- Corresponding Author:
- A. Srinatha
Department of Pharmaceutics, National College of Pharmacy, Shimoga-577 201, India
E-mail: asrinatha@gmail.com
| Date of Submission | 26 February 2014 |
| Date of Revision | 31 August 2014 |
| Date of Acceptance | 2 September 2014 |
| Indian J Pharm Sci 2014;76(5):437-444 |
Abstract
Mucoadhesive microbeads of low methoxyl pectin were prepared, either alone or in combinations with hydroxypropyl methyl cellulose, sodium carboxymethyl cellulose, methyl cellulose and carbopol 934P, by ionotropic gelation. The influence of copolymers on mucoadhesivity, microbeads characteristics and in vitro drug release was investigated. Spherical microbeads with 78.69±0.59 to 85.84±0.78% drug entrapment and of a size of 791.90±4.58 to 960.88±4.61 μm were prepared. The concentration of cross linking agent affects the encapsulation efficiency of microbeads. Mucoadhesiveness of microbeads was dependent on the concentration of copolymers. The formulations exhibiteda pH-dependent release and followed diffusion-controlled first-order kinetics.
Keywords
Pectin, hydroxypropyl methylcellulose, ionotropic gelation, mucoadhesion, microbeads
Microbeads amongst other approaches have garnered much interest in researchers around the world [1,2]. However, they have been less successful owing to their short residence time in gastrointestinal (GI) tract on oral administration. One approach to enhance the GI residence time is to increase the intimate contact of the microbeads with biological barrier membrane. Mucoadhesive systems are developed to increase the GI residence time of dosage form to enhance the bioavailability of drugs [3–5] and provide precise control over drug release rate [6].
Pectin, a natural ionic polysaccharide, consists of linearly connected α-(1-4)-D-galacturonic acid residues. It is classified based on degree of esterification (DE) which is expressed as a percentage of esterified or amidated carboxyl groups [7,8]. Low methoxyl pectin (<50% DE, LMP) cross-links with multivalent ions like Ca2+ and form rigid gel.Microbeads are produced by intermolecular cross-linking between divalent metal ions and the negatively charged carboxyl group of LMP. On cross-linking with divalent ions, pectin produces an insoluble and strong gel bead [9]. The cross-linking reaction proceeds with formation of an ‘egg-box model’ by interaction between pairs of carboxylic groups on adjacent helices [10]. LMP is hydrophilic polymer containing a large number of carboxyl groups which form hydrogen-bond with functional groups in mucus.
Multiparticulate systems of amidated pectin with indomethacin and sulphamethoxazole exhibited a pH and solubility release profiles [11]. The drug release profile was modulated by complexation with chitosan [12]. Similarly, the effect of HPMC on the properties of pectin tablet formulations are reported previously [13,14]. Madziva et al. [15] reported that by varying the concentration of pectin and alginate in the microbeads it is possible to achieve different release patterns.
Glimepiride is a second generation sulfonylurea, mainly used in management of type-2 diabetes mellitus [16]. It has been suggested previously by Ilic et al. [17] that by use of hydrophilic polymers as matrix formers dissolution of poorly soluble drugs can be enhanced. If the drug solubility is low, it is advised to use a carrier material which is highly water soluble or highly permeable to water [18].
The present work was designed with an aim of preparing mucoadhesive microbeads of glimepiride using low methoxyl pectin as the primary polymer. The effect of hydroxypropyl methylcellulose (HPMC), carboxymethyl cellulose sodium (NaCMC), methylcellulose (MC) and carbopol 934P on the microbeads properties, drug release and mucoadhesion property was also studied.
Materials and Methods
Glimepiride was obtained ex gratis from Apex Formulations Pvt. Ltd, Ahmedabad, India. Low methoxy pectin (LMP, DE–28 to 32%, methoxy content–5 to 6% and degree of amidation −0%) derived from citrus fruits was obtained from Krishna Pectin Pvt. Ltd, Jalegoan, India. Hydroxypropyl methylcellulose K15M (HPMC K15M, Mw 120 kDa) was obtained from Colorcon Industries, India. Sodium carboxymethyl cellulose (NaCMC, Mw 90 kDa) and methyl cellulose (MC, Mw ~60 kDa) were gifted by Torrent Research Center, Ahmedabad, India. Carbopol 934P (Mw ~800 kDa) and calcium chloride were procured from S.D. Fine-Chem Ltd, Mumbai, India. All other chemicals used were of analytical grade.
Preparation of microbeads
Microbeads were prepared by ionotropic gelation method as described previously with minor modifications [19–21]. Briefly, a polymeric solution (3% w/v) of LMP, either alone orin combination with HPMC, NaCMC, MC or carbopol 934P, was prepared (Table 1). Glimepiride (25% w/w of polymer weight) was dispersed in the polymeric solution under constant stirring on magnetic stirrer to form a smooth viscous dispersion keeping a drug to polymer ratio of 1:4. This dispersion was added dropwise using a syringe fitted with needle (24G) into 5% w/v calcium chloride solution maintained under gentle agitation. The added droplets were retained in calcium chloride solution for 15 min to complete the reaction to produce spherical rigid microbeads. The microbeads were collected by filtration and washed twice with distilled water (25 ml) and dried at 40° for 24 h.
| Polymer combination | Particle size (µm)a | Drug content (mg)a | Entrapment efficiency (%)a | |
|---|---|---|---|---|
| N | LMP (10:0) | 791.90±4.58 | 16.78±0.12 | 83.83±0.59 |
| N1 | LMP: HPMC (9:1) | 922.58±5.63 | 16.59±0.17 | 82.97±0.85 |
| N2 | LMP: HPMC (8:2) | 942.62±4.52 | 15.92±0.11 | 79.59±0.57 |
| N3 | LMP: HPMC (7:3) | 960.88±4.61 | 15.74±0.12 | 78.69±0.59 |
| N4 | LMP: NaCMC (9:1) | 834.97±5.88 | 16.60±0.47 | 83.01±2.35 |
| N5 | LMP: NaCMC (8:2) | 866.31±6.10 | 16.42±0.29 | 82.11±1.43 |
| N6 | LMP: NaCMC (7:3) | 917.64±5.31 | 16.03±0.17 | 80.14±0.83 |
| N7 | LMP: MC (9:1) | 889.89±3.13 | 16.63±0.17 | 83.14±0.87 |
| N8 | LMP: MC (8:2) | 918.34±5.61 | 16.30±0.15 | 81.49±0.76 |
| N9 | LMP: MC (7:3) | 939.01±4.50 | 16.25±0.07 | 81.25±0.36 |
| N10 | LMP: Carbopol (9:1) | 796.78±4.62 | 16.73±0.12 | 83.66±0.61 |
| N11 | LMP: Carbopol (8:2) | 812.83±3.75 | 16.92±0.37 | 84.59±1.86 |
| N12 | LMP: Carbopol (7:3) | 828.59±4.80 | 17.17±0.16 | 85.84±0.78 |
aValues are mean±SD, n=3
Table 1: Formulation composition and Physico-chemical properties of prepared Microbeads
Microbeads size determination
The size of prepared microbeads was determined with an optical microscope fitted with a calibrated eye piece micrometer. The mean of 100 microbeads was noted as microbeads size. All the studies were carried out in triplicate and the mean (±SD) was noted.
Scanning electron microscopy (SEM)
Morphological examination of the surface and internal structure of microbeads was performed using a scanning electron microscope (JSM-840A, Jeol, Japan). The sample was sputter coated conductive metal and the observations were made under vacuum.
Entrapment efficiency
Microbeads (100 mg) were crushed in a glass mortar and suspended in minimal amount of distilled water for dissolving the coat shell of microbeads. The suspension was suitably diluted with methanol in a graduated flask and filtered to separate the shell fragments. Drug content was analyzed after suitable dilution using UV-spectrophotometer at 228 nm (UV-1601, Shimadzu, Japan) against a suitable blank. From the absorbance values, amount of drug in the aliquot was calculated from a calibration curve plotted from pure glimepiride with a concentration range of 2-16 μg/ml. All the studies were conducted in triplicate and the average values (±SD) are reported. The percent drug entrapment efficiency (EE) of each formulation was calculated using the Eqn., % EE=(practical drug content/theoretical drug content)×100
Swelling studies
Swelling ratio of the microbeads was determined in simulated gastric fluid (SGF; 0.1 N HCl- pH 1.2) and simulated intestinal fluid (SIF; phosphate buffer- pH 7.4). 100 mg of microbeads were taken in a wire mesh basket and immersed in the respective test medium. At different time intervals the weight of the swollen microbeads was recorded after wiping the excess of liquid with a tissue paper. The swelling ratio was calculated using the following Eqn., swelling ratio = (Wt-Wo)/Wo, where, Wo is the initial weight of microbeads and Wt is the weight of the microbeads at time ‘t’.
