- *Corresponding Author:
- I. Ahmad
Department of Pharmaceutical Sciences, Faculty of Pharmacy, Universitas Indonesia, Depok, Indonesia
E-mail: islamudinahmad@farmasi.unmul.ac.id
| Date of Submission | 11 November 2016 |
| Date of Revision | 26 March 2017 |
| Date of Acceptance | 25 September 2017 |
| Indian J Pharm Sci 2017;79(6): 1013-1017 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Extraction of the polyphenolic content from Peperomia pellucida (L) Kunth herb (Piperaceae family) using 1-ethyl-3-methylimidazolium bromide as a solvent was attempted. The herbs were extracted using the ionic liquid-based microwave-assisted extraction method with some combination factors such as extraction time, microwave power, liquid-solid ratio, and ionic liquid concentration. The optimum yields of total polyphenolic content (13.750 µg GAE/g sample) were obtained by using a microwave power of 30 % Watt, extraction time of 10 minutes, the liquid-solid ratio of 14 ml/g, and ionic liquid concentration of 0.7 mol/l. Based on the results, compared to conventional organic solvent, the solvent of 1-ethyl-3-methylimidazolium bromide could provide higher extraction yields of polyphenolic content. Moreover, the extraction of a secondary metabolite from this herb becomes more rapid, easy, and efficient.
Keywords
Peperomia pellucida (L). Kunth, 1-ethyl-3-methylimidazolium bromide, ionic liquid, microwave assisted extraction, total polyphenolic content
The use of ionic liquid solvent was done over the last decade. Various techniques, cost, and their complexity have been developed to extract the active components of the plant [1]. Ideally, the use of extraction method aimed to produce the secondary metabolites optimally, by considering the extraction method, which is a simple, rapid, safety, economical, eco-friendly and can be reproducible [2-4]. The heat reflux extraction (HRE) method is a conventional method and most appropriate to use an ionic liquid solvent compared with other. However, the use of the non-conventional methods has proven to be more effective and efficient to extract the active constituents of the plants [5,6]. An ionic liquid solvent combined with the use of non-conventional extraction methods, such as ionic liquid-based microwave-assisted extraction (IL-MAE), negativepressure cavitation-assisted extraction (IL-NPCE), ultra-high pressure assisted extraction (IL-UPE), supercritical fluid extraction (IL-SFE), and ultrasoundassisted extraction (IL-UAE) [7,8]. Although all of the methods have special requirements, moreover in some cases has been performed a comparative analysis of these methods.
1-Ethyl-3-methylimidazolium bromide (EMIMBr) is one type of ionic liquid solvent. It used as a solvent for extracting compounds from the natural products. Study of ionic liquid as a solvent had reported such as the trans-resveratrol extraction from Polygonum cuspidatum using IL-MAE [9]. Phenolic compound extraction from Arctium lappa L using IL-UAE and IL-MAE methods [10], extraction of rhiosin and rhodionin from Rhodiola rosea using IL-UAE methods [11].
Peperomia pellucida (L.) Kunth is a herb, belongs to a family of Piperaceae. The herbs are traditionally utilized to treat various diseases such as hypertension, diabetes mellitus, gout, headache and pain (abdominal pain) [12,13]. Some studies had reported the plant has pharmacological properties as angiotensin converting enzyme (ACE) inhibitory [14,15], gastroprotective [16], antiinflamatory [17], antisickling [18], sunscreen [19], antimicrobial and antioxidant [20]. Polyphenol compounds have been isolated including; quercetin [15], secolignan [21], pelusidin A [22] and chromene [23]. However, to isolate the leading compounds (dereplication) or new compounds are difficult, it was due to the yield (particularly phenolic content) of this plant is poor. Therefore, the development of extraction methods conducted with the application of the green chemistry principles by using an ionic liquid solvent. Application of the IL-MAE for the secondary metabolite extraction from this plant has reported in the preliminary study previously [24]. The use of EMIMBr as a solvent for the extraction of phenolic constituents has not reported. The use of ionic liquid solvent aims to obtain the extracts with maximum phenolic constituents.
Samples of P. pellucida herb were collected from North Mamuju, West Sulawesi, Indonesia on June 10-20, 2016. The voucher specimens identified at the Herbarium Bogoriense, Bogor, West Java, Indonesia. The sample was washed, dried and powdered using a grinder. The chemical materials were employed, such as EMIMBr as a solvent purchased from Shanghai Chen Jie Chemical, China. Sodium carbonate, Folin- Ciocalteu reagent, and gallic acid standard purchased from Sigma-Aldrich, UK. Aqua DM, methanol, n-hexane and ethyl acetate were purchased from PT. SmartLab Indonesia, Indonesia.
