- *Corresponding Author:
- Anubha Khale
M. E. S. Instititute’s, H. K. College of Pharmacy, Pratikshanagar, Jogeshwari (W), Mumbai-400 102, India
E-mail: scientific.cell@hkcollege.ac.in
| Date of Submission | 11-Nov-2010 |
| Date of Revision | 11-Oct-2011 |
| Date of Acceptance | 16-Oct-2011 |
| Indian J Pharm Sci , 2011, 73 (5): 543-549 |
Abstract
In the present study attempts were made to prepare metered dose inhalation of salbutamol in solution form and compared it with the marketed metered dose inhalation in suspension form. Solution form of the drug was found better than marketed suspension formulation with respect to homogeneity and content uniformity. Propellant blend P-11 and P-12 in the proportion 30:70 was selected as it gave optimum vapour pressure. Surfactant oleic acid in concentration 10 mg per can was selected as it gave best results with clarity, spray pattern, vapour pressure, content per spray and rate of evaporation. Ethyl alcohol 2 ml per can was used as a cosolvent to give a clear solution, optimum vapour pressure, maximum content per spray and fair rate of evaporation. The selected formulation was subjected to the physico-chemical evaluation tests as per the standard pharmacopoeial procedures and the characteristics of the formulations were further compared with a conventional marketed formulation. In vitro study reveled the net respirable fraction was better than marketed preparation.
Keywords
Cosolvent, net respirable fraction, propellant blend, spray pattern
Introduction
Asthma represents a profound worldwide public health problem. B2 agonists are the most effective antiasthmatic drugs currently available which control asthma in 90 to 95% patients [1]. Asthma is characterized by epithelial damage and basement membrane thickening. The principal symptom of asthma is dyspnea (breathlessness), wheezing, cough and chest tightness which leads to nocturnal awakening [2]. Lower airway remodeling process plays important role in pathophysiology of asthma. Salbutamol (albuterol) is a short-acting ß2- adrenergic receptor agonist. Salbutamol is used as a bronchodilator in the management of disorders involving reversible airway obstruction such as asthma and in some patients with chronic obstructive airway diseases. Chemically salbutamol [3] is (RS)-1-(4- hydroxy-3-hydroxymethylphenyl)-2-(tert-butylamino) ethanol.
Metered dose inhalation (MDI) aerosols are formulated either as suspensions or solutions. In case of suspension formulations, the substances that are insoluble in the propellant and solvent are dispersed in suitable propellant vehicle. Particle size, solubility of active ingredient and surfactants or dispersing agents is the important factors to be considered in formulating MDI suspension formulations. Solution formulations of MDI consist of the active ingredient dissolved in a pure or mixture of propellants. In case of poor solubility of the drug in the propellants, a solvent miscible with propellant system can be added to solubilize the drug. Solution aerosols are relatively easy to formulate provided the ingredients are soluble in the propellant or propellant-solvent system. MDI suspensions tend to flocculate and have a potential for physical instability. Moreover, in suspensions, the particle size is limited due to micronization of drug where as in solutions it is governed by the initial droplet size, the concentration of the non-volatile component and the propellant evaporation rate. The specialized techniques are required for MDI suspension formulation. Also formulation should have a better pulmonary deposition pattern in vitro. If the volatility of the formulation is increased, better respiratory tract penetration can be achieved by solutions as compared to the suspensions. Hence attempts were made to develop a solution formulation and compare it with marketed MDI in the suspension form, with respect to physico-chemical characteristics and net respirable fraction (in vitro study).
Materials and Methods
Salbutamol was a gift sample from Cipla India Limited. Marketed MDI was purchased from local market. Ethyl alcohol, oleic acid were obtained from Qualigens fine chemicals. All the other chemicals used were of analytical grade.
