- *Corresponding Author:
- Chandana sengupta
Division of Pharmaceutical Chemistry, Department of Pharmaceutical Technology, Jadavpur University,Kolkata - 700 032, India
E-mail: csgjupt@yahoo.com
| Date of Submission | 1 June 2005 |
| Date of Revision | 13 February 2007 |
| Date of Acceptance | 18 May 2007 |
| Indian J Pharm Sci, 2007, 69 (3): 378-383 |
Abstract
Attempt has been made to evaluate free radical scavenging activity of water extract of Spirulina platensis on cisplatin-induced lipid peroxidation using some common laboratory markers. In this present study goat liver has been used as lipid source. This in vitro evaluation was done by measuring the malondialdehyde, 4-hydroxy-2-nonenal, reduced glutathione and nitric oxide content of tissue homogenates. The results suggest that cisplatin could induce lipid peroxidation to a significant extent and it was also found that water extract of the algae has the ability to suppress the cisplatin-induced toxicity.
Keywords
Lipid peroxidation, Spirulina platensis, cisplatin, malondialdehyde, reduced glutathione, 4-hydroxy- 2-nonenal, nitric oxide.
Spirulina also called arthospira is a microscopic and filamentous cyanobacterium (blue green algae) that has a long history of use as food [1-3]. Spirulina is 50- 70% protein by weight and contain a rich source of vitamins especially vitamin B12, β-carotene (provitamin A), vitamin E. It also contains carbohydrates like rhamnose, fructose, ribose, mannose and some minerals like copper, magnesium, zinc, potassium and iron. Beside γ-linolenic acid (GLA), it also contains a host of other phytochemicals that have potential health benefits [4-5]. Spirulina contains phycocyamin (7% dry weight basis) and polysaccharides, both of them have antioxidant properties. They have a direct effect on reactive oxygen species. It also contains an important enzyme superoxide dismutase (1700 units/g) that acts indirectly by slowing down the rate of oxygen radical generating reactions5. It has been reported that spirulina has a protective role in cisplatin-induced nephrotoxicity in rats6. It was found that besides antioxidant effects spirulina had properties like immunomodulation effects [7], anticancer effects [8].
Cisplatin [cis-diaminedichloroplatinum(II)] is one of the widely used antineoplastic drugs. However it has major side effects such as acute tubular necrosis [9]. It has also strong side effects on gastrointestinal tract [10].It has been found that cisplatin impairs the respiratory function and DNA of mitochondria in renal proximal tubules and small intestinal mucosal cells, thereby inducing apoptosis of epithelial cell [10]. It has been suggested that cisplatin induced toxicity is closely associated with an increase in lipid peroxidation [11]. Lipid peroxidation is oxidative deterioration of polyunsaturated lipid that occurs through free radical chain reaction [12]. Free radicals are generated inside the body and cause several damages to vital cellular organs. To control and reduce lipid peroxidation antioxidants have been proven helpful to a significant extent.
In the ongoing search of the present authors for antioxidant that may reduce drug-induced lipid peroxidation [13-24], the present work has been carried out in vitro to evaluate the antioxidant effect of water extract of Spirulina platensis on cisplatin-induced lipid peroxidation.
Materials and Methods
The study had been performed on the goat (capra capra) liver using some common laboratory markers of lipid peroxidation like measurement of the malondialdehyde (MDA), 4-hydroxy-2-nonenal (4- HNE), reduced glutathione (GSH) and nitric oxide (NO) content of the tissue. The goat liver was selected because of its easy availability and close similarity to the human liver in its lipid profile [25].
Preparation of water extract of Spirulina platensis
Spirulina was obtained from Indo Leena Biotech Private Ltd., Spirulina Farm, Namakkal, Tamil Nadu. Attempt was made to determine the maximum concentration of the algae in water extract. For this purpose, first 2.5 g of spirulina powder was weighed accurately and taken in a beaker. Then 200 ml of water was added to it. The mixture was heated cautiously in a steam bath until the volume was reduced to 50 ml. The hot solution was filtered at a suction pump using single filter paper. After that the filtrate was again filtered at a suction pump using double filter paper. Then the filtrate was transferred in a 50 ml volumetric flask and the volume was made up to the mark with double distilled water. The concentration of the solution was determined as follows: At first a clean petridish was weighed accurately. Then 1 ml of the extracted solution was placed on it. Then the solution was heated on a steam bath to remove water and last traces of water were removed by drying in hot air oven. It was then kept in a desiccator to cool to room temperature. The weight of the petridish along with the solid material was weighed. Then further 1 ml of the extract was added and same procedure was done. In this way a total of 5 ml of extract was added to petridish and water was evaporated. Finally the weight of the petridish and solid material was taken. The amount of solid present in 5 ml extract was calculated by difference from the empty weight of petridish. The concentration of the water extract determined in this way was 0.92% w/v. The same procedure was followed with 4, 5, 6 and 7 g of spirulina powder and the concentrations were 1.4, 1.7, 1.7 and 1.7% w/v respectively. It was found that the maximum extractable concentration of the algae using 200 ml of water would be 1.7% w/v. The λmax of the water-extracted solution was found at 259 nm.
Preparation of tissue homogenate
Goat liver was collected from Kolkata Municipal Corporation (KMC) approved outlet. Goat liver perfused with normal saline through hepatic portal vein was harvested and its lobes were brieß y dried between filter papers to remove excess blood and thin cut with a heavy-duty blade. The small pieces were then transferred in a sterile vessel containing phosphate buffer (pH 7.4) solution. After draining the buffer solution as completely as possible, the liver was immediately grinded to make a tissue homogenate (1 g/ml) using freshly prepared phosphate buffer (pH 7.4). The homogenate was divided into four equal parts, which were then treated differently as mentioned below.
Incubation of tissue homogenate with drug and/or antioxidant
The tissue homogenate was divided into four parts of 50 ml each. The first portion was kept as control (C), while the second portion was treated with cisplatin (D) at a concentration of 0.0244 mg/g tissue homogenate. The third portion was treated with cisplatin at a concentration of 0.0244 mg/g tissue homogenate and water extract of spirulina at a concentration of 0.1666 mg/g tissue homogenate (DA) and the fourth one was treated with water extract of spirulina alone at a concentration of 0.1666 mg/g-tissue homogenate (A). After treatment with cisplatin and/or water extract of spirulina, the liver homogenates were shaken for 1 h and incubated at 180±20 for a period of maximum 4 h for further work.
Estimation of malondialdehyde (MDA) level from tissue homogenate
The extent of lipid peroxidation was measured in terms of MDA content using thiobarbuturic acid (TBA) method26. The estimation was done at 2, 4 h of incubation and repeated in five animal sets. In each case three samples of 2.5 ml of incubation mixture were treated with 2.5 ml of 10% (w/v) trichloroacetic acid (TCA) and centrifuged at 1200 g for 30 min to precipitate protein. Then 2.5 ml of the filtrate was treated with 5 ml of 0.002 (M) TBA solution and the volume was made up to 10 ml with distilled water. The mixture was heated on a boiling water bath for 30 min and then tubes were cooled to room temperature and the absorbance was measured at 530 nm against a TBA blank (prepared from 5 ml of TBA solution and 5 ml of distilled water) using Elico Mini Spec (SL 171). The concentrations of MDA were determined from standard curve, which was constructed as follows. Different aliquots from standard 1,1,3,3-tetrahydroxypropane (TEP) solution were taken in graduated stoppered test tubes and volume of each solution was made up to 5 ml. To each solution, 5 ml of TBA solution was added and the mixture was heated in a steam bath for 30 min. The solutions were cooled to a room temperature and their absorbances were measured at 530 nm against TBA as blank. By plotting absorbances against concentrations a straight line passing through the origin of grid was obtained. The best-fit equation is A=0.007086 M, where M= nanomoles of MDA, A= absorbance, r= 0.995, SEE= 0.006.
Estimation of reduced glutathione (GSH) level from tissue homogenate
Reduced glutathione (GSH) was measured in accordance to the Ellman’s method [27]. The estimation was done at 1, 2 h of incubation and repeated in five animal sets. In each case three samples of 1 ml of incubation mixture were treated with 1 ml of 5% TCA in 1 mM EDTA centrifuged at 2000 g for 10 min. After that, 1 ml of the filtrate was mixed with 5 ml of 0.1M phosphate buffer (pH 8.0) and 0.4 ml of 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB, 0.01% in phosphate buffer) was added to it. The absorbances of the solutions were measured at 412 nm against blank (prepared from 6.0 ml of phosphate buffer and 0.4 ml of DTNB). The concentrations of reduced glutathione were determined from standard curve.
Estimation of 4-hydroxy-2-nonenal (4-HNE) level from tissue homogenate
The estimation was done only at 2 h of incubation and it was repeated in 5 animal sets. In each case three samples of 2 ml of incubation mixture were treated with 1.5 ml of 10% TCA solution and centrifuged at 1200 g for 30 min. Then 2 ml of the filtrate was treated with 1 ml of 2,4 dinitrophenyl hydrazine (100 mg/100 ml of 0.5 M HCl) and kept for 1 h at room temperature. After that the samples were extracted with hexane and the extract was evaporated to dryness under argon at 400. After cooling to a room temperature, 2 ml of methanol was added to each sample and the absorbance was measured at 350 nm against methanol as blank [28]. The concentrations of 4-HNE content were determined from the standard curve.
Estimation of nitric oxide (NO) level from tissue homogenate
The estimation was done at 1, 2 h of incubation and it was repeated in five animal sets. NO content was determined by reaction with Griess reagent [29]. In each case three samples of 4.0 ml of tissue homogenate were treated with 2.5 ml of 10% TCA solution and centrifuged at 1200 g for 30 min. Then 5 ml of the filtrate were treated with 0.5 ml Griess reagent. After 10 min the absorbances of the solutions were measured at 540 nm against blank (prepared from 5.0 ml of distilled water and 0.5 ml of Griess reagent). The concentrations of NO content were calculated from standard curve.
Results and Discussion
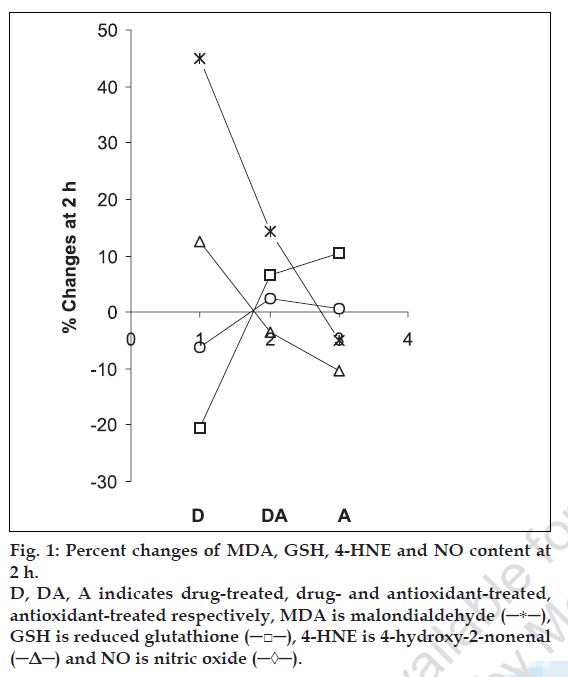
The percent changes in MDA, GSH, 4-HNE and NO level at different h of incubation were calculated with respect to the control of the corresponding h of incubation and the changes in MDA/GSH/4-HNE/NO level was considered as indicator of the extent of lipid peroxidation. The averages of percent changes in MDA/GSH/4-HNE/NO content of five animal sets along with standard error are listed in Table 1. Interpretation of the results is supported by analysis of variance and also by statistical multiple comparison analysis using least significant different procedure [30-31].
| Parameters | Incubation (h) | % Changes due to treatment samples (Average±SE) |
Analysis of variance and multiple comparison | ||
|---|---|---|---|---|---|
| D | DA | A | |||
| MDA | 2 | 44.81 (±9.84) |
14.38 (±6.1 2) |
-5.04 (±5.49) |
F1=13.24 [df=(2,8)], F2=1.45[df=(4,8)] Pooled variance (S2)*=238.45, Critical difference, (p=0.05)# LSD=29.07, Ranked means** (D) (DA, A) |
| 4 | 23.7 (±10.24) |
-11.07 (±5.17) |
-18.89 (±10.70) |
F1=8.77[df=(2,8)], F2=2.22[df=(4,8)] Pooled variance (S 2)* =287.30, Critical difference, (p=0.05)# LSD =31.91, Ranked means**(D) (DA, A) |
|
| GSH | 1 | -11.33 (±3.95) |
02.69 (±4.05) |
03.50 (±3.27) |
F1=4.13 [df=(2,8)], F2=0.538[df=(4,8)] Pooled variance (S2)* =84.19, Critical difference, (p=0.05)# LSD =17.27, Ranked means** (D, DA, A) |
| 2 | -20.39 (±2.62) |
06.67 (±8.06) |
10.42 (±4.24) |
F1=21.27[df=(2,8)], F2=4.76[df=(4,8)] Pooled variance (S2)* =66.43, Critical difference, (p=0.05)# LSD =6.65, Ranked means** (D) (DA, A) |
|
| 4-HNE | 2 | 12.45 (±2.86) |
-03.61 (±1.75) |
-10.39 (±4.62) |
F1=10.89[df=(2,8)], F2=0.586[df=(4,8)] Pooled variance (S2)* =63.17, Critical difference, download (p=0.05)# LSD =14.96, Ranked means** (D) (DA, A) |
| NO | 1 | -09.79 (±1.66) |
5.83 | 12.73 | F1=16.72 [df=(2,8)], F2=3.01 [df=(4,8)] Publications2 Pooled variance (S )*=39.78, Critical difference (p=0.05)#.LSD =11.87, Ranked means**(D) (DA, A) |
| 2 | -06.35 (±1.35) |
02.49 (±0.525) |
06.82 (±2.22) |
F1=15.10 [df=(2,8)], F2=0.375 [df=(4,8)] Pooled variance (S2)*=14.90, Critical difference (p=0.05)# LSD=7.27, Ranked means**(D) (DA, A) |
|
Theoretical values of F: P=0.05 level F1=4.46 [df=(2,8)], F2=3.84 [df=(4,8)], P=0.01 level F1=8.65 [df=(2,8)], F2=7.01 [df=(4,8)]. F1 and F2 corresponding to variance ratio between groups and within groups respectively. D, DA, A indicate drug treated, drug and antioxidant treated, antioxidant treated respectively. SE= Standard Error (df=4); df= degree of freedom, *Error mean square, #Critical difference according to least significant procedure (31). **Two means not included within same parenthesis are statistically significantly different at P=0.05 level.
Table 1: Effects of Water Extract of Spirulina Platensis On Cisplatin-Induced Lipid Peroxidation: Changes In Mda/Gsh/4-Hne/No Profile
Incubation of tissue homogenates with cisplatin resulted an increase in MDA content with respect to corresponding control (Table 1). This observation suggests lipid peroxidation induction potential of the cisplatin. MDA is a highly reactive three-carbon dialdehyde produced as a biproduct of polyunsaturated fatty acid peroxidation and arachidonic acid metabolism. Increase in the accumulation of MDA in cells can result into cellular degradation, some biochemical and functional changes and even cell death32. It was further found that MDA content was significantly reduced when tissue homogenates were treated both with cisplatin and water extract of Spirulina platensis. This implies the free radical scavenging property of the water extract of the algae. When tissue homogenates were treated only with the water extract of algae then there is also depletion of MDA level in comparison to control.
The average percent changes in reduced glutathione (GSH) level of five animal sets are shown along with statistical analysis in Table 1. Incubation of tissue homogenates with cisplatin decreased the GSH level with respect to corresponding controls. Glutathione is a small protein composed of three amino acid, such as cysteine, glutamic acid and glycine [33]. It is an important antioxidant and plays a very important role in the defence mechanism for tissue against the reactive oxygen species [34]. When the tissue homogenates were treated both with cisplatin and water extract of the algae then GSH level was increased in comparison to drug treated group. Incubation of tissue homogenates only with water extract of algae also enhances the GSH level. These observations suggest that increase in GSH level may be due to antioxidant property of the extract.
The average percent changes in 4-HNE levels of five animal sets are shown along with statistical analysis in Table 1. Incubation of tissue homogenates with cisplatin caused significant increase in 4-HNE content with respect to control. 4-HNE is a specific and stable end product of lipid peroxidation. It can diffuse within or even escape from the cell and attack targets far from the site of the original free radical event35-36. 4- HNE can be produced from arachidonic acid, linolenic acid or their hydroperoxide in concentration of 1 μM to 5 nM in response to oxidative stress35. When tissue homogenates were treated both with cisplatin and water extract of the algae, 4-HNE content was significantly reduced in comparison to the drug treated group. Incubation of tissue homogenates only with water extract of algae also reduces the 4-HNE levels. This implies that water extract of Spirulina platensis inhibits cisplatin-induced lipid peroxidation to a significant extent.
The average percent changes in nitric oxide (NO) level of five animal sets are shown along with statistical analysis in Table 1. Incubation of tissue homogenates with cisplatin reduced the NO content with respect to corresponding controls. Nitric oxide has versatile role in biology because it can be a signaling molecule in vasodilation [37], a toxin [38], a prooxidant [39] and a potential antioxidant [40-43]. It was further found that incubation of tissue homogenates with cisplatin and water extract of algae resulted increase in NO content with respect to drug treated group. Incubation of tissue homogenates only with water extract of algae also enhances NO level with respect to corresponding controls. These results suggests that water extract of spirulina could inhibit lipid peroxidation to a significant extent. It has been proposed that NO causes chain termination reactions during lipid peroxidation as observed in lowdensity lipoprotein oxidation as well as in chemical systems [40-43].
The data presented in this work demonstrate the lipid peroxidation induction potential of cisplatin. The results also suggest the antiperoxidative effects of water extract of spirulina and demonstrate its potential to reduce cisplatin-induced lipid peroxidation. The antioxidant effect is attributed due to its various constituents working individually or in synergy. From fig. 1 it is found that that the % changes of MDA and 4-HNE follow similar pattern while those of GSH and NO show similar trend for all three samples (D, DA and A). The results indicate that spirulina merits further extensive studies to explore potential to reduce cisplatin-induced toxicity.
Acknowledgements
The authors wish to thank Biological Evans Ltd., Hyderabad for providing the free gift sample of cisplatin. Helpful discussion with Prof. A U De is also thankfully acknowledged.
References
- Abdulqader G, Barsanti L, Tredici M. Harvest of Arthrospira platensis from lake kossorom (chad) and its household usage among the kanembu. J Appl Phycol 2000;12:493-8.
- Belay A, Ota Y, Miyakawa K, Shimamatsu H. Production of high quality spirulina at earthrise farms. In: Phang SM, editor. Algal Biotechnology in the Asia PaciÞc Region. Kuala Lumpur: University of Malaya; 1994. p. 92-102.
- Bealy A, Kato T, Ota Y. Spirulina (Arthrospira): Potential application as an animal feed supplement. J Appl Phycol 1996;8:303-11.
- Foster S, Tyler VE. Tyler’s honest herbal: A sensible guide to the use of herbs and related remedies. 4th ed. New York; Haworth Herbal Press: 1999.
- Belay A. The potential application of spirulina (Arthrospira) as a nutritional and therapeutic supplement in health management. J Am Nutraceutical Assoc 2002;5:26-48.
- Mohan IK, Khan M, Shobha JC, Rao Naidu MU, Prayag A, Kutala VK. Protection against cisplatin-induced nephrotoxicity by spirulina in rats. Cancer Chemother Pharmacol 2006;58:802-8.
- Hayashi O, Katoh T, Okuwaki Y. Enhancement of antibody production in mice by dietary Spirulina platensis. J Nutr Sci Vitaminol 1994;40:431-41.
- Mathew B, Sankarnarayanan R, Nair P, Varghese C, Somanathan T, Amma P et al. Evaluation of chemo prevention of oral cancer with Spirulina fusiform. Nutr Cancer 1995;24:197-202.
- Parlakpinar H, Sahna E, Ozer MK, Ozugurlu F, Vardi N, Acet A. Physiological and pharmacological concentrations of melatonin protect against cisplatin-induced acute renal injury. J Pineal Res 2002;33:161-6.
- ChangdownloadB, Nishikawa M, Sato E, Utsumi K, Inoue M. L-carnitine inhibits cisplatin-induced injury of the kidney and small intestine. Arch Biochem BiophysPublications2002;405:55-64.
- 0 Ulubas B, Cimen.MY, Apa DD, Saritus E, Muslu N, Cimen OB. The free protective effect of acetyl salicylic acid on free radical production in cisplatin-induced nephrotoxicity: An experimental Rat model. Drug Chem Toxicol 2003;26:259-70.
- Halliwell B, Gutteridge JM. Free radicals in biology and medicine. 2nd. com) ed. Oxford; Oxford University Press: 1989.
- Sengupta M, De AU, Sengupta C. Correlation of biological activity Medknow (therapeutic and toxic) with solvochromic properties of metronidazole, emetine hydrochloride and diloxanide furoate. Indian J Biochem Biophys 1995;32:302-7.
- Dutta H, De AU, Sengupta C. Effects of two cardiac glycosides, dogitoxin and digoxin on blood lipids. Indian J Biochem Biophys. medknow1996;33:76-80.
- Roy K, Rudra S, De AU, Sengupta C. In vitro studies on effects of cefotaxime sodium and metoprolol tartrate on whole goat blood phospholipids. Indian J Pharm Sci 1998;60:153-7.
- Roy K, Rudra S, De AU, Sengupta C. Evaluation of ascorbic acid as inhibitor of lipid peroxidation induced by cefotaxime sodium and metoprolol. Indian J Pharm Sci 1999;61:44-7.
- Roy K, Saha A, De K, Sengupta C. Ceftriaxone induced lipid peroxidation and its inhibition with various antioxidants: Part II. Evaluation of glutathione and Probucol as antioxidants. Acta Pol Pharm Drug Res 2000;57:385-90.
- Saha A, Roy K, De K, Sengupta C. Effects of oral contraceptive norethindrone on blood-lipid and lipid peroxidation parameters. Acta Pol Pharm Drug Res 2000;57:443-7.
- De K, Roy K, Saha A, Sengupta C. Evaluation of alpha tocopherol, probucol and ascorbic acid as suppressor of digoxin induced lipid peroxidation. Acta Pol Pharm Drug Res 2001;58:391-400.
- Saha A, Roy K, De K, Sengupta C. Effect of desogestrol on blood lipid in relation to its biological activities. Acta Pol Pharm Drug Res 2002;59:65-9.
- Roy K, Saha A, De K, Sengupta C. Evaluation of probucol as suppressor of ceftizoxime induced lipid peroxidation. Acta Pol Pharm Drug Res 2002;59:231-4.
