- *Corresponding Author:
- Ana Tomas
Department of Pharmacology, Toxicology and Clinical Pharmacology, Faculty of Medicine, University of Novi Sad, Serbia
E-mail: anatomas@uns.ac.rs
| Date of Submission | 01 June 2017 |
| Date of Revision | 06 February 2018 |
| Date of Acceptance | 09 August 2018 |
| Indian J Pharm Sci 2018;80(5):858-867 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Dietary supplements based on soybean extracts are widely used for a myriad of indications, but there is evidence that isoflavones, components of these supplements affect cytochrome activity. Alteration in pharmacodynamics of cytochrome-substrate drugs after co-administration with dietary supplements based on soybean extracts offers information on clinically significant herb-drug interactions that can cause unanticipated adverse reactions or therapeutic failures. Moreover, herbal drugs can contribute to the development and severity of drug-induced liver injury. Therefore, the aim of this study was to examine the effect of commercially available dietary supplements based on soybean extracts on liver and renal function, oxidative stress and pharmacodynamics of several conventional drugs. After quantification of isoflavones in the supplement using HPLC-DAD, pharmacological tests such as hot-plate, rotarod, pentobarbital-induced sleeping time were performed on Swiss albino mice treated with soybean extracts and several central nervous system acting drugs. Liver, renal function, and oxidative status in liver homogenates were determined in healthy and Wistar rats subjected to oxidative stress with CCl4. Dietary supplements based on soybean extracts weakened the analgesic activity of codeine, significantly potentiated diazepam-induced motor coordination impairment at 10th and 30th minute after diazepam administration, but had the opposite effect on alprazolam effect. Soybean extracts pretreated group also exhibited significantly shorter pentobarbital sleep induction and sleeping time. Soybean extracts administration did not affect the liver and renal function and ameliorated oxidative stress caused by CCl4. Despite exhibiting no negative effects on liver and renal function and demonstrated antioxidant in vivo potential, the safety of soybean extracts in addition to conventional drugs is questionable. The results of our study implicate the potential of dietary supplements based on soybean extracts for serious herb-drug interactions.
Keywords
Phytoestrogens, soybean extract, drug interactions, cytochrome P-450, conventional drugs, liver function, antioxidant
Soy (Glycine max. L.) is a plant that has been used since ancient times due to its high nutritive value and health benefits. To this day, soy and soybeans products, such as tofu, soy milk, soy sauce, miso, tempeh represent an important component of human diet [1]. In addition to their high nutritive value, soybeans also contain biologically active compounds and vitamins, which includes isoflavones, tocopherols, vitamin C, saponins, and sterols [2,3]. Isoflavones are non-steroidal, polyphenolic compounds that are present in unconjugated form (aglycones), and as different types of glycoside conjugates (β-glucosides, malonyl glucosides and acetyl glucosides). The most important representatives of isoflavones are daidzein, genistein and glycitein [1,4]. Consumption of foods rich in phytoestrogens is reported to be associated with reduced risk of prostate and breast cancer [1]. Soybean isoflavones and other phytoestrogens play an important role in reducing the incidence of cardiovascular diseases, hormone-dependent cancers, osteoporosis as well as alleviation of hot flushes [5]. Different nutritional or nutraceutical supplement products have been produced from soybeans with the ones containing vitamin-E, lecithin and isoflavone, being the most popular ones [6]. Soybean-based supplements could become an alternative to the hormone replacement therapy, a treatment of choice to relieve menopausal symptoms and reduce incidence of osteoporosis in postmenopausal women, which is avoided by many women because of the perceived increased risk of breast cancer [6-8].
With an increase in the popularity and use of herbal supplements, potential for adverse drug reactions also increases as these are seldom used alone, and more often as an adjunct to conventional drugs. Conventional drugs that are metabolized by cytochrome P450 (CYP) enzymes are particularly susceptible to herb-drug interactions, which can be attributed to the ability of many herbal compounds to induce or inhibit these enzymes [9]. These potential interactions between herbs and conventional drugs could lead to two major problems, toxicity or failure of the treatment. Roberts-Kirchhoff et al. showed that isoflavones were metabolized by microsomal liver enzymes (CYP 450), affecting the activity of these enzymes at the same time [10]. Number of studies showed that soy isoflavones affect the expression and activity of drug metabolizing enzymes [10-12] and multiple studies have demonstrated the regulation of several isoforms of CYP1, 2, and 3 subfamily members by isoflavones in rodent models [13-16]. A study which evaluated the influence of genistein, one of the most active soy isoflavones on cytochrome enzymes reported potentiation of the activity of CYP3A isoenzyme and P-glycoprotein [11]. When administered to experimental animals, soy isoflavones have potentiated gene expression for the synthesis of CYP1A1 and 2D1 and inhibited synthesis of 2D2 and 3A1 isozymes [12]. Li et al. determined that the concurrent intake of soy isoflavones with prescription medication may affect the pharmacokinetic profile and hence bioavailability of such co-administered drugs, due to CYP3A4 induction, an enzyme critically important in drug metabolism, being responsible for the metabolism of ∼50 % of all prescription drugs [17]. However, it is known that even though during pharmacokinetic interactions significant changes in drugs concentrations can occur, this must not always be followed with a significant change in the pharmacodynamic response [18]. There is no data if this effect on the enzymatic complex and subsequent changes in pharmacokinetics is related to actual changes in drug effect. Therefore, possible serious clinically relevant herb-drug interactions might occur during co-administration of soybean-based supplements and conventional drugs whose metabolic pathways included isozymes reported to be affected by the main isoflavones in the supplement. To mention a few, benzodiazepines are metabolized via CYP450 enzymatic system. Diazepam oxidative metabolism through demethylation (cytochrome 2C9, 2C19, 2B6, 3A4) and hydroxylation (cytochrome 3A4 and 2C19) results in formation of several active metabolites, while alprazolam biotransformation in CYP 3A4 produces less potent metabolites [19]. In experimental pharmacology, pentobarbital, a well-known hypnotic is used to establish the effect of xenobiotics on pentobarbitalinduced sleep as a measure of pharmacodynamics of this drug [20]. Pentobarbital is almost completely metabolized into inactive metabolites through hydroxylation by CYP2B6 and CYP2D6. Changes in codeine activity can also help understand the span of effects soybean supplementation has on microsomal liver enzymes, as codeine metabolism is strongly influenced by the activity of this enzymatic complex. Codeine is transformed into active metabolites through CYP2D6, and these metabolites then undergo glucuronidation and are excreted through urine [21].
Besides the risk of interactions, herbal drugs can also significantly affect liver function, while some may even cause drug-induced liver injury [22]. The main mechanism of drug-induced hepatotoxicity involves the generation of hepatotoxic metabolites and accumulation of reactive oxygen species (ROS). The disturbance in the balance between cellular pro- and antioxidant activities and increased ROS levels damage DNA, proteins, enzymes, and lipids and induce apoptosis and necrosis of hepatocytes [23]. Reports on the effects of soybean-based supplement on liver function are scarce, but as the incidences of liver injuries due to herbal and dietary supplements continue to increase, it is necessary to determine the effect of soybean-derived supplements on liver function, in both normal and pathological conditions. Furthermore, renal dysfunction is common in liver diseases, since concomitant renal and liver dysfunction may share common pathogenic mechanisms. Renal failure can be functional, secondary to liver dysfunction or acute or chronic liver disease may complicate with intrinsic renal abnormalities, therefore renal function also needs to be monitored in order to completely grasp the potential of xenobiotics causing liver injury. Despite the increase in the use of products containing isoflavones, currently there is not enough evidence from previous preclinical and clinical studies that would fully clarify and confirm efficiency and safety of these products. Lack of information about interactions with other drugs also imposes a need for further examination of active constituents of soybean. Taking all this into account, the aim of this study was to examine the effect of commercially available dietary supplement containing soybean extract on pharmacodynamics of diazepam, alprazolam, codeine and pentobarbital and to determine the effect on liver and renal function in healthy animals and animals subjected to oxidative stress.
Materials and Methods
Dietary supplement based on soybean extract (DSU) that is commercially available on Serbian market was used. Hard capsules with standardized isoflavone content of 40 % isoflavones/100 mg of soy extract i.e. 40 mg isoflavone/capsule; 1 capsule contains 100 mg of soybean extract. Recommended daily dose is 1 capsule. Isoflavone standards (daidzein, glycitein and genistein) and diazepam, alprazolam, codeine and pentobarbital were purchased from Sigma Aldrich Life Sciences (Germany). Saline solution was obtained from Hemofarm (Serbia).
Quantification of isoflavones in the commercially available dietary supplement
The content of isoflavones per capsule was determined. Content of 10 capsules was pulverized, mixed and analysed in triplicate. The extraction was conducted with 40 ml of 80 % methanol for 2 h, at 40° with constant stirring. After extraction, volume of the extract was made up to 50 ml with 80 % methanol, the extract was then diluted 10 times using the same solvent. Before high-performance liquid chromatography (HPLC) analysis, extracts were filtered through a membrane filter (0.45 μm) in the dark vials. An Agilent (Palo Alto, CA, USA) model 1100 series HPLC equipped with a binary pump, degasser, auto sampler, and diodearray detector (DAD) was used to separate, identify, and quantify isoflavones. Analysis of isoflavones was conducted according to the slightly modified method described by Lee et al. [24]. Isoflavones were separated using a Zorbax SB C18 reversed-phase HPLC column (4.6×150 mm, 5 μm) with a Zorbax SB C18 guard column (12.5×4.6 mm, 5 μm, 25°, 0.6 ml/min, 10 μl, 270 nm). Mobile phase gradients were formed between solvent A (1 % v/v acetic acid in water), and solvent B (100 % acetonitrile). Gradient conditions were as follows, 0-5 min 15 % solvent B, 5-44 min from 15 to 35 % solvent B, 44-45 min from 15 to 35 % solvent B and 45-50 min 15 % solvent B. A post-time period of 20 min was applied with solvent A:B is 85:15 %. Isoflavones were identified by comparing the retention times and UV spectra (nm) of samples with those of standards and literature data [24,25]. Isoflavones were quantified by the method of external standards. Five-point regression curves of daidzein, glycitein and genistein standard compounds were used. Method was proved to be linear (r≥0.9998). Standard solutions were prepared by dissolving standard compounds in mixture of methanol:water (4:1, v/v). Calibration curve range for daidzein and genistein were 0.002-0.03 mg/ml, while for glycitein it was 0.002-0.006 mg/ml. As only standards of phytoestrogen aglycones were used, the content of corresponding glucoside forms was obtained by calculation, and their identification was confirmed by comparing retention times with literature data. For this purpose, calibration curves of corresponding aglycone compounds were used and corrections for differences in molecular weight between aglycones and glucosides were applied following the pattern given by Andlauer et al. [25], c (glucoside) = c (corr. aglycone)× Mr (glucoside)/Mr (corr. aglycone). The total isoflavone content was expressed as aglycone equivalents, by summarizing the masses of the corresponding components without applying the correction for differences in molecular weight between aglycones and glucosides.
Experimental animals and treatment
Examination of potential interactions using standard pharmacodynamics tests was carried out on adult, sexually mature Swiss albino mice of both sexes, weighing 20-40 g. For examination of liver function and antioxidant potential, 7-8 w old male Wistar rats, weighing 200-250 g were used. All animals were obtained from the Military Technical Institute, Belgrade, Serbia. Animals were housed in Ehret Uni Protect laboratory cages at a controlled temperature (23 ± 1°) and humidity (55 ± 1.5 %) under standard circadian rhythm (day/night), with free access to pelleted food and water. Animal care and experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals edited by Commission of Life Sciences, National Research Council (USA). The experimental procedures were approved by Ethics Committee for Animal Use in Experiments of the University of Novi Sad (I-2013-03). The doses of DSU used in the experiment were adapted for the experimentation on mice using FDA guidance [26] according to the recommended human daily dosed for a male with standard weight of 70 kg.
Examination of potential interactions
All animals were divided into 9 experimental groups, each containing 6 animals, and treated as follows, CONS, saline 1 ml/kg per os (p.o.) for 7 d, where the last dose was applied 30 min before intraperitoneal (i.p.) administration of saline solution on the d 7; CODS, saline 1 ml/kg p.o. for 7 d, where the last dose was applied 30 min before i.p. administration of codeine (30 mg/kg) on d 7; PENS, saline 1 ml/kg p.o. for 7 d, where the last dose was applied 30 min before i.p. administration of pentobarbital (40 mg/kg) on d 7; DIAS, saline 1 ml/kg p.o. for 7 d, where the last dose was applied 30 min before intramuscular (i.m.) administration of diazepam (2 mg/kg) on d 7; ALPS, saline 1 ml/kg p.o. for 7 d, where the last dose was applied 30 min before i.m. administration of alprazolam (2 mg/kg) on d 7; CODDSU, DSU p.o. 170 mg/kg for 7 d, where the last dose was applied 30 min before i.p. administration of codeine (30 mg/kg) on d 7; PENDSU, DSU p.o. 170 mg/kg for 7 d, where the last dose was applied 30 min before i.p. administration of pentobarbital (40 mg/kg) on d 7; DIADSU, DSU p.o. 170 mg/kg for 7 d, where the last dose was applied 30 min before i.m. administration of diazepam (2 mg/kg) on d 7; ALPDSU, DSU p.o. 170 mg/kg for 7 d, where the last dose was applied 30 min before i.m. administration of alprazolam (2 mg/kg) on d 7.
Examination of in vivo antioxidative potential
To determine the effects of DSU on in vivo antioxidant capacity, Wistar rats were subjected to pro-oxidative compound, CCl4. All animals were divided into 4 experimental groups, each containing 6 individuals, and treated as follows: CONS, saline 1 ml/kg p.o. for 7 d; CONCCl4, saline 1 ml/kg p.o for 7 d+a single CCl4 dose 2 ml/kg i.p. DSU; DSU p.o. for 7 d; CCl4DSU: DSU p.o. for 7 d, where the last dose was applied 1 h before administration of a single CCl4 dose 2 ml/kg i.p.
DSU in the dose of 170 mg/kg was dissolved in 10 ml of saline and was applied by per oral gavage to laboratory animals. The control group received an equivalent volume of saline solution. Codeine hydrochloride (30 mg/kg) and pentobarbital (40 mg/kg) were administered i.p. 30 min after the last DSU solution intake [20,27]. Diazepam (2 mg/kg) and alprazolam (2 mg/kg) were applied via i.m. injection 30 min after the last DSU solution intake. Alprazolam was suspended in 1 % Tween 80 in water because alprazolam is not freely soluble in saline. CCl4 was dissolved in olive oil solution to the final concentration of 50 %. All experiments were carried out during the daytime.
Hot plate test
The hot plate test was performed by placing the animal’s individually on hot plate and assessing their response to the thermal stimulus. The temperature of the metal plate enclosed by Plexiglas walls was maintained at 52.5°. The response time was measured in seconds at which the animal licked or flinched one of the hind paws, or jumped off the plate. To prevent tissue damage, a cut-off time was used as a double value of latencies measured before drug application. Response latencies were first determined two times before the application of the tested compound in order to determine a pre-treatment response for each animal, and then 5, 10, 20, 30, 60, 90 min following the drug administration. After responding or reaching the cutoff time, animals were removed from the plate [20,27]. Analgesic effect determined in seconds was expressed as percent of prolongation of measured reaction time compared to control reaction time.
Rotarod performance test
Evaluation of motoric coordination of experimental animals was performed using the rotarod performance test. Horizontally oriented rotating cylinder was placed on the height of 50 cm, so that animals would be discouraged to jump from it and would not be injured when falling down. Rotation speed was set to 12 rpm. Only those animals able to maintain balance on the rod for 5 min were included in the experiment. The length of time (s) that animals stayed on this rotating rod was measured in the intervals of 10, 20, 30 and 50 min after administration of diazepam and alprazolam (retention time) [20]. A depression of motoric activity was calculated as a percent of time changed before and after the administration of diazepam and alprazolam, according to the following formula: % depression = 100–(A×100)/B, where A is a retention time (s) and B is maximum time (300 s) [27].
Sleeping time test
Sleeping time test was used to determine potential interactions in the depressive effects on central nervous system between pentobarbital and commercially available DSU [20,27]. After administration of tested substances, animals were observed for onset of sleep. A mouse was considered to be asleep if it was placed on its back and displayed a loss of righting reflex for 5 min. The latency of the loss of the righting reflex after pentobarbital administration (sleep induction time) and the time between the loss and the recovery of the righting reflex, duration (sleeping time) were determined for each animal.
Determination of oxidative status of the liver and serum biochemical parameters
Following CCl4 administration, animals were sacrificed after 24 h and 1 g liver samples were collected and homogenized with Tris-HCl buffer (pH=7.4) in 1:3 ratio to determine the parameters of oxidative stress. Levels of total liver proteins, lipid peroxidation intensity (LPX), activity of glutation peroxidase (GPX), peroxidase (PX), glutation reductase (GR), catalasis (CAT) and gluthatione reducatase (GR) levels were determined. The procedures were performed on Agilent 8453 UV/Vis (Santa Clara, USA) spectrophotometer. The LPX activity was estimated through measurement of malondialdehyde levels [28]. XOD activity was measured by the rate of the reduction of methylene blue [29]. CAT activity was estimated by determination of the rate of decomposition of H2O2 at 240 nm [30], and PX activity through application of guaiacol as the enzyme’s substrate [31]. The activities of GPX and GR were determined by the measuring the decrease in absorbance caused by the oxidation of NADPH at 340 nm [32,33].
Total and direct bilirubin, urea, uric acid and creatinine levels and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities, were determined in serum using commercially available kits. All analyses were performed in triplicate on the Olympus AU 400 autoanalyser (Hamburg, Germany) according to the manuals supplied.
Statistical analysis
The level of significance between the groups was assessed with the Student’s t-test for small independent samples using MedCalc 9.2.0.1 software. All data were expressed as a mean ± standard deviation (SD). A value of p<0.05 was considered to be statistically significant.
Results and Discussion
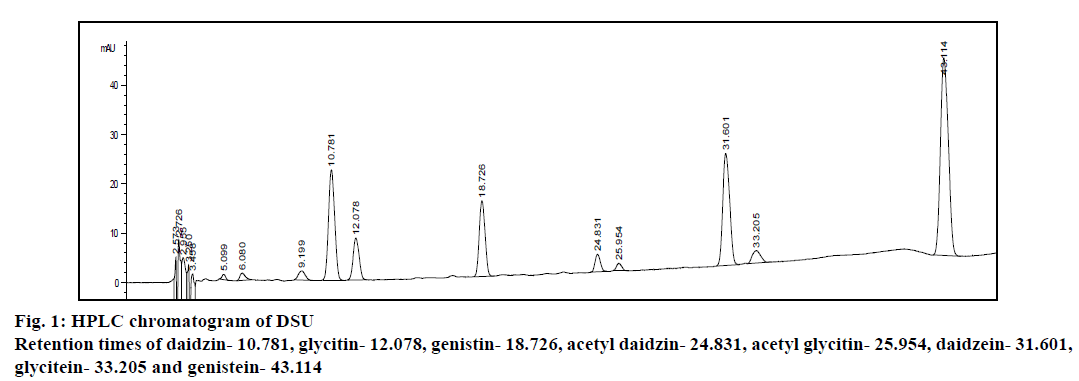
Analysis of DSU used in this study was performed on HPLC (Tables 1 and 2, Figure 1). It has been show that daidzein and its conjugates were present to the highest extent in this dietary supplement (25.59 mg/ capsule). The second most frequent was genistein type of isoflavones (10.67 mg/cps). Glycitein was the least present in the analysed supplement (3.83 mg/capsule). It was observed that the content of total isoflavones (40.09 mg/capsule) was in accordance with label claim (40 mg/capsule) with deviation from the declared content of only +0.225 %. The concentration of total isoflavones in supplement analysed in the present study was 87.5 mg per gram of supplement.
| Measured compounds |
Content (mg/cps) |
|---|---|
| Total daidzein | 25.59 |
| Total glycitein | 3.83 |
| Total genistein | 10.67 |
| TI* determined | 40.09 |
| TI declared | 40 |
DSU is dietary supplement based on soybean extract. *TI- total isoflavones
Table 1: Isoflavone content in DSU as aglycone equivalents
| Measured compounds | Content (mg/cps) |
|---|---|
| Daidzein | 13.11 |
| Daidzin | 18.19 |
| ac-Daidzin | 2.47 |
| Glycitein | 0.96 |
| Glycitin | 3.92 |
| ac-Glycitin | 0.65 |
| Genistein | 7.47 |
| Genistin | 5.12 |
Table 2: Individual isoflavone composition of DSU with mass correction
Pretreatment with DSU reduced analgesic activity of codeine at the beginning of the experiment, in 5th and 10th min after codeine administration, analgesic effect of codeine was significantly lower in the group pretreated with DSU (CODDSU) compared to the control (p<0,05). On the other hand, 20 min post administration, percentage of pain depression was significantly higher in CODDSU group (Table 3). In other measured time points (30', 60' and 90') pretreatment with DSU has potentiated analgesic effect of codeine, however, this difference was not statistically significant.
| Time (min) | Pain depression (%) CODS | Pain depression (%) CODDSU |
|---|---|---|
| 5 | 35.11 ± 18.89 | 10.90 ± 5.57* |
| 10 | 42.93 ± 11.93 | 16.63 ± 3.12* |
| 20 | 31.70 ± 10.82 | 58.51 ± 7.57* |
| 30 | 32.35 ± 19.88 | 37.79 ± 15.39 |
| 60 | 11.16 ± 34.29 | 17.56 ± 37.53 |
| 90 | 40.52 ± 16.14 | 42.54 ± 55.21 |
Pain depression (%, mean ± SD) induced by the codeine (30 mg/kg) in animals pretreated with saline solution (10 ml/kg) or DSU (dietary supplement based on soybean extract, 170 mg/kg) for seven days. *P<0.05 compared to the CODS group
Table 3: Effect of DSU on analgesic activity of codeine in the hot-plate test
The retention time of animals in DIADSU group was significantly shorter in 10th and 30th min after diazepam administration compared to the DIAS group (p<0.05). In other time points, DIADSU and DIAS groups exhibited no statistically significant differences. Unlike with diazepam, seven-day pretreatment with DSU (ALPDSU) significantly prolonged the retention time of animals in 20th and 30th min after alprazolam administration (p<0.05; Table 4).