In vitro drug release
In vitro drug release studies were performed using USP 23 dissolution test apparatus I at 50 rpm. The dissolution studies were performed in 900 ml of either SGF or SIF containing sodium lauryl suplhate (0.5% w/v) maintained at 37±0.5º. Further, at predetermined time intervals an aliquot of 5 ml was withdrawn and replenished with fresh medium. Amount of drug in each aliquot was assayed on a UV-spectrophotometer (UV-1601, Shimadzu, Japan) at 228 nm using a suitable blank. All trials were conducted in triplicate and the average (±SD) reading was noted.
Release kinetics and mechanism
In vitro drug release data was fitted to Zero order, First order equations and Higuchi square root model [22]. Drug release mechanism was further analyzed using the Korsmeyer-Peppas empirical power law Eqn. [23], Mt/M∞=Ktn, where, Mt/M∞ is the fraction of drug released at time‘t’, K is the structural and geometrical constant and ‘n’ is the release exponent.
Ex vivo mucoadhesion test
Freshly excised piece of sheep intestinal mucosa was collected from slaughter house. The mucosa was cut to a dimension of 2×2 cm2 and mounted on glass slides (3×1 inches) with cyanoacrylate glue. Approximately 50 microbeads were spread onto each wet rinsed tissue specimen, and immediately the slide was hung onto the arm of USP tablet disintegrating test apparatus. The tissue specimen was given a slow, regular up and down movement in the beaker containing SGF or SIF maintained at 37±0.5º. At predetermined time intervals the machine was stopped and the number of microbeads still adhering to the tissue was counted [19,21].
Results and Discussion
Preliminary batches of low methoxyl pectinmicrobeads were prepared using 1-5% w/v LMP. Microbeads prepared with low concentrations of LMP gave burst release of drug (data not shown) and complete drug was released within 2 h. Glimepiride loaded LMP microbeads prepared using 3% w/v LMP were found to be satisfactory and this was selected as the maximum pectin concentration, above which the solution was highly viscous and was difficult to extrude through a needle of 24 G size. The external cross-linking was a result of pectin reacting with calcium ions present in the coagulation fluid resulting in the formation of calcium pectinate beads.
Microbeads were found to be large, discrete and free flowing. The mean size of microbeads without any added co-polymer was 791.90±4.58 μm (Table 1). With addition of the co-polymers, the mean microbeads size increased upto 960.88±4.61 μm. Further, the mean size of the microbeads increased with increase in the concentration of the co-polymers in the microbeads. Among the co-polymers studied, HPMC had significantly increased the microbeads size in comparison to NaCMC, MC and carbopol. The increase in mean sizes of microbeads with addition of co-polymers could be attributed to increase in viscosity of the polymeric dispersions which eventually leads to formation of larger size of microbeads. In comparison to other co-polymers, no significant increment in the particle size was noticed when carbopol was used as co-polymer.
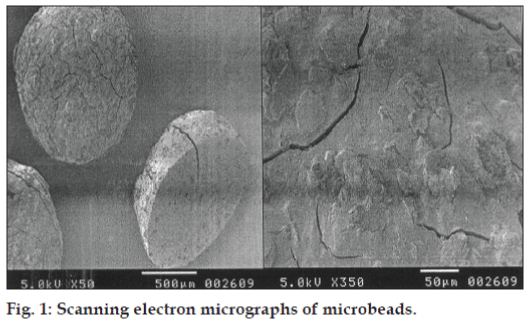
The SEM photograph (fig. 1) of microbeads with LMP indicated that the microbeads were almost spherical with rough surface. The formation of the rough surface was possibly due to higher concentration of drug uniformly dispersed at molecular level in the calciumpectinate matrices. Surface morphology also revealed deposits of crystalline matter possibly of drug on the microbeads surface.
Drug content of the formulations was found to be in the range of 15.74±0.12 to 17.17±0.16 mg (Table 1). The drug content was independent of the polymer matrix as no significant difference in the drug content of the prepared formulations was observed. Maximum drug content was observed with formulation N12 containing pectin-carbopol at 7:3 ratio.
Entrapment efficiency of the preliminary batchesof microbeads prepared using 2.5, 5, 7.5 and 10% calcium chlorideas cross-linking agent was 71.55±0.98%, 83.83±0.59%, 77.90±0.37% and 73.83±0.57%, respectively. The decrease in EE was observed with increasing the calcium chloride concentration above 5% (i.e. 7.5 and 10%). This phenomenon can be best attributed to the weakening of surface gel strength due to an excess of calcium ions. It has been shown previously by Pawar et al. [10] that when a graph of calcium concentration against gel strength is plotted, it reaches a point further which increase in calcium concentration will not cause any increment in gel strength indicating a point of calcium saturation. On increasing calcium concentration beyond this point, the gel strength decreases. The possible reason could be the formation of non-homogenous gel structure caused by pregelation as a result of a rapid interaction of pectin with calcium ions [10]. Considering these facts, further batches of microbeads were prepared using 5% w/v calcium chloride solution as the optimum crosslinking concentration. The prepared microbeads formulations exhibited entrapment efficiency in the range of 78.69±0.59 to 85.84±0.78% (Table 1). The entrapment efficiency of pectin-HPMC, pectin-NaCMC and pectin-MC microbeads was marginally lower as compared to formulation prepared using low methoxyl pectin alone. This was possibly due to non-availability of sufficient polymer for crosslinking and to encapsulate the drug with increasing concentration of co-polymers [10]. In contrast, the entrapment efficiency was found to increase marginally with pectin-carbopol 934P microbeads which may be due to the formation of dense polymer network structure.
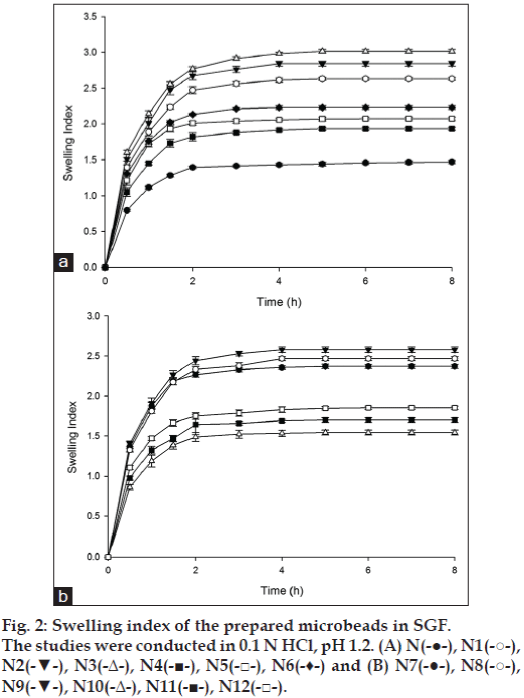
Swelling studies were performed in SGF and SIF to study the influence of pH of dissolution medium on swelling character of the microbeads. Swelling of the microbeads of LMP, either alone or in combinations with co-polymers, was less in SGF (fig. 2). The swelling ratio of formulation prepared using low methoxyl pectin was found to be 1.47±0.02 in 8 h. In solutions of low pH (pH 1.2) ionization of carboxylate groups on LMP might be repressed and there is less water incorporation into interchain entanglements due to closer network structure of hydrogel which leads to microbeads with less swellable properties [8]. The swelling index of the microbeads prepared with co-polymers was higher in comparison to LMP microbeads (fig. 2). The microbeads showed maximum swelling within 3-4 h and then a gradual but slow change in swelling pattern was observed. This initial high rate of swelling may be attributed to the hydrophilic nature of the co-polymers used which hydrate rapidly on contact with the dissolution fluid.
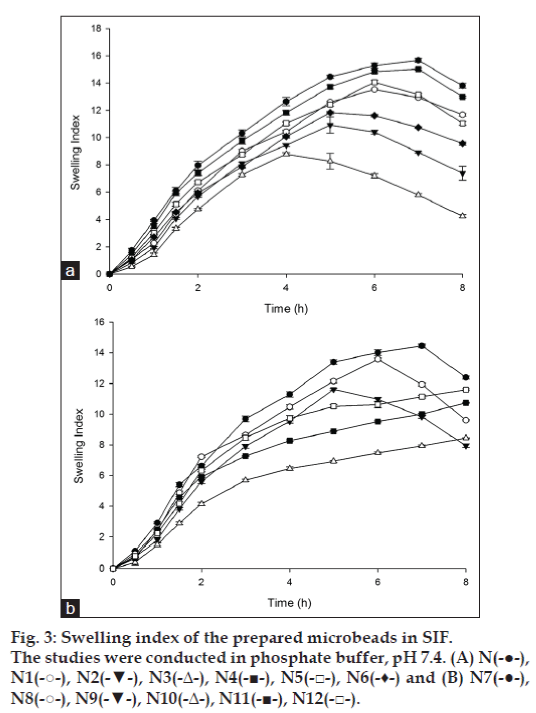
The swelling index of the microbeads was higher in SIF (pH 7. 4) in comparison to SGF (pH 1.2). In SGF, swelling ratio reached a steady plateau after an initial swelling phase as shown in fig. 2. However, in SIF swelling ratio reached a maximum value and decreased as the time progressed (fig. 3). The observed pattern of swelling in SIF can be attributed to the slow and gradual erosion of the hydrogel network which was probably predominant during the latter phase of swelling index studies. Microbeads prepared using LMP alone showed highest swelling (i.e. 15.66±0.14) in 7 h in comparison to the microbeads prepared with copolymers. Ester, hydroxyl and carboxylate groups present in low methoxyl pectin on hydration form hydrogen bonds with water molecules forming a more swellable network structure [24]. The highest swelling of pectin microbeads in SIF may be attributed to ionization of carboxylate groups on pectin favored by basic pH of dissolution fluid. With the addition of copolymers such as HPMC, NaCMC and MC to the microbeads matrix the swelling index decreased in comparison to the swelling index of pectin microbeads. Swelling index of all the prepared microbeads with pectin alone or in combinations with HPMC, NaCMC and MC increased steadily to a saturation point after which the swelling index declined (fig. 3). This phenomenon is possibly due to the erosion of the polymeric matrix being the predominant factor after equilibrium swelling. As the microbeads swell, the matrix experiences intra-matrix swelling force which promotes erosion and leaching of the drug leaving a highly porous matrix. Water influx weakens the network integrity of the polymer, thus influencing structural resistance of the swollen microbeads, which in turn results in pronounced erosion of the loose gel layer [25]. The water soluble hydrophilic polymers like NaCMC and MC hydrates rapidly, creating pores. These pores are filled by the solvent which diffuses into the microbeads and there by accelerate the erosion of the gel layer [25]. However, the microbeads with carbopol as co-polymer in the matrix was an exception as even at the end of 8 h study, swelling index continued to increase.
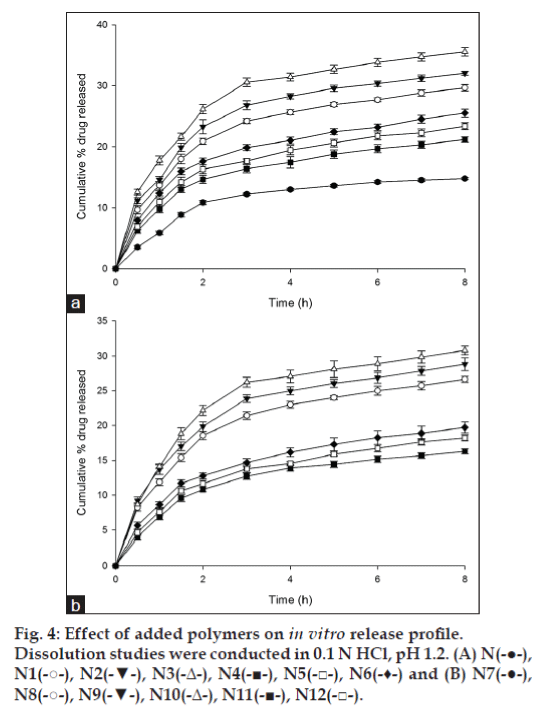
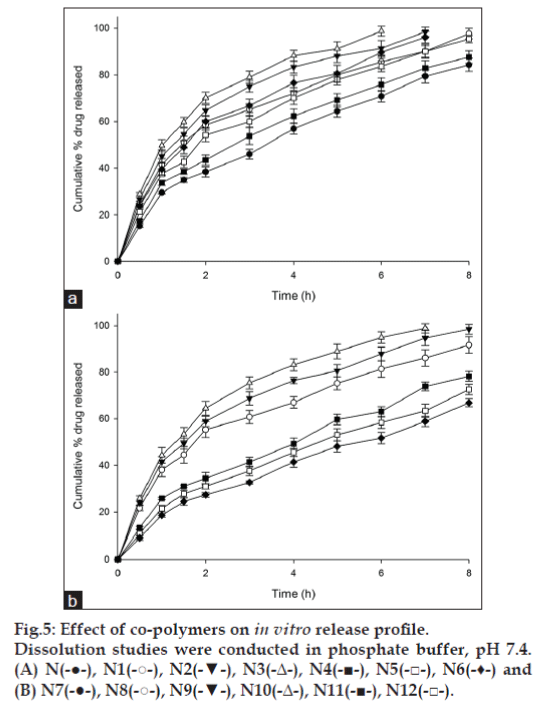
The release of glimepiride from microbeads was studied in dissolution media mimicking gastric fluid (SGF; 0.1N HCl -pH 1.2) and intestinal fluid (SIF: phosphate buffer - pH 7.4). Drug release from the pectin microbeads was less in SGF with 14.78±0.19% drug released in 8 h (fig. 4). The drug release increased with addition of HPMC, NaCMC, MC and carbopol 934P to microbeads matrix (fig. 4). In SIF glimepiride release was faster and higher as compared to drug dissolution in SGF. At intestinal pH, the ionization of the carboxyl groups on LMP was favored leading to the increased swelling of microbeads and decreased mechanical rigidity of the calcium pectinate matrix due to erosion, which accelerates the drug release [24]. Among the prepared microbeads, formulation with low methoxyl pectin showed a drug release of 84.31±2.82% in 8 h (fig.5). Addition of HPMC, NaCMC and MC increased drug release from the microbeads as compared to microbeads with pectin matrix. However, in case of microbeads with carbopol, drug release was lesser than drug released by pectin microbeads. In case of formulations N1, N2 and N3 prepared using HPMC, glimepiride release from the microbeads was found to be 97.70±2.27% in 8 h, 98.48±2.03% in 7 h and 98.77±2.13% in 6 h, respectively. HPMC as a copolymer, exhibited poor release retardant effect due to the presence of extensive amount of hydroxyl groups, which leads to highly swollen matrix causing loose gel layer formation, followed by erosion. It has been reported previously that incorporation of pectin in HPMC matrix lead to faster matrix erosion causing increased drug release. This was reportedly due to weakening of HPMC hydrogel matrix [26]. In our study, calcium pectinate was the predominant release retardant and HPMC probably interfered with the formation of stronger calcium pectinate matrix. In case of formulations N4, N5 and N6 prepared using NaCMC, release of glimepiride from the microbeads was 87.64±2.67% in 8 h, 95.42±1.57% in 8 h and 96.14±2.51% in 7 h, respectively. During dissolution, formulations prepared using NaCMC as a copolymer swelled forming a gel layer on the exposed surface of microbeads. The loosely bound polymer molecules in these microbeads were readily eroded, allowing the easy and faster release of glimepiride [25]. Formulations prepared using MC showed very low control over the release of glimepiride from the microbeads. The reason may be attributed to the highly hydrophilic nature of the MC which induces channeling effect causing the formation of micro-cavities in the matrix, which lead to the leaching of the drug through porous matrix resulting in increased drug release. Formulations prepared using carbopol 934P (N10, N11 and N12), resulted in better control over the release of glimepiride with 78.23±2.13%, 72.53±2.29% and 66.86±1.70% drug dissolution in 8 h, respectively (fig.5). Although no definite reasons could be attributed to the low drug release from pectincarbopol microbeads, it could possibly due to the lesser ionization of the polymeric matrix in intestinal pH as supported by the low swelling index.
The effect of progressive change in pH on the release pattern of glimepiride from the microbeads was further evaluated on formulations prepared using pectin and pectin-carbopol (7:3). The study was performed in SGF for first 2 h followed by SIF for the rest of dissolution study periods. As expected very low drug release was observed from pectin and pectin-carbopolmicrobeads in SGF with a release of 9.57±0.21% and 11.07±0.42% in first 2 h followed by faster drug release in SIF with 76.57±1.17% and 59.13±1.12% in 8 h (data not shown). The observed pH-responsive release is believed to be due to the carboxyl groups of LMP which were shifted from unionized form in pH 1.2 (SGF) to the ionized form in pH 7.4 (SIF), leading to the faster drug release.
In vitro dissolution data were fit to various models to analyse the kinetics of drug release. The regression co-efficient (r2) values for formulations were higher for first-order equations than for zero-order kinetic equations. The regression co-efficient value of Higuchi’s model was in the range of 0.9286-0.9665 in SGF and 0.9762-0.9965 in SIF. These values indicated linearity towards diffusion mechanism of drug from the microbeads matrix. Further, the data treatment using Korsmeyer-Peppas equation indicated that drug release from the microbeads in SGF was by Fickian diffusion. The release exponent ‘n’ was less than 0.5 for the prepared microbeads in SGF indicating Fickian diffusion as release mechanism, where the polymer dissociation was almost negligible in the dissolution medium. However, the drug release from microbeads with carbopol and NaCMC in SIF followed non- Fickian (Anomalous) mechanism (n>0.5) while other formulations followed Fickian mechanism. Anomalous mechanism of drug release from microbeads indicated a combination of drug diffusion as well as polymeric chain relaxation. This was expected as pectin has a tendency of swelling and erosion in aqueous solution with higher pH values.
