- Corresponding Author:
- M. P. Jadhav
Department of Pharmaceutics, Bombay College of Pharmacy, Kalina, Santacruz, Mumbai - 400 098
E-mail: manojpjadhav@yahoo.com
| Date of Submission | 3 September 2010 |
| Date of Revision | 24 February 2011 |
| Date of Acceptance | 27 February 2011 |
| Indian J Pharm Sci, 2011, 73 (1): 57-64 |
Abstract
In the present study, we formulated long circulating liposomes for amphotericin B and characterized them. The formulation was optimized using 23 factorial designs. Pegylated liposomal formulation showed favorable results with reference to particle size (247.33±9.60 nm), percent entrapment efficiency (94.55±3.34%). TEM studies revealed that the liposomes were essentially spherical, hollow, and appeared like powder puff structures. From DSC study it was concluded that the pegylated formulation containing Amp B showed better stability and membrane integrity of the formulation. During the stability studies the formulation was found to be stable. When subjected to gamma scintigraphy kinetic tracer studies the formulation showed longer residence time in the blood in BALB/C mice.
Keywords
Amphotericin B, BALB/c mice, liposomes, tracer kinetic studies
There has been a steady increase in the incidence of invasive fungal infections in immunocompromised patients. These are among the most important causes of morbidity and mortality in patients with cancer and among other severely immunocompromised hosts. Because the overall prognosis for patients with invasive fungal infections remains poor, there is a critical need to improve methods for treating these infections. Amphotericin B (Amp B) has a broad and potent activity against fungi that cause aspergilosis, candidasis and cryptococcosis, and is expected to exhibit good efficacy in deep-seated mycoses. However, it is difficult to use it for protracted periods or in high doses due to its toxic effects, limiting its clinical use [1,2].
One of the promising approaches is to use of Amp B incorporated into liposomes or other lipid carriers [3-5]. However, liposomes are easily trapped by the reticuloendothelial system (RES) [6,7]. Until, now it has not been known whether a long blood residence time is of importance for the improved efficacies of Amp B liposomes. The ability to achieve a significantly longer blood circulation time of liposomes opens new ways to achieving improved delivery of antimicrobial agents to infected tissues including infections in nonmononuclear phagocyte system [8]. Long circulating liposomes are prepared by coating the liposome surface using hydrophilic phosphatidylethanolamine derivatives of monomethoxypolyethyleneglycols (PEGPE) can effectively prolong their blood residence time [9-12]. These are also called sterically stabilized liposomes. The distinctive properties of sterically stabilized liposomes makes them excellent candidate for many therapeutic applications.
In the present study, we formulated long circulating liposomes for Amp B and characterized them. In order to get idea about its long residence time we conducted tracer kinetic study using Tc99m as radionuclide. A pharmacokinetic study to correlate above findings was also carried out in healthy BALB/c mice.
Materials and Methods
Amphotericin B was a gift sample form Cipla Ltd., Mumbai, India. MPEG2000-DSPE, (Lipoid, Inc., Germany) was gifted by Sun Pharmaceuticals Ltd., Ahmedabad. Soya Phosphatidylcholine was procured from Lipoid Inc., Germany. Cholesterol was purchased from S.D., Fine Chemicals, Mumbai, India. 99Molybdenum was procured from Board of Radiation and Isotopes Technology (BRIT), Mumbai, India. Sodium pertechnate (99mTc) was extracted from 99Molybdenum by solvent extraction. All other reagents and solvents namely, disodium hydrogen phosphate, EDTA (ethylenediaminetetraacetic acid), potassium dihydrogen phosphate, sodium hydroxide, sodium chloride, stannous chloride, methanol, acetonitrile, dimethyl sulfoxide (DMSO), chloroform were either HPLC or of analytical grade. Freshly prepared double distilled and cooled water was used for HPLC analysis.
Preparation of liposomes (conventional and long circulating liposomes)
Liposomes were prepared by simple film hydration method. Empty and drug loaded liposomes were prepared by minor modification of film hydration method described by Bangham et al. [13]. A 23 factorial designs were used to optimize the entrapment efficiency and particle size of liposomes containing amphotericin B. Required quantities of soyaphosphotidylcholine, cholesterol (1:1, 2:1), MPEG 2000-DSPE (5 mol%) [14] and Amp B (0.2 mg/ml) in appropriate molar ratios were dissolved in chloroform. Glass beads (10 g) were added to increase surface area available for film formation. Chloroform was evaporated under reduced pressure on a rotary evaporator (Buchi Rotavap Evaporator) to form a thin film on the inner surface of the flask. Lipid film was hydrated using above gel to liquid crystalline phase transition temperature (65°) of the lipids and cholesterol for two minutes and flask was manually shaken vigorously for 5 min for formation of liposomes followed by heating it again for 2 min for annealing of lipsomes. The dispersion was sonicated using bath sonicator (Expo India) for 15 or 45 s to get the liposomes of smaller size. Empty as well as drug loaded liposomes were stored at 2-8° until analysis.
Analytical method development and validation
A modified reverse phase HPLC method was developed for the analysis of Amp B on the basis of method reported by Eldem et al. [15]. The analytical method for estimation of Amp B was validated in both aqueous as well as plasma matrix. The following chromatographic conditions were optimized. The mobile phase (acetonitrile-EDTA (20 mM), (40:60 v/v) at pH 5.5) was degassed by filtration under vacuum through a nylon filter (0.4 μm pore size, Pall, Gelman Laboratