used in each test. Samples (5 ml) were withdrawn through a filter (0.45 μ) at different time intervals over 24 h and were assayed at 238 nm for nifedipine using a Shimadzu UV-150 double-beam spectrophotometer. The sample (5 ml) taken at each sampling time was replaced with fresh dissolution medium (5 ml). The drug release experiments were conducted in triplicate.
Results and Discussion
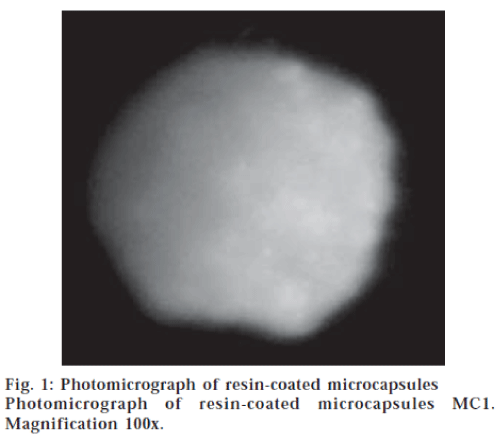
An emulsification-solvent evaporation method was developed for microencapsulation of nifedipine by the resin. The method involves emulsification of the polymer (resin) solution in chloroform containing the dispersed drug particles in an immiscible liquid medium (0.5% w/v solution of sodium CMC) as microdroplets, followed by removal of the solvent chloroform by continuous stirring to form rigid microcapsules. Resin-coated microcapsules of nifedipine could be prepared by the emulsification-solvent evaporation method developed. The microcapsules were found to be discrete, spherical and free flowing. The nature of the method of preparation indicated that the microcapsules were of multinucleate and monolithic type. Microphotographs (fig. 1) indicated that the microcapsules were spherical with smooth surface and completely covered with the polymer (resin) coat.
The sizes could be separated by sieving and a more uniform size range of microcapsules could readily be obtained. The sieve analysis of different microcapsules showed that a large proportion of microcapsules (60-70%) in a batch were in the size range of –20 +30 (715 μm) mesh. A log-normal size distribution of the microcapsules was observed in all the batches prepared.
Low coefficient of variation in percent drug content (< 1.0%) indicated uniformity of drug content in each batch of microcapsules (Table 1). The microencapsulation efficiency was in the range (98-105%). Drug content of the microcapsules was found to be the same in different sieve fractions. As the microcapsules are spherical, the theoretical average wall thickness of the microcapsules was calculated as per Luu et al.7. Microcapsules prepared with various ratios of core: coat was found to have different wall thickness (Table 1).
| Microcapsules (Size) | Nifedipine content (%) | Micro-encapsulation efficiency (%) | Wall thickness (µ) | T50(h) | Ko(mg/h) | K1(h-1) | |
|---|---|---|---|---|---|---|---|
| MC1 (20/30) | 89.13 (0.12)* | 99.03 | 74.0 | 10.1 | 3.281 | 0.064 | |
| MC1 (30/50) | 88.30 | (0.11) | 98.11 | 47.2 | 9.4 | 3.426 | 0.078 |
| MC1 (50/80) | 88.29 | (0.11) | 98.1 | 25.3 | 9.0 | 3.476 | 0.099 |
| MC2 (20/30) | 83.14 | (0.14) | 104.92 | 87.4 | 16.4 | 2.562 | 0.041 |
| MC2 (30/50) | 84.06 | (0.11) | 105.07 | 53.1 | 12.0 | 2.942 | 0.057 |
| MC2 (50/80) | 80.25 | (0.11) | 100.31 | 30.4 | 10.0 | 3.446 | 0.080 |
*Figures in parentheses are coefficient of variation (CV) values, T50 is the time for 50% release and K 0 and K 1 are zero order and first order release rate constants, respectively.
Table 1: Nifedipine content, microencapsulation efficiency, wall thickness and release characteristics of resin coated microcapsules.
Nifedipine release from the microcapsules was studied in phosphate buffer of pH 6.8 containing 1% w/v sodium lauryl sulphate as prescribed for nifedipine extended release tablets in USP XXIV. Nifedipine release from the microcapsules was slow and spread over a period of more than 24 h and depended on core: coat ratio, wall thickness and size of the microcapsules. As the proportion of the coat was increased, nifedipine release rate was decreased. Smaller microcapsules gave higher release rates due to increased surface area.