- Chakraborty S, Bhuti PD, Ray S, Sengupta C, Roy K. A study on ceftriaxone-induced lipid peroxidation using 4-hydroxy-2-nonenal as model markers. Acta Pol Pharm Drug Res 2005;62:141-3.
- Ray S, Sengupta C, Roy K. Evaluation of ascorbic acid as suppressor of cyclophosphamide-induced lipid peroxidation using common laboratory markers. Acta Pol Pharm Drug Res 2005;62:145-51.
- Ray S, Roy K, Sengupta C. Cisplatin-induced lipid peroxidation and its inhibition with ascorbic acid. Indian J Pharm Sci 2006;68:199-204.
- Hilditch TP, Williams PN. The chemical constituents of fats. London; Chapman and Hall: 1964.
- Ohkawa H, Ohishi N, Yagi K. Assay of lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8.
- Ellman GL. Tissue Sulfhydryl groups. Arch Biochem Biophys 1959;82:70-7.
- Kinter M. Quantitave analysis of 4-hydroxy-2-nonenal. In: Punchard NA, Kelly FJ, editors. Free radicals- A practical approach. Oxford; Oxford University Press: 1996. p. 133-45.
- Sastry KV, Moudgal RP, Mohan J, Tyagi JS, Rao GS. Spectrophotometric determination of serum nitrite and nitrate by coppercadmium alloy. Anal Biochem 2002;306:79-82.
- Snedecor GW, Cochran WG. Statistical methods. New Delhi; Oxford and IBH Publishing Co. Pvt. Ltd: 1967.
- Bolton S. Statistics. In: Gennaro AR, editor. Remington: The science and practice of pharmacy. 20th ed. Volume I. Philadelphia; Lippincott Williams and Wilkins: 2000. p. 124-58.
- Winrow VR, Winyard PG, Morris CJ, Black DR. Free radicals in inß ammation: Second messengers and mediators of tissue destruction. Br Med Bull 1993;49:506-22.
- Sen CK. Nutritional biochemistry of cellular glutathione. J Nutr Biochem 1997;8:660-72.
- Kosower EM, Kosower NS. Glutathione metabolism and function. Raven Press: New York; 1976.
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related articles. Free Radic Biol Med 1991;11:81-128.
- Uchida K, Szweda LI, Chae HZ, Stadtman ER. Immunochemical detection of 4-hydroxynonenal proteins adducts in oxidized hepatocytes. Proc Natl Acad Sci USA 1993;90:8742-6.
- Ignarro LJ, Byrans RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radicals. Circ Res 1987;61:866-79.
- Moncada S, Higgs A. L-arginine-nitric oxide pathway. N Engl J Med 1993;329:2002-12.
- Beckman JS, Beckman TW, Chen J, Marshal PA, Freeman BA. Apparent hydroxyl radical production by peroxinitrite: Implication for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 1990;87:1620-4.
- Hogg N, kalyanaraman B, Joseph J, Struck A, Parthasarathy S. Inhibition of low density lipoprotein oxidation by nitric oxide: Potential role in atherogenesis. FEBS Lett 1993;334:170-4.
- Rubho H, Parthasarathy S, Barnes S, Kirk M, Kalyanaraman B, Freeman BA. Nitric oxide inhibition of lipoxygenase-dependent liposome and low-density lipoprotein oxidation: Termination of radical chain propagation reactions and formation of nitrogen containing oxidized lipid derivatives. Arch Biochem Biophys. 1995;324:15-25.
- Yamanaka N, Oda O, Nagao S. Nitric oxide released from zwitterionic polyamine/NO adducts inhibit Cu2+-induced low-density lipoprotein oxidation. FEBS Lett 1996;398:53-6.
- Odonnel VB, Chumley PH, Hogg N, Bloodsworth A, Freeman BA. Nitric oxide inhibition of lipid peroxidation: Kinetics of reaction with lipid peroxyl radicals and comparison with alpha-tocopherol. Biochemistry 1997;36:15216-23.