Formulation development of MDI solutions
MDI solution formulations were prepared to get clear solution of the drug in the propellants. However, the propellants are non-polar and poor solvents for medicinal agents. A solvent or a combination of solvents can be used to achieve desired solubility of the active ingredient. Ethyl alcohol in concentration ranging between 30 to 50% has found of greatest use for this purpose. In solution formulations various surfactants are mixed to give the proper HLB value. Propellant is selected to give the desired vapour pressure, spray characteristics and particle size distribution. The vapour pressure of propellant has significant effect on the output particle size. A lowering of vapour pressure results in the production of larger particles. Thus, if the volatility of the solution formulation is increased by controlling the concentration of the nonvolatile component, it leads to an increased output of particles having smaller particle size.The atmospheric conditions were maintained throughout preparation. The temperature was maintained at 20-22° and humidity not more than 40% RH. The following procedure was followed. Metered Dose Inhalations of Salbutamol were prepared using propellants, P11 and P12.
Procedure for filling/sealing canisters
In a closed vessel maintained in an ice bath, drug was dissolved in the required quantity of the cosolvent, ethyl alcohol and mixed with the predetermined quantity of the propellant, P-11. Propellant system plays a major role in an aerosol formulation. To each canister, required quantity of the above product concentrate was added. The canisters were crimped immediately using crimping machine. The sealed cans were then sonicated in the bath sonicator containing cold water at 10° for 15 min. The propellant filling unit was used to fill propellant P-12 under pressure with an accuracy of ±1 cc. The aerosol filling unit was attached to a propellant storage cylinder along with pressure burette capable of metering small volumes of liquefied gas. The storage cylinder provided with suitable valve, allowed purging of the canister and also permitted required quantity of the liquid propellant to be charged from the storage cylinder into the canister. Thus Propellant P-12 was purged into each canister till the desired weight was achieved. The filling of the propellant was carried out with the help of filling unit operated under nitrogen pressure 15 lbs/Kg cm2. The canisters were checked for any leakage.
Development of MDI solution formulation
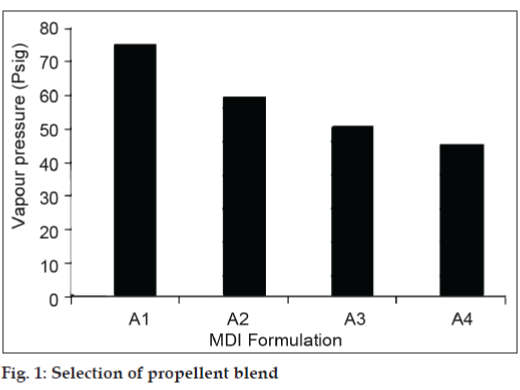
Requires appropriate propellant system, so as to get the optimum vapour pressure to expel the drug from the canister in the form of a fine spray, when pressure within the canister is released on actuation of metering valve. A series of formulations A1, A2, A3 and A4 was prepared by taking different proportions of propellants P-11 and P-12 such as, 30:70, 40:60, 50:50 and 60:40. The formulations were checked for the vapour pressure (fig. 1). The blend giving optimum vapour pressure, in the range of 70-75 psig was selected for further development of salbutamol formulations.
Optimization of concentration of cosolvent
Since salbutamol has poor solubility in propellant system, various concentrations of ethyl alcohol as cosolvent used in the range of 1-3 ml were used in the formulations D1 to D5 (Table 1). These formulations were evaluated on the basis of physicochemical parameters such as clarity, evaporation rate, vapour pressure and content per spray.
| Formulation | Ethyl alcohol | Clarity | Vapour | Evaporation | Content per |
|---|---|---|---|---|---|
| Conc.(ml/can) | pressure (psig) | rate | spray (%) | ||
| D1 | 1.0 | Turbid | 70-75 | +++ | 105.6 |
| D2 | 1.5 | Slightly turbid | 70-75 | ++ | 105.9 |
| D3 | 2.0 | Clear | 70-75 | ++ | 106.4 |
| D4 | 2.5 | Clear | 70-75 | + | 99.3 |
| D5 | 3.0 | Clear | 70-75 | + | 99.01 |
| +++ » Excellent; ++ » Good; + » Poor | |||||
Table 1: Effect Of Concentration Of Ethyl Alcohol On Salbutamol MDI Solution Formulation
Selection of surfactant
In order to stabilize the solution system, the effect of surfactants on the formulations was studied by using various surfactants such as oleic acid, ethyl oleate, span 20, tween 80 and tween 20 in formulations E1- E6 (Table 2). Clarity, evaporation rate, vapour pressure and content per spray were studied to evaluate performance of these formulations
| Formulation | Clarity | Spray | Vapour | Evaporation | Content per |
|---|---|---|---|---|---|
| pattern | pressure (psig) | rate | spray (%) | ||
| E1 | Clear | +++ | 70-75 | +++ | 102.32 |
| E2 | Slight yellow tinge | ++ | 60-65 | ++ | 100.26 |
| E3 | Slight yellow tinge | ++ | 60-65 | ++ | 100.15 |
| E4 | Yellowish | + | 60-65 | ++ | 98.22 |
| E5 | Yellowish | ++ | 60-65 | ++ | 99.17 |
| E6 | Clear | ++ | 65-70 | +++ | 100.12 |
| +++ » Excellent; ++ » Good; + » Poor | |||||
Table 2: Effect Of Surfactants On Salbutamol MDI Solution Formulations
Physico-chemical evaluation
Since MDIs are pressurised packages, many quality tests are necessary to ensure proper performance of the final package and safety during storage and use. Various factors like actuator design, formulatory ingredients, vapour pressure of the propellants, the orifice diameter and the particle size of the spray emerging from the MDI are found to affect the performance of the inhalation aerosols. The canisters used in experiment were of the same dimensions as of marketed MDI. The components of the MDI pack viz. canister, adaptor (actuator) and the mouthpiece cap were separated and measured individually for length, breadth and height. Uniformity of their dimensions was assessed using vernier calipers. The flame extension test was performed to check if the product is inflammable [4]. Following Quality Control tests were performed on MDI solution formulation E1 and results were compared with marketed formulation M1.
Spray pattern
Spray pattern is useful in determining the performance of a specific formulation, valve combination and ensures therapeutic performance and batch to batch uniformity [5]. The spray delivered through MDI was impinged onto a glass plate containing activated silica gel-dye mixture. The MDI was held at a distance of 3 cm from the plate. The spots formed were observed under the UV light (Table 3).
| Test | Solution formulation | Marketed |
|---|---|---|
| (E1) | formulation (M1) | |
| Spray pattern | Round spots with | Round spots with |
| violet colour | violet colour | |
| Partcle size | ||
| istribution | ||
| (%) <3 μ | _ | 97 |
| 3-5 μ | _ | 3 |
| Vapour pressure | 70-75 | 75-80 |
| (psig) | ||
| Evapouration rate | Excellent | Excellent |
| No of deliveries | 202 | 228 |
| per container | ||
| Avg. weight per | 87.60±0.054 | 87.80±0.078 |
| metered dose (mg) |
Table 3: Qualitative and Quantitative Tests
Particle size distribution
The parameter influences the in vivo performance of the aerosol system as it is one of the governing factors affecting respirable dose of the drug [6]. For optimum therapeutic performance the aerosolised particles should be less than 5 μm in size [7]. The particle size distribution of the aerosol suspension was initially determined by the optical microscopy. The MDI under evaluation was sprayed on a glass slide, previously rinsed with carbon tetrachloride (CCl4). Particle size was measured by using 100X magnification with oil immersion method. 100 particles in 25 different fields were measured. The results are tabulated in Table 3 as the percentage number of particles ≤3 μm and between 3-5 μm.
Vapour pressure
The test is applied at the initial stages of development of MDI formulation as a tool to determine the basic formulatory requirement for performance of a MDI product. Vapour pressure is a very important parameter to be determined at the developmental stage as well as at the finished product stage. Sufficient development of pressure within the container is absolutely essential so that the desired quantities of the contents propel into the metering valve and then expelled outside as a fine spray. The test is applied to the finished product, helps to establish the integrity of the pack, as any leakage would be detected by a drop in vapour pressure [8]. It was measured using ‘Comes Pressure Gauge’ (Table 3).
Evaporation rate
With one single actuation of MDI, the formulation was sprayed on a dark surface (for visibility) and the time taken by it to disappear was measured. The evaporation performance was rated as excellent (+++), good (++) and poor (+) based on the time taken for evaporation. When the time taken for the evaporation was less than 30 s, it was considered as having excellent rate of evaporation and times between 30- 60 s and more than 1 min were rated as good and poor evaporation rates respectively.
Number of deliveries per container
These were checked by counting the total number of actuations, till the contents of the canister were exhausted completely (Table 3).