| Time (min) | Retention time (s) DIAS | Retention time (s) DIADSU | Retention time (s) ALPS | Retention time (s) ALPDSU |
|---|---|---|---|---|
| 10 | 296.8 ± 1.92 | 224.8 ± 49.43* | 29.33 ± 12.01 | 38.83 ± 48.59 |
| 20 | 271.4 ± 60.63 | 281.8 ± 35.74 | 77.67 ± 29.04 | 167.17 ± 52.58a |
| 30 | 293.8 ± 3.03 | 267.8 ± 49.22* | 80.67 ± 28.34 | 215.83 ± 45.12a |
| 50 | 294.6 ± 3.05 | 293.6 ± 2.70 | 103.33 ± 32.5 | 184.83 ± 54.13 |
Retention time (S, mean ± SD) after diazepam (2 mg/kg) and alprazolam (2 mg/kg) administration in animals pretreated with saline solution (10 ml/kg) or DSU (dietary supplement based on soybean extract, 170 mg/kg) for seven days *p<0.05 compared to the DIAS group, ap<0.05 compared to the ALPS group
Table 4: Effect of DSU on diazepam- and alprazolam-induced changes retention time in the rotarod test
It was observed that percentage of motoric coordination depression was significantly higher in 10th and 30th min in DIADSU group, compared to the control group that was treated with saline solution (DIAS group), p<0.05. Depression of motoric activity of mice in ALPS group was significantly lower in 20th and 30th min after alprazolam administration than in ALPDSU group (Table 5). In general, animals treated with alprazolam showed a higher rate of motoric coordination depression in all measured time points compared to the animals treated with diazepam.
| Time (min) | Motor depression (%) DIAS | Motor depression (%) DIADSU | Motor depression (%) ALPS | Motor depression (%) ALP DSU |
|---|---|---|---|---|
| 10 | 1.07 ± 0.64 | 25.07 ± 16.50* | 90.22 ± 10.01* | 87.06 ± 16.20b |
| 20 | 9.53 ± 20.21 | 6.07 ± 11.91 | 74.11 ± 15.24* | 44.28 ± 21.20a,*,b |
| 30 | 2.07 ± 1.01 | 10.73 ± 16.49* | 73.11 ± 20.84* | 28.06 ± 9.62a |
| 50 | 1.80 ± 1.02 | 2.13 ± 0.90 | 65.56 ± 15.56* | 38.39 ± 10.23b |
Depression of motor coordination (%, mean ± SD) induced by diazepam (2 mg/kg) and alprazolam (2 mg/kg) in animals pretreated with saline (10 ml/kg) or DSU (dietary supplement based on soybean extract, 170 mg/kg) for seven days. *P<0.05 compared to the DIAS group, ap<0.05 compared to the ALPS group, bp<0.05 compared to the DIA DSU group
Table 5: Effect of DSU on diazepam- and alprazolam-induced coordination impairement in the rotarod test
Pentobarbital-induced sleeping time in group pretreated with DSU (PENDSU) was significantly shorter compared to the control group (PENS; Table 6), and PENDSU group also exhibited significant prolongation of pentobarbital-induced sleeping time (p<0.05).
| PARAMETER | PENS | PEN DSU |
|---|---|---|
| Induction time (min) | 7.20 ± 3.49 | 4.80 ± 0.79* |
| Sleep duration time (min) | 43.60 ± 18.78 | 96.60 ± 36.38* |
Table 6: Effect of DSU on pentobarbital (40 mg/kg)-induced sleep time
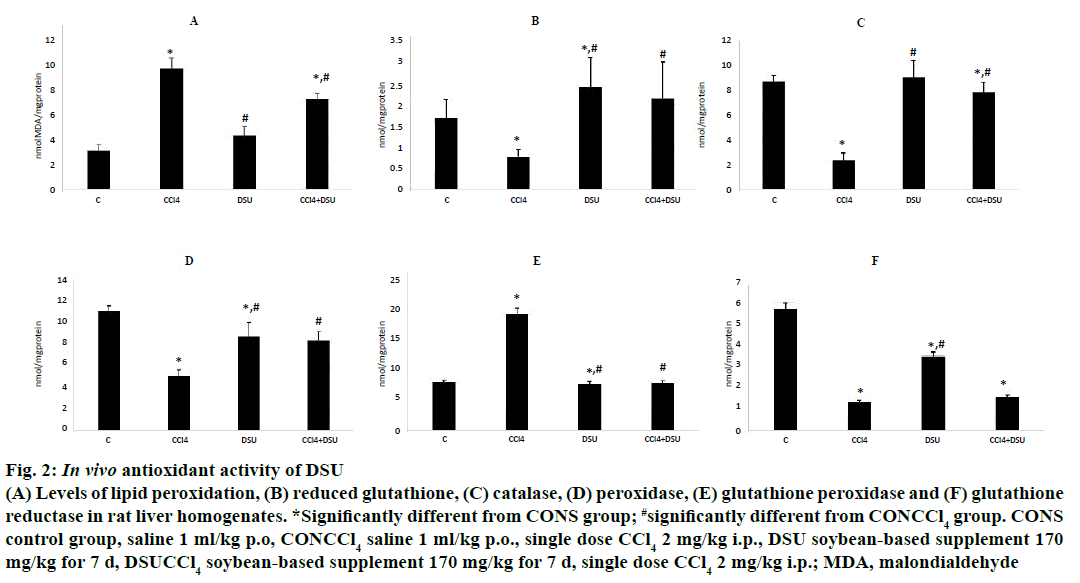
Single dose of CCl4 induced 3.5-fold increase in MDA level when compared to control group. This was accompanied by the significant reduction of GSH concentration (0.7 ± 0.11 nmol vs. 1.68 ± 0.44 nmol GSH/mg of proteins in control group), and reduced activity of all examined antioxidant enzymes, except GPX for which the increased activity in liver homogenates was noted (Figure 2). Administration of DSU did not enforce oxidative damage on liver cells, the activity of tested antioxidant enzymes did not differ significantly compared to control group. Pretreatment with DSU alleviated the toxic effects of CCl4- the levels of measured parameters were neared the values of the control group.