In the conventional extraction method, the dried powder of sample (3 g) was macerated with n-hexane 50 ml, allowed to stand for 24 h, and filtered and then evaporated to obtain a dry extract. Furthermore, the residue was macerated using ethyl acetate in the same procedure. In the non-conventional extraction method [24], the dried powder of sample (3 g) was mixed with an ionic liquid solvent then extracted using an ILMAE (Modena 900 Watt, with slight modification), which operated under some conditions. The residue and extract solution was separated by filtering using a cotton swab and cooled at room temperature. The obtained extract solution was left for 10 to 12 h to precipitate the desired extract.
Determination of the total polyphenolic content using a microplate reader 96 well method [25,26]. A total of 20 μl (1000 ppm) of the extract solution or the standard solution were added to 100 μl reagent 25 % Folin- Ciocalteu solution, homogenized for one minute and then allowed to stand for 4 min. Then a 75 μl sodium carbonate solution was added and homogenized for one minute. Absorbance measured at a 750 nm wavelength using a 96 well microplate reader after incubated for 2 h at room temperature in the dark. Gallic acid solutions (200, 100, 50, 25, and 12.5 μg/l, respectively) were used as standards.
As far as we know, the selection of solvent to obtain extracts with maximum yield (total polyphenolic content) is a crucial step, especially for the optimization of extraction methods that aim to acquire a target compound. The use of EMIMBr as a solvent is expected to attract to the optimum polyphenolic constituents by using the IL-MAE method. Some factor considered in this method, among others, the extraction time, the microwave power, the liquid-solid ratio, and the ionic liquid concentration shown in Table 1. Each extract with the yields of total polyphenolic content compared with a conventional method.
| Factor | Unit | Symbol | Range and level | ||
|---|---|---|---|---|---|
| Low | Medium | High | |||
| Extraction time | Min | A | 10 | 15 | 20 |
| Microwave power | %W | B | 10 | 30 | 50 |
| Ionic liquid concentration | mol/l | C | 0.2 | 0.7 | 1.2 |
| Liquid-solid ratio | ml/g | D | 10 | 12 | 14 |
Table 1: Experimental Factors of an Il-MAE Using Emimbras a Solvent
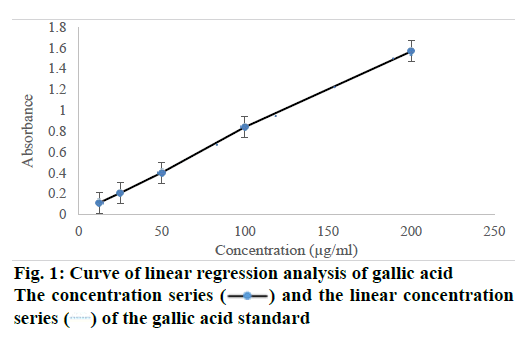
A standard calibration curve was performed using the microplate reader was showen in Table 2. Based on the results of linear regression, analysis has obtained automatically from the microplate reader (VersaMax™ ELISA Microplate Reader). The result was in accordance with the results had been reported in the previous studies [25,27,28], and the Eqn. was Y=0.023+7.812X with a correlation coefficient (R2) of 0.999 (as shown in Figure 1). Where Y is the yields of total phenolic content, and X is the concentration of standard or sample. The equation was applied to determine the total polyphenolic content from herb samples using different types of solvents and extraction methods.
| Concentration (µg/ml) | Absorbance | Approximate absorbance | Standard deviation |
|---|---|---|---|
| 12.5 | 0.112 | 0.116 | 0.004 |
| 0.118 | |||
| 0.119 | |||
| 25 | 0.205 | 0.209 | 0.004 |
| 0.212 | |||
| 0.210 | |||
| 50 | 0.415 | 0.405 | 0.009 |
| 0.405 | |||
| 0.395 | |||
| 100 | 0.874 | 0.843 | 0.027 |
| 0.832 | |||
| 0.823 | |||
| 200 | 1.559 | 1.57 | 0.045 |
| 1.531 | |||
| 1.619 |
Table 2: Results of Absorbance Measurement from Gallic Acid Standard
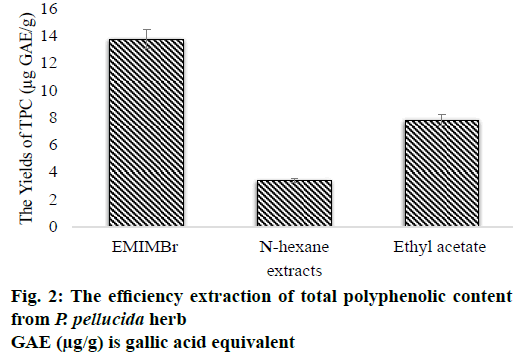
Based on the results of the absorbance measurements of the sample obtained from both the conventional and non-conventional methods, there are differences in the yields of total phenolic content. The optimum yields were obtained using non-conventional extraction methods in the range from 5.529 to 13.750 μg GAE/g sample such as the ionic liquid concentration of 0.7 mol/l; extraction time of 10 min; liquid-solid ratio of 14 ml/g and microwave power of 30 % Watt (Table 3). Whereas the yields were acquired using conventional extraction methods, both the n-hexane and ethyl acetate extracts were 3.408 and 7.823 μg GAE/g sample, respectively. Based on the above results, the use of EMIMBr solvent is higher compared to the organic solvent (Figure 2).