Average weight per metered dose (shot weight test)
This test determines the capacity of the metering valve in terms of the weight of the delivered substance. Density of the propellant used mainly affects the shot weight. The canisters under evaluation were first separated from the adaptor and their weights were recorded. The first five sprays were fired in air. (‘Test Firing’). After the test fire, the weights of canisters were recorded (W1). Five successive deliveries were sprayed from the inhaler after placing the canisters back into their actuators. The canisters were subsequently removed from the adaptors and the valve stem and the orifice were wiped clean. The containers were weighed again and their weights were recorded (W2). Average weight per Metered Dose =W1–W2/5. The test was performed in triplicate to check the reproducibility of the results. The results are given under Table 3.
Content per spray
The amount of drug delivered per spray was determined by a spectrophotometric method of analysis and the average of 6 actuations was checked for the compliance with their label claims. In a beaker containing 75 ml methanol, ten deliveries were fired below the surface of the methanol from MDI. Each time the canister was shaken thoroughly keeping 5 s gap between each spray. Absorbance of the resulting solution was measured at 246 nm and the salbutamol content was determined by the method described below.
Sample preparation
The solution obtained after firing ten deliveries from the MDI was collected in a beaker containing methanol. This was introduced into a 100 ml volumetric flask which contained 10 ml of 0.1N NaOH solution. The volume was made up to 100 ml mark using methanol and the absorbance of the resulting solution, (conc. 10 μg/ml) was recorded at 246 nm (I). To the remaining contents of the volumetric flask, 0.5 ml of conc. HCl was added and the absorbance was again measured at 246 nm (II). The net reading was obtained by subtracting (II) from (I).
A standard solution was prepared by weighing approximately 50 mg of standard salbutamol (I.P) in a 100 ml volumetric flask containing 10 ml of 0.1N NaOH. The volume was made up using methanol. From the resulting solution, a 2 ml aliquot was taken and diluted to 100 ml with methanol. The resulting solution (conc.10 μg/ml) was measured for its absorbance at 246 nm (I). To the remaining solution 0.5 ml of conc. HCl was added and the absorbance was measured again at 246 nm (II). The net reading was obtained by subtracting (II) from (I). The content per spray of the MDI was calculated using formula: Content per spray = (Sample Abs/Std. Abs)×(Std. Wt./100)×(2/100) ×(100/10)×1000. The results are recorded in the Table 4.
| Sample | % Salbutamol per Spray | |
|---|---|---|
| no. | E1 | M1 |
| 1 | 101.23 | 104.33 |
| 2 | 102.64 | 103.68 |
| 3 | 100.88 | 101.74 |
| 4 | 102.79 | 103.12 |
| 5 | 103.35 | 104.61 |
| 6 | 101.08 | 105.55 |
| Average | 102.00 | 103.84 |
| Std. Dev | 1.054 | 1.320 |
Table 4: Content Per Spray From The Salbutamol MDI
Content uniformity
The uniformity of content of salbutamol in 10 doses
was measured using the method similar to that
mentioned for content per spray. In order to check the
extent of variation in 1st, 25th, 50th, 100th, 150th, 200
th doses sprayed from MDI. These sprays were analysed
for the content of salbutamol per spray (Table 5).
| Sample no. | % Salbutamol per Spray | |
|---|---|---|
| (Actuation no.) | E1 | M1 |
| 1st | 98.56 | 97.11 |
| 25th | 103.45 | 102.63 |
| 50th | 104.77 | 100.95 |
| 100th | 102.51 | 103.67 |
| 150th | 101.75 | 102.24 |
| 200th | 102.13 | 101.55 |
| Average | 102.20 | 101.36 |
| Std. Dev (±) | 2.084 | 2.280 |
Table 5: Content Uniformity Of Thesalbutamol MDI Packs
In vitro evaluation for net respirable fraction
Therapeutic efficacy of MDI is determined by the amount of drug deposited in the lungs by measuring net respirable fraction using twin stage impinger [9,10]. Apparatus, an apparatus that mimics the respiratory tract in terms of deposition of the particulate matter which consists of two stage reservoirs; wherein Stage I represents the extent of drug deposited in the oropharyngeal region and not available for lung deposition (Non Respirable Fraction) and stage II represents the extent of drug deposition in the lungs called as Net Respirable Fraction. The two collecting chambers were filled with the required amount of distilled water (7 ml in stage I and 30 ml in stage II). The MDI was attached to the device by means of the rubber collar. 10 sprays were fired into the apparatus.