Figure 2: In vivo antioxidant activity of DSU
(A) Levels of lipid peroxidation, (B) reduced glutathione, (C) catalase, (D) peroxidase, (E) glutathione peroxidase and (F) glutathione
reductase in rat liver homogenates. *Significantly different from CONS group; #significantly different from CONCCl4 group. CONS
control group, saline 1 ml/kg p.o, CONCCl4 saline 1 ml/kg p.o., single dose CCl4 2 mg/kg i.p., DSU soybean-based supplement 170
mg/kg for 7 d, DSUCCl4 soybean-based supplement 170 mg/kg for 7 d, single dose CCl4 2 mg/kg i.p.; MDA, malondialdehyde
Administration of DSU alone did not affect the levels of measured biochemical parameters (Table 7). Treatment of animals with CCl4 induced statistically significant increase in both total and direct bilirubin levels in serum. The impaired excretory function caused by CCl4 was exacerbated after the co-treatment with DSU. AST and ALT activities were increased 25- and 20-fold, respectively in CONCCl4 group compared to saline-treated group. The treatment of animals with DSU attenuated these parameters of hepatotoxicity significantly, even though the reduction was not to physiological levels. The values of both total and direct bilirubin in the group of animals treated with supplement alone were not significantly elevated in relation to control. In addition, the concentration of total and direct bilirubin was significantly lower in the DSU-treated group than in the group of animals treated only with CCl4. But, both direct and total bilirubin concentrations were significantly higher in the group treated with the combination of CCl4 and DSU in comparison to CCl4 alone. Serum concentrations of urea, creatinine and uric acid, as biomarkers of renal function, were also examined. Administration of DSU for 7 d did not the affect the hepatic and renal function. These biochemical parameters were increased in CCl4- treated group, indicating its nephrotoxicity, but the co-administration of DSU lowered the levels of these parameters.
| Parameter | CONS | CONCCL4 | DSU | CCL4+DSU |
|---|---|---|---|---|
| Bilirubin total (mM) | 2.16 ± 0.17 | 4.21 ± 0.42* | 2.67 ± 0.12# | 5.24 ± 0.74*# |
| Bilirubin direct (mM) | 0.38 ± 0.09 | 1.38 ± 0.31* | 0.62 ± 0.08# | 2.78 ± 0.34*# |
| AST | 117.40 ± 5.10 | 2827.80 ± 510.50* | 138.70 ± 8.10# | 1512.10 ± 121.30*# |
| ALT | 48.50 ± 1.30 | 983.70 ± 231.50* | 59.60 ± 3.80# | 817.30 ± 173.20* |
| Urea | 7.31 ± 0.31 | 7.04 ± 0.21 | 7.78 ± 0.29 | 6.29 ± 0.27# |
| Creatinine | 46.10 ± 1.72 | 54.10 ± 0.64* | 48.20 ± 1.12 | 47.80 ± 0.86# |
| Uric acid | 58.90 ± 2.31 | 102.40 ± 13.10* | 61.70 ± 3.24# | 77.40 ± 7.31 |
DSU is dietary supplement based on soybean extract. *P<0.05 compared to control, #p<0.05 compared to CONCCL4
Table 7: effect of DSU pretreatment on serum biochemical, liver and renal parameters
In the present study, selected isoflavones were quantified and their effect on liver and renal function, their potential to ameliorate liver injury induced by pro-oxidant agent CCl4 and herb-drug interactions with commercial DSU. Based on the patient information sheet, this supplement is used for relief of menopausal symptoms such as hot flashes, insomnia and nervousness. The beneficial effects of DSUs been attributed to presence of isoflavones, the most examined group of phytoestrogens from a nutritional and health point of view, with daidzein and genistein having the greatest effect on oestrogen receptors [3,34-36]. The analysis of examined DSU also confirmed that daidzein and genistein were present in the highest amount in the supplement.
As mentioned before, there is evidence that isoflavones affected the expression and activity of several drugmetabolizing enzymes [10-12], which implied a risk of clinically significant interactions between DSU and conventional drugs metabolized through these enzymes. Our results showed that DSU administration affected pharmacodynamics of all tested conventional drugs. Pretreatment with DSU had notably potentiated pharmacodynamic effect of diazepam and had shown an increased impairment of motor coordination in the rotarod test. The opposite effect on alprazolam activity was observed. Furthermore, statistically significant decrease of time of induction and prolongation of sleep duration after pretreatment with DSU was detected in the pentobarbital-induced sleeping time test [37]. The obtained results were in accordance with current literature data, confirming that soybean isoflavones have the ability to potentiate the change the activity of microsomal enzymes and in this way activate the drug metabolism, thus changing the pharmacological profile of studied central nervous system depressors [11,17,38]. It is suggested that soybean isoflavones, inhibit microsomal enzymes responsible for degradation of pentobarbital to inactive metabolites, thus potentiating its hypnotic effect while having opposite effect on the isoforms responsible for the benzodiazepine metabolism. DSU treatment influenced pharmacodynamics of codeine in an interesting manner. Following 7 d treatment with commercially available DSU, analgesic effect of codeine in 5th and 10th min of the hot-plate test decreased. Contrary to that, in 20th min and afterwards, the analgesic effect of codeine in the experimental group became stronger compared to control group. Decrease of codeine’s analgesic effect at the beginning of hot plate test can be explained by inhibition of codeine metabolism via CYP2D6 by soybean extract, and subsequent decrease in the production of its active metabolites [39,40], while the following potentiation of analgesia can be the consequence of elevated glucuronyltransferase activity. Glucuronidation of codeine produces morphine-6-glucuronide, which analgesic effect is even stronger than morphine [40].
The results of liver and renal function tests, demonstrated that 7 d pretreatment with DSU alone did not negatively affect the liver and renal excretory function. The values of biochemical parameters measured in serum did not differ between the control and DSU-treated group and supplementation exhibited no negative effects on excretory capacity and liver function of treated animals. Pretreatment with DSU also exhibited protective effects on the extent of parenhimatose organ damage induced by CCL4. Supplementation had positive effect on serum transferase levels, implicating better preservation of liver cellular integrity that can be explained by the marked in vivo antioxidant activity of tested supplement. As noticeable in Figure 2, DSU showed ability to prevent CCl4-induced increase in MDA level, one of the well-known secondary products of lipid peroxidation, an indicator of cell membrane injury. The accumulation of ROS after CCL4 challenges the endogenous antioxidant capacities, leading to depletion of PX, GSH, CAT and GR levels. The administration of DSU restored the levels of these enzymes to those of control group. The hepatoprotective potential of soy-derived products has been previously documented. Kim et al. examined the effects of fermented soybean extract on tert-butyl hydroperoxide-induced oxidative stress in HepG2 cells and rat liver. The fermented soy extract demonstrated antioxidant activity and the ability to decrease the serum levels of clinical markers for liver function [41]. Tofu made from fermented soy also exhibited protective effects in CCl4-induced liver injury [42], and soyasaponins-rich extract from soybean prevented the increases in serum transaminases and markers of oxidative stress in alcohol-induced liver damage [43]. Besides the ability to restore the antioxidant capacity in liver tissue homogenates, the levels of AST and ALT were significantly lower in animals treated with DSU compared to CONCCl4, even though the DSU did not restore these parameters to physiological levels. Nephroprotective effects of DSU were also noted, as there was statistically significant decrease in urea and creatinine levels compared to CONCCl4 group.
Despite all these effects, supplementation failed to prevent CCl4-induced increase in bilirubin levels. High levels of both direct and total bilirubin with concomitant physiological levels of aminotransferases, indicative of cholestatic liver injury, were measured and especially pronounced in the CCl4DSU group. Cholestasis occurring after CCl4 administration appeared to be mediated through changes in the activity of several efflux transporters. One of these efflux pumps, a MRP3 transporter, responsible for the occurrence of conjugated hyperbilirubinemia [44], was shown to be major factor in phytoestrogens disposition [45], which might explain the aggravation of already disrupted bilirubin secretion noted in CCl4 and DSU-treated animals.
Despite several beneficial effects noted in animals subjected to oxidative stress, large potential for interactions renders this supplement suitable for further examination with regard to safety and clinically relevant interactions. Administration of DSU concomitantly to several central nervous system acting drugs significantly changed pharmacological profiles of conventional drugs, depending on their metabolic pathways and biotransformation.
Commercially available DSU significantly changed pharmacological profile of diazepam, alprazolam, pentobarbital and codeine. This suggested that isoflavones modify the activity of microsomal enzymes, there by affecting the metabolic processes and subsequent pharmacological effects of the conventional drugs studied. Hence, the risk of possible side effects and interactions between these conventional drugs and dietary supplements based on soybean extract cannot be excluded. Our study confirmed that there is a need for further research in this field to obtain new preclinical, safety and toxicity data, with a special focus on interactions with conventional drugs. This would ensure safe use of DSUs by the patients, especially taking into consideration its therapeutic benefit and increased use by the patients.
Acknowledgments
This work was supported by the HORIZON 2020 project MEDLEM id 690876, H2020-MSCARISE- 2015 and by the Ministry of Science and Technological Development, Republic of Serbia, project No. 41012.
Conflict of interest
The authors declare that they have no competing interests.
References
- Cvejić J, Bursać M, Atanacković M. Phytoestrogens: "Estrogen-like" phytochemicals. In: Rahmann A, editor. Studies in Natural Products Chemistry, Bioactive Natural Products. 1st ed. Oxford, UK: Elsevier; 2012. p. 1-35.
- Andlauer W, Martena MJ, Furst P. Determination of selected phytochemicals by reversed-phase high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. J Chromatogr A 1999;849:341-8.
- Romani A, Vignolini P, Galardi C, Aroldi C, Vazzana C, Heimler D. Polyphenolic content in different plant parts of soy cultivars grown under natural conditions. J Agric Food Chem 2003;51(18):5301-6.
- Cvejić J. Disease preventing effect of soy and phytoestrogens. Pharmaca Serbica 2009;1(3-4):17-22.
- Tham DM, Gardner CD, Haskell WL. Clinical review 97: Potential health benefits of dietary phytoestrogens: a review of clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab 1998;83:2223-35.
- He FJ, Chen JQ. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. FSHW 2013;3:146-61.
- Burke GL, Vitolins MZ, Bland D. Soybean isoflavones as an alternative to traditional hormone replacement therapy: are we there yet? J Nutr 2003;130:664S-5S.
- Liu MM, Huang Y, Wang J. Developing phytoestrogens for breast cancer prevention. Anticancer Agents Med Chem 2012;12:1306-13.
- Posadzki P, Watson L, Ernst E. Herb-drug interactions: an overview of systematic reviews. Br J Clin Pharmacol 2013;75:603-18.
- Roberts-Kirchhoff ES, Crowley JR, Hollenberg PF, Kim H. Metabolism of genistein by rat and human cytochrome P450s. Chem Res Toxicol 1999;12:610-6.
- Xiao CQ, Chen R, Lin J, Wang G, Chen Y, Tan ZR, et al. Effect of genistein on the activities of cytochrome P450 3A and P-glycoprotein in Chinese healthy participants. Xenobiotica 2012;42:173-8.
- Mrozikiewicz PM, Bogacz A, Czerny B, Karasiewicz M, Kujawski R, Mikolajczak PL, et al. The influence of a standardized soybean extract (Glycine max) on the expression level of cytochrome P450 genes in vivo. Ginekol Pol 2010;81:516-20.
- Ronis MJ, Chen Y, Jo CH, Simpson P, Badger TM. Diets containing soy protein isolate increase hepatic CYP3A expression and inducibility in weanling male rats exposed during early development. J Nutr 2004;134:3270-6.
- Ronis MJ, Rowlands JC, Hakkak R, Badger TM. Altered expression and glucocorticoid-inducibility of hepatic CYP3A and CYP2B enzymes in male rats fed diets containing soy protein isolate. J Nutr 1999;129:1958-65.
- Mezei O, Chou CN, Kennedy KJ, Tovar-Palacio C, Shay NF. Hepatic cytochrome p450–2A and phosphoribosylpyrophosphate synthetase-associated protein mRNA are induced in gerbils after consumption of isoflavone-containing protein. J Nutr 2002;132:2538-44.
- Backlund M, Johansson I, Mkrtchian S, Ingelman-Sundberg M. Signal transduction-mediated activation of the aryl hydrocarbon receptor in rat hepatoma H4IIE cells. J Biol Chem 1997;272:31755-63.
- Li Y, Ross-Viola JS, Shay NF, Moore DD, Ricketts ML. Human CYP3A4 and Murine Cyp3A11 Are Regulated by Equol and Genistein via the Pregnane X Receptor in a Species-Specific Manner. J Nutr 2009;139:898-904.
- Schentag JJ. Assessment of Pharmacokinetic Drug Interactions in Clinical Drug Development. In: Yacobi A, Skelly JP, Shah VP, Benet LZ, editors. Integration of Pharmacokinetics, Pharmacodynamics, and Toxocokinetics in Rational Drug Development. 1st ed. New York: Springer US; 1993. p. 153.
- Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand 2008;118:69-86.
- Vogel GH, Vogel WH. Psychotropic and neurotropic activity. In: Vogel GH, Vogel WH, editors. Drug Discovery and Evaluation: Pharmacological Assays. 2nd ed. Germany: Springer-Verlag: Berlin/Heidelberg; 2002. p. 398-496.
- Anderson GD, Rosito G, Mohustsy MA, Elmer GW. Drug interaction potential of soy extract and Panax ginseng. J Clin Pharmacol 2003;43(6):643-8.
- David S, Hamilton JP. Drug-induced liver injury. US Gastroenterol Hepatol Rev 2010;6:73.
- Cesaratto L, Vascotto C, Calligaris S, Tell G. The importance of redox state in liver damage. Ann Hepatol 2004;3(3):86-92.
- Lee J, Renita M, Fioritto RJ, St Martin SK, Schwartz SJ, Vodovotz Y. Isoflavone characterization and antioxidant activity of Ohio soybeans. J Agric Food Chem 2004;52:2647-51.
- Andlauer W, Martena MJ, Furst P. Determination of selected phytochemicals by reversed-phase high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. J Chromatogr A 1999;849:341-8.
- Food and drug administration, FDA. Guidance for Industry, Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Available from: https://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf%23search=%27guidekines+for+industry+sfe+starting%27.
- Raskovic A, Horvat O, Jakovljevic V, Sabo J, Vasic R. Interaction of alcoholic extracts of hops with pentobarbital and diazepam in mice. Eur J Drug Metabol Pharmacokinet 2007;32:45-9.
- Fleischer S, Parker L. Methods in Enzymology. New York: Academic Press; 1978.
- Bergmayer HU. Methoden der enzymatischen Analyse. Weinhem: Verlag Chemie GmbH; 1970. p. 483-4.
- Beers RFJ, Sizer IW. Spectrophotometric method for measuring of breakdown of hydrogen peroxide by catalase. J Biol Chem 1950;195:133-40.
- Simon LM, Fatrai Z, Jonas DE, Matkovics B. Study of metabolism enzymes during the development of Phaseolus vulgaris. Plant Physiol Biochem 1974;166:389-93.
- Beutler E. Red Cell Metabolism: A manual of biochemical methods. New York: Grune and Stratton; 1984.
- Goldberg DM, Spooner RJ. Glutathione reductase. In: Bergmeyer HU, Bergmeyer J, GraRI M, editors. Methods of enzymatic analysis. 3rd ed. Weinhem: Verlag Chemie GmbH; 1983. p. 258-65.
- Dean M, Murphy BT, Burdette JE. Phytosteroids beyond estrogens: Regulators of reproductive and endocrine function in natural products. Mol Cell Endocrinol 2016;442:98-105.
- Miquel J, Ramírez-Boscá A, Ramírez-Bosca JV, Alperi JD. Menopause: a review on the role of oxygen stress and favorable effects of dietary antioxidants. Arch Gerontol Geriatr 2006;42:289-306.
- Krebs EE, Ensrud KE, MacDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol 2004;104:824-36.
- Tsuji R, Isobe N, Kurita Y, Hanai K, Yabusaki Y, Kawasaki H. Species difference in the inhibition of pentobarbital metabolism by empenthrin. Environ Toxicol Pharmacol 1996;2:331-7.
- Ronis MJ, Chen Y, Badeaux J, Laurenzana E, Badger TM. Soy protein isolate induces CYP3A1 and CYP3A2 in prepubertal rats. Exp Biol Med 2006:231:60-9.
- Vree TB, Verwey-van Wissen CP. Pharmacokinetics and metabolism of codeine in humans. Biopharm Drug Dispos 1992;13:445-60.
- Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro 2006;20:187-210.
- Kim EY, Park Y, Suh HJ. Protective Effect of Germinated and Fermented Soybean Extract Against tert-butyl Hydroperoxide-Induced Hepatotoxicity in the Rats. FASEB J 2015;1:LB343.
- Lien DTP, Hoang CTK, Hanh NT, Chu DX, Tram PTB, Toan HT. Hepatoprotective effect of tofu processed from germinated soybean on carbon tetrachloride induced chronic liver injury in mice. J Food Health Sci 2017;3:1-11.
- Yang X, Dong C, Ren G. Effect of soyasaponins-rich extract from soybean on acute alcohol-induced hepatotoxicity in mice. J Agric Food Chem 2011;59:1138-44.
- Keppler D. The roles of MRP2, MRP3, OATP1B1, and OATP1B3 in conjugated hyperbilirubinemia. Drug Metab Dispos. 2014;42:561-5.
- van de Wetering K, Feddema W, Helms JB, Brouwers JF, Borst P. Targeted metabolomics identifies glucuronides of dietary phytoestrogens as a major class of MRP3 substrates in vivo. Gastroenterology 2009;137:1725-35.