Ex vivo mucoadhesion test of microbeads were studied to evaluate the mucoadhesive performance of the prepared microbeads. The wash off test results showed that time required for wash off of the prepared microbeads at SGF conditions was less in comparison to SIF conditions. Almost complete microbeads wash-off was observed within 5 h during the study under simulated gastric conditions compared to 8 h under simulated intestinal conditions. In case of microbeads prepared using pectin alone, the washoff time was ~2 h, indicating low mucoadhesiveness of low methoxyl pectin in simulated gastric conditions (pH 1.2). Pectin consists of several carboxylic groups which causes mucoadhesion by interacting with the mucin present in mucus layer [27]. The low mucoadhesion of pectin microbeads could be a result of weaker hydrogen bonding formed with muscin layer. The prepared pectin-HPMC, pectin-NaCMC, pectin-MC and pectin-carbopol 934P microbeads showed a slow wash-off from the mucous membrane indicating increased mucoadhesivenss of the prepared microbeads. The observed increase in mucoadhesive properties in microbeads with HPMC, NaCMC and MC was due to the wetting of the copolymers which gets hydrated on contact with the fluid resulting in the hydrogen bond formation with mucin. Carbopol 934P at pH 1.2 contains large number of carboxylic groups which can form hydrogen bonds with the negatively charged mucus layer followed by the formation of physical entanglements [28]. This theory was supported by the fact that microbeads prepared using pectincarbopol 934P in 7:3 showed the slowest wash-off time with ~14% adhering to the mucous membrane even at the end of 5 h.
In comparison to the results of the wash-off test under simulated gastric conditions the ex vivo washoff time under simulated intestinal conditions was more. The mucoadhesivity of the prepared pectin, pectin-HPMC, pectin-NaCMC, pectin-MC and pectincarbopol 934P microbeads increased in comparison to gastric conditions. The pectin molecules interact with mucin by interpenetration of pectin-mucin chains via hydrogen bond formation. Due to ionization of the carboxyl groups of both pectin and mucin under intestinal pH conditions both pectin and mucin show stronger mucoadhesive properties. The mucoadhesive property of the microbeads increased with the addition of the copolymers. Amongst the prepared microbeads, pectin-MC microbeads exhibited least mucoadhesivity which may be due to disruption of structural integrity of the polymeric matrix [28]. The mucoadhesive property of pectin- HPMC wasless than that of formulations prepared using NaCMC and carbopol 934P. This was due to the formation of thick and viscous-swollen gel which is not continuous and forms localized pockets of polymer [28]. Pectin-NaCMC microbeads showed moderate mucoadhesion proceeded by faster hydration of polymeric matrix that promotes interpenetration of the polymer chain with the tissue.The mucoadhesion is primarily due to the carboxylic group of pectin can form hydrogen bonds with tissue. Whereas pectin-carbopol 934P microbeads showed good mucoadhesive properties, which may be due to the ionization of the carboxylate groups of carbopol 934P resulting in a uncoiled structure with more mucin penetrability with lower intramolecular hydrogen bonding than coiled form due to unionized carbopol [28]. The observed results were in line with a previous report wherein carbopol 934P has shown highest mucoadhesivenss followed by NaCMC [29].
In this study mucoadhesive microbeads were studied for physical and in vitro drug release properties. The swelling character, mucoadhesivenss and in vitro drug release was influenced by addition of copolymers and pH of the dissolution fluid. At simulated gastric conditions, swelling, mucoadhesivity and drug release was low while it was higher under simulated intestinal conditions. Based on the results, it can be concluded that the LMP microbeads can be specifically prepared to target the release to select segment in GIT. The drug release rate could easily be modulated by incorporation of co-polymers in the microbeads matrix.
References
- Kondo A. Microparticle processing and technology. New York: Marcel Dekker; 1979.
- Gutcho MH. Microcapsules and microcapsulation technique. Park Ridge, New Jersey: Noyes Data Corporation; 1976.
- Ikeda K, Murata K, Kobayashi M, Noda K. Enhancement of bioavailability of dopamine via nasal route in beagle dogs. Chem Pharm Bull 1992;40:2155-8.