The side arm tube was connected to the vacuum pump. The flow rate was adjusted to 60 (l/min) with the help of a flowmeter. This mimics the respiratory or inspiratory flow rate of normal individual. A gap of 5 s was maintained between two successive sprays. The reservoir was rinsed with the distilled water. The amount of drug deposited on the adaptor, collar and valve is indicated under ‘device’. Stage I represents the fraction of the drug that is not available for inhalation or the ‘Non-respirable Fraction’. The amount of drug deposited in stage II, is the amount of drug available to the lungs and represents the ‘Net Respirable Fraction’ (Table 6).
| Deposition at | % Drug Deposition (Average±SD) | |
|---|---|---|
| E1 | M1 | |
| Dervice | 13.55±0.825 | 12.25±0.680 |
| Stage I | 39.41±0.723 | 45.20±0.653 |
| Stage II | 47.04±0.675 | 42.55±0.532 |
| Average of six readings | ||
Table 6: Net Respirable Fraction By Twin Impinger Technique
Results and Discussion
Different proportions of propellants, P-11 to P-12 viz. 30:70, 40:60, 50:50 and 60:40 were tried in formulations A1, A2, A3, A4 respectively. The vapour pressure in the formulation was found to be, 75, 60, 50, 45 psig respectively. Fig. 1 represents the comparative study of the propellant blends in terms of their vapour pressure. The formulation A1 showed desired vapour pressure 70-75 psig. Hence this proportion, P-11: P-12, 30:70 was further used in the MDI formulations. Ethyl alcohol in concentration range 1 ml to 3 ml of was used (Table 1) Formulations D1 and D2, with 1 ml and 1.5 ml ethyl alcohol did not give clear solution. Formulations D4 and D5 with higher concentration of ethanol showed poor vapour pressure and poor evaporation rate as increase in the concentration of the cosolvent is found to increase the non-volatile component of the MDI formulation. Formulation D3 with 2 ml of ethanol gave comparatively better results and hence it was selected as the optimum concentration for preparation of MDI solution formulation. Oleic acid, ethyl oleate, span 20, tween 20 and tween 80 were the various surfactants tried in the solution formulations E1 to E6, in the concentration of 2 mg per can was used. It was observed from Table 2 that formulations E4 and E5 with tween, turned yellow and showed poor content per spray. The formulations E2 and E3 containing ethyl oleate and span 20 had developed slight yellow tinge and had poor evaporation rate. The formulations E1 and E6 with oleic acid and with no surfactant respectively were better formulations in terms of all the physico-chemical parameters. Both the formulations were found to be clear and had good evaporation rate. Content per spray was 102.32 and 100.12 respectively. However, Formulation E1 showed better spray pattern as compared to E6. Hence formulation E1 was finally selected for further studies.
Spray pattern is an important test to determine actuator and valve performance. Size and shape of the actuator orifice as well as valve affect the spray characteristics. Smaller the diameter of the spots formed on the silica gel plate lower is the emitted particle droplet size. Table 3 indicates average diameters of the spots along with their description. Both the formulations showed good spray pattern with the spot diameter in the range of 10-12 mm. The spots were round to oval in shape with distinct violet colour at the centre and faint pink colour at the periphery. The anatomy and physiology of the respiratory tract makes the therapy difficult as it restricts entry of the particulate matter. Smaller particles in the range of 1-5 µm, settle in the lower airways. Table 3 shows the particle size distribution of formulations under study. The test is applicable to the suspension formulations. Hence the MDI formulation M1 was studied by this method. The average of three readings in 25 different fields showed 97% of the particles below 3 μm; and 3% of the particles were found in the range 3-5 μm.