| Run | Factor A | Factor B | Factor C | Factor D | ABS | Yield actual |
|---|---|---|---|---|---|---|
| 1 | 15 | 30 | 0.7 | 12 | 1.048 | 10.931 |
| 2 | 15 | 10 | 0.2 | 12 | 0.663 | 6.824 |
| 3 | 10 | 30 | 0.7 | 14 | 1.312 | 13.750 |
| 4 | 20 | 30 | 0.7 | 14 | 0.922 | 9.590 |
| 5 | 10 | 30 | 0.7 | 10 | 1.094 | 11.428 |
| 6 | 15 | 50 | 0.7 | 14 | 1.061 | 11.076 |
| 7 | 15 | 30 | 0.2 | 10 | 0.541 | 5.529 |
| 8 | 10 | 30 | 0.2 | 12 | 0.672 | 6.923 |
| 9 | 15 | 50 | 0.2 | 12 | 0.857 | 8.897 |
| 10 | 20 | 30 | 1.2 | 12 | 1.060 | 11.062 |
| 11 | 15 | 30 | 0.7 | 14 | 1.029 | 10.728 |
| 12 | 20 | 50 | 0.7 | 12 | 0.983 | 10.244 |
| 13 | 20 | 30 | 0.2 | 12 | 1.280 | 13.412 |
| 14 | 15 | 50 | 0.7 | 10 | 0.972 | 9.593 |
| 15 | 15 | 10 | 1.2 | 12 | 0.955 | 11.684 |
| 16 | 15 | 50 | 1.2 | 12 | 0.922 | 10.536 |
| 17 | 10 | 50 | 0.7 | 12 | 1.118 | 14.131 |
| 18 | 20 | 30 | 0.7 | 10 | 1.017 | 11.755 |
| 19 | 15 | 10 | 0.7 | 10 | 1.348 | 10.276 |
| 20 | 10 | 10 | 0.7 | 14 | 1.125 | 8.651 |
| 21 | 10 | 10 | 0.7 | 12 | 0.986 | 10.604 |
| 22 | 20 | 10 | 0.7 | 12 | 0.883 | 11.531 |
| 23 | 15 | 30 | 1.2 | 10 | 0.834 | 8.989 |
Table 3: Results of Absorbance Measurement and Determination of total Polyphenol content of P. Pellucida extract obtained using IL-MAE
This study is an early stage in the development of extraction methods to obtain the target compound from medicinal plants rapidly, easily and efficiently. Furthermore, from the further study is in progress including the optimization of extraction method, isolation of biomarker compound, and screening of activity.
Acknowledgements
This study supported by grants via “PITTA 2017” from Directorate of Research and Humanity Engagement (DRPM), Universitas Indonesia.
Conflict of interest
The authors declare that they have no conflict of interest.
Financial support and sponsorship
Nil.
References
- Espino M, Fernández MA, Gomez FJV, Silva MF. Natural designer solvents for greening analytical chemistry. Trends Anal Chem 2016;76:126-36.
- Bogdanov MG, Kantlehner W. Simple prediction of some physical properties of ionic liquids: the residual volume approach. Z Naturforsch B 2009;64:215-22.
- Bogdanov MG, Petkova D, Hristeva S, Svinyarov I, Kantlehner W. New guanidinium-based room-temperature ionic liquids. Substituent and anion effect on density and solubility in water. Z Naturforsch B 2010;65:37-48.
- Jessop PG, Jessop D, Fu D, Phan L. Solvatochromic parameters for solvents of interest in green chemistry. Green Chem 2012;14:1245-59.
- Smith RM. Extractions with superheated water. J Chromatogr A 2002;975:31-46.
- Khoddami A, Wilkes M, Roberts T. Techniques for analysis of plant phenolic compounds. Molecules 2013;18:2328-75.
- Chemat F, Vian MA, Cravotto G. Green extraction of natural products: Concept and principles. Int J MolSci 2012;13:8615-27.