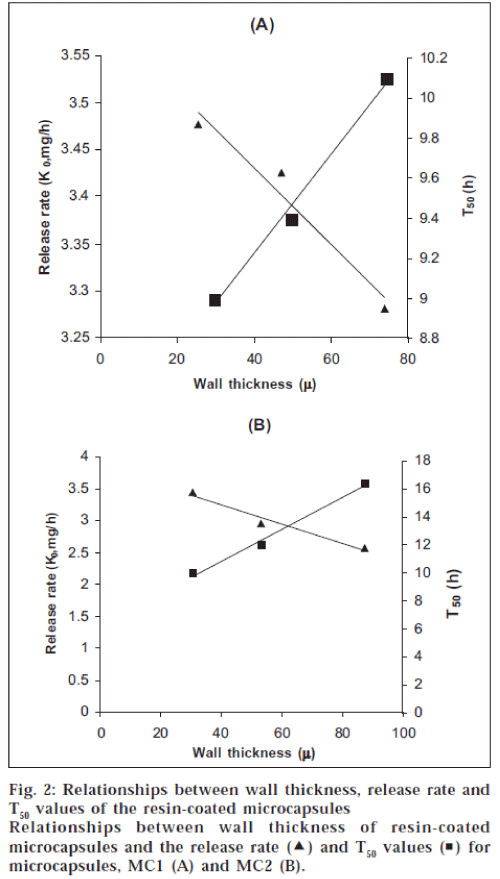
Analysis of the release data as per zero and first order kinetic models indicated that both the models are equally applicable to describe the release data of the microcapsules. Correlation coefficient (r) values in the two models were nearly the same. When the release data was analyzed as per Peppas equation [8], the release exponent ‘n’ was in the range of 0.554 to 0.716 with all the microcapsules indicating non-fickian diffusion as the release mechanism. Plots of percent released Vs square root of time were found to be linear (r>0.9820) indicating that the drug release from the microcapsules was diffusion controlled. Linear relationships were observed between wall thickness of the microcapsules, release rate (K0) and T50 (time for 50% release) values (fig. 2).
Spherical resin-coated microcapsules of nifedipine could be prepared by the emulsification-solvent evaporation method developed. The method is industrially feasible as it involves emulsification and removal of the solvent, which can be controlled precisely. Microencapsulation efficiency was found to be in the range 98-105%. Nifedipine release from the resin-coated microcapsules was slow and extended over longer periods of time and depended on core: coat ratio, wall thickness and size of the microcapsules. Drug release from the resin-coated microcapsules was by non-fickian diffusion mechanism. Good linear relationships were observed between wall thickness of microcapsules, release rate (K0) and T50 values. Thus, olibanum resin was found suitable as microencapsulating agent and the resin-coated microcapsules exhibited good controlled release characteristics and were found suitable for oral controlled release products. Olibanum is reported [9] as non-toxic. Since the resin is of natural origin, it is biocompatible and cheaper.
References
- Kondo, A., Eds., In; Microcapsule Processing and Technology, Marcel Dekker, Inc., New York, 1979, 18.
- Gutcho, M.H., Eds., In; Microcapsules and Microencapsulation Techniques, Noyes Data Corporation, New Jersey, 1976, 236.
- Nigam, S.K. and Mitra, C.R., Indian Drugs, 1979, 16, 80.
- Srinivas, R.S. and Madhu, B., Indian J. Chem., 1976, 176, 168.
- Reynolds, J.E.F., Eds., In; Martindale, The Extra Pharmacopoeia, 29th Edn., The Pharmaceutical Press, London, 1989, 1510.
- The United States Pharmacopoeia XXIV, The United States Pharmacopocial Convention, Inc., Rockville, MD, 2000, 2644.
- Luu, S.N., Carlier P.F., Delort P., Gazzola, J. and Lanfont, D., J. Pharm. Sci., 1973, 62, 452.
- Ritger, P.L. and Peppas, N.A., J. Control. Release, 1987, 5, 37.
- Joerg, G., Thomas, B. and Christof, J., Eds., In; PDR for Herbal Medicines, 2nd Edn., Medical Economics Company, Montvale, New Jersey, 2000, 319.