Vapour pressures of the test formulations were measured and the results are tabulated under Table 3. MDI formulations E1 and M1 had vapour pressure in the range of 70-80 psi, which is optimum for an aerosol product with good spray performance. Evaporation rate is an important physical characteristic that indirectly determines the spray pattern, and drug deposition in the respiratory tract. The performance was rated as excellent, good and poor, based on the time required for evaporation. Both the formulations under study had excellent evaporation rate which was less than 30 s. Number of deliveries per container test is performed to confirm the proper filling as well as sealing of the containers. Any leakage from the container would not give complete actuations. The canisters contain 200 metered doses. The number of deliveries possible after actuating container valves till the contents were exhausted, (Table 3) for formulations E1 and M1. The numbers of deliveries were found to be, 203 and 228 respectively. The excess number of deliveries, in case of marketed preparation shows about 15% filling overages. The formulations pass the test.
In case of MDIs the metering valve determines the dose. For correct dosing one has to ensure proper design and functioning of the valve. Table 3 shows the average of the six doses delivered from the MDIs. The average weight per metered dose or shot weight was found to be 87.6-87.8 mg with standard deviation less than 0.1.
Content per spray test confirms delivery of the drug as per the labeled claim. Average of six readings is given in Table 4 and indicated that the test formulations pass the pharmacopoeial limits, not less than 80% of the labelled claim and not more than 120% of the labelled claim. The formulation E1 and M1 showed 102.0% and 103.84% of the drug per spray with standard deviation 1.054 and 1.320 respectively.
The drug content in the spray was determined at various intervals of actuation to confirm uniform delivery of the drug at each time till the entire content was used up. Content uniformity was assessed at 1st, 25th, 50th, 100th, 150th and 200th actuation. The results recorded in Table 5, indicated that the average content of the specified actuations for all the three formulations was found satisfactory, in the range 100-102% with almost same standard deviation, 2-2.3%. The formulations confirm the uniformity of drug content till the last dose is actuated out of the container. In in vitro evaluation for net respirable fraction, it was observed that the average net respirable fraction was 47.04% and 42.55% for the formulations E1 and M1 respectively. The solution formulation of MDI showed the maximum respirable fraction indicating comparatively better therapeutic performance (Table 6). Hence it can be concluded that, the formulated solution formulation were comparable with marketed MDI formulation. The solution formulations of MDI were better than marketed suspension formulation as they had shown better pulmonary deposition pattern in vitro. Also it required less specialized techniques compared to MDI suspension formulation.
Acknowledgements
The authors would like to thank to S. N. D. T. University’s, C. U. Shah College of Pharmacy, for providing laboratory facilities and Cipla India Limited for sending a gift sample of salbutamol.
References
- Caramori G, Groneberg D, Ito K, Casolari P, Adcock IM, Papi A. New Drugs targeting Th2 Lymphocytes in asthma. J Occup Med Toxicol 2008;3:S6.
- Christoptier W, Cheristopher H, Edusin C. Respiratory Disorders. In: Davidson’s Principles and Practice of Medicine. 17th ed. New York: ELBS and Churchill Livingstone; 1995. p. 323-35.
- Mashru RC, Sutaria VB, Shankalia MG. Development and evaluation of fast dissolving film of salbutamol sulphate. Drug Develop Ind Pharm 2005;31:25-34.
- Leung WP, Rawlins P. Pharmaceutical Aerosols. In: Rawlins EA, editor. Bentley’s Textbook of Pharmaceutics. 8th ed. London: Baillie`reTindall; 1977. p. 680-2.
- Benjamin EJ, Korten J, Shek E. Characterization of spray patterns of inhalation aerosols using thin-layer chromatography. J Pharm Sci 1983;72:380-5.
- Byron PR. Pulmonary targeting with aerosols. Pharm Tech 1987;11:42-56.
- Byron PR, Gonda I. Perspectives on the biopharmacy of inhalation aerosols. Drug Develop Ind Pharm 1978;4:243-59.
- Haywood PA, Martin-Smith M, Smith IJ. Establishing more meaningful specifications and tests for metered dose pressurised inhalers formulated as suspensions. Pharm Forum 1989;15:5193-202.
- Hallworth GW, Westmoreland DG. The twin impinger: A simple device for accessing the delivery of drugs from metered dose pressurized aerosol inhalers. J Pharm Pharmacol 1987;39:966-72.
- Miller NC, Marple VA, Schultz RK, Poon WS. Assessment of twin impinger for size measurement of metered dose inhaler sprays. Pharm Res 1992;9:1123-7.