- Chemat F, Vian MA. Green chemistry and sustainable technology: Alternative solvents for natural products extraction. New York: Springer US; 2014.
- Du F, Xiao X, Li G. Application of ionic liquids in the microwave-assisted extraction of trans-resveratrol from RhizomaPolygoniCuspidati. J Chromatogr A 2007;1140:56-62.
- Lou Z, Wang H, Zhu S, Chen S, Zhang M, Wang Z. Ionic liquids based simultaneous ultrasonic and microwave assisted extraction of phenolic compounds from burdock leaves. Anal ChimActa 2012;716:28-33.
- Zhu S, Ma C, Fu Q, Hu L, Lou Z, Wang H, et al. Application of ionic liquids in an online ultrasonic assisted extraction and solid-phase trapping of rhodiosin and rhodionin from Rhodiolarosea for UPLC. Chromatographia 2013;76:195-200.
- Hariana A. Medicinal Plants and Their Efficacy. 3rd ed. Jakarta: PenebarSwadaya; 2006 (in Bahasa).
- Heyne K. The Useful Indonesia Plants. 3rd ed. Jakarta: DepartemenKehutanan, YayasanSaranaWana Jaya; 2007.
- Saputri F, Mun’im A, Lukmanto D, Aisyah S, Rinandy J. Inhibition of angiotensin converting enzyme (ACE) activity by some Indonesia edible plants. Int J Pharm Sci Res 2015;6:1054-9.
- Kurniawan A, Saputri F, Ahmad I, Mun’im A. Isolation of angiotensin converting enzyme (ACE) inhibitory activity quercetin from Peperomiapellucida. Int J PharmTech Res 2016;9:115-21.
- Roslida AH, Aini NZ. Evaluation of gastroprotective effect of the ethanolic extract of Peperomiapellucida(L) Kunth. Pharmacologyonline 2009;2:678-86.
- Arrigoni-Blank MF, Dmitrieva EG, Franzotti EM, Antoniolli AR, Andrade MR, Marchioro M. Anti-inflammatory and analgesic activity of Peperomiapellucida (L.) HBK (Piperaceae). J Ethnopharmacol 2004;91:215-8.
- Abere TA, Okpalaonyagu SO. Pharmacognostic evaluation and antisickling activity of the leaves of Peperomiapellucida (L.) HBK (Piperaceae). Afr J Pharm Pharmacol 2015;9:367-74.
- Ahmad I. Penentuan nilai persentase eritema dan pigmentasi ekstrak herba Suruhan (Peperomia pellucida L.) secara in vitro. J Sains dan Kesehatan 2015;1:90-5.
- Oloyede GK, Onocha PA, Olaniran BB. Phytochemical, toxicity, antimicrobial and antioxidant screening of leaf extracts of Peperomiapellucidafrom Nigeria. Adv Environ Biol 2011;5:3700-9.
- Xu S, Li N, Ning MM, Zhou CH, Yang QR, Wang MW. Bioactive compounds from Peperomiapellucida. J Nat Prod 2006;69:247-50.
- Bayma JDC, Arruda MSP, Müller AH, Arruda AC, Canto WC. A dimeric ArC2 compound from Peperomiapellucida. Phytochemistry 2000;55:779-82.
- Susilawati Y, Nugraha R, Muhtadi A, Soetardjo S, Supratman U. (S)-2-Methyl-2-(4-methylpent-3-enyl)-6-(propan-2-ylidene)-3,4,6,7-tetrahydropyrano[4,3-g]chromen-9(2H)-one. Molbank 2015;2015:1-6.
- Ahmad I, Yanuar A, Mulia K, Mun’im A. Application of ionic liquid based microwave-assisted extraction of the secondary Metabolite from Peperomiapellucida(L) Kunth. Pharmacogn J 2017;9:227-34.
- Zhang Q, Zhang J, Shen J, Silva A, Dennis DA, Barrow CJ. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J ApplPhycol 2006;18:445-50.
- Bobo-García G, Davidov-Pardo G, Arroqui C, Vírseda P, Marín-Arroyo M, Navarro M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity of polyphenolic extracts, and comparison with conventional spectrophotometric methods. J Sci Food Agric 2014;95:204-9.
- Medina-Remón A, Barrionuevo-González A, Zamora-Ros R, Andreas-Lacueva C, Estruch R, Martinez-Gonzalez A, et al. Rapid Folin-Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal ChimActa 2009;634:54-60.
- Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin – Ciocalteu reagent. Nat Protoc 2007;2:875-7.
 ) and the linear concentration
) and the linear concentration ) of the gallic acid standard
) of the gallic acid standard