- Tsuneji N, Yuji N, Naoki N, Yoshiki S, Kunio S. Powder dosage form of insulin for nasal administration. J Control Release 1984;1:15-22.
- Ilium L, Farraj N, Critchley H, Davis SS. Nasal administration of gentamicin using a novel microsphere delivery system. Int J Pharm 1988;46:261-5.
- Ibrahim HM, Ahmed TA, Lila AE, Samy AM, Kaseem AA, Nutan MT. Mucoadhesive controlled release microcapsules of indomethacin: Optimization and stability study. J Microencapsul 2010;27:377-86.
- Sriamornsak P, Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery. Int J Pharm 1998;160:207-12.
- Sriamornsak P. Chemistry of pectin and its pharmaceutical uses: A review. SilpakornUnivInt J 2003;3:206-28.
- Liu L, Fishman ML, Kost J, Hicks KB. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003;24:3333-43.
- Pawar AP, Gadhe AR, Venkatachalam P, Sher P, Mahadik KR. Effect of core and surface cross-linking on the entrapment of metronidazole in pectin beads. Acta Pharm 2008;58:78-85.
- Munjeri O, Collett J, Fell J. Hydrogel beads based on amidatedpectins for colon-specific drug delivery: The role of chitosan in modifying drug release. J Control Release 1997;46:273-8.
- Bigucci F, Luppi B, Cerchiara T, Sorrenti M, Bettinetti G, Rodriguez L, et al. Chitosan/pectin polyelectrolyte complexes: Selection of suitablepreparative conditions for colon-specific delivery of vancomycin. Eur J Pharm Sci 2008;35:435-41.
- Turkoglu M, Ugurlu T. In vitro evaluation of pectin-HPMC compression coated 5-aminosalicylic acid tablets for colonic delivery. Eur J Pharm Biopharm 2002;53:65-73.
- Ugurlu T, Turkoglu M, Gurer US, Akarsu BG. Colonic delivery of compression coated nisin tablets using pectin/HPMC polymer mixture. Eur J Pharm Biopharm 2007;67:202-10.
- Madziva H, Kailasapathy K, Phillips M. Alginate-pectin microcapsules as a potential for folic acid delivery in foods. J Microencapsul 2005;22:343-51.
- Sweetman CS. Martindale: The complete drug reference. 36th ed. London: Pharmaceutical Press; 2002.
- Ilić I, Dreu R, Burjak M, Homar M, Kerc J, Srcic S. Microparticle size control and glimepiride microencapsulation using spray congealing technology. Int J Pharm 2009;381:176-83.
- Sekiguchi K, Obi N. Studies on absorption of eutectic mixture. I. A comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem Pharm Bull 1961;9:866-72.
- Chowdary KP, Rao YS. Design and in vitro and in vivo evaluation of mucoadhesive microcapsules of glipizide for oral controlled release: A technical note. AAPS PharmSciTech 2003;4:E39.
- Chowdary KP, Mohapatra P, Murali Krishna M. Evaluation of olibanum resin as microencapsulating agent for controlled drug delivery. Indian J Pharm Sci 2006;68:461-4.
- Singh C, Jain K, Kumar C, Agarwal K. Design and in vitro evaluation of mucoadhesive microcapsules of pioglitazone. J Young Pharm 2009;1:195-8.
- Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 1963;52:1145-9.
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 1983;15:25-35.
- Chung JT, Zhang Z. Mechanical characterization of calcium pectinate hydrogel for controlled drug delivery. Chem Ind 2003;57:611-6.
- Semalty M, Semalty A, Kumar G. Formulation and characterization of mucoadhesivebuccal films of glipizide. Indian J Pharm Sci 2008;70:43-8.
- Wu B, Chen Z, Wei X, Sun N, Lu Y, Wu W. Biphasic release of indomethacin from HPMC/pectin/calcium matrix tablet: I. Characterization and mechanistic study. Eur J Pharm Biopharm 2007;67:707-14.
- Sriamornsak P, Wattanakorn N, Nunthanid J, Puttipipatkhachorn S. Mucoadhesion of pectin as evidence by wettability and chain interpenetration. CarbohydrPolym 2008;74:458-67.
- El-Kamel AH, Sokar MS, Naggar VF, Al-Gamal SS. Bioadhesive controlled release metronidazole vaginal tablets. Acta Pharm 2002;52:171-9.
- Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev Ind Pharm 2004;30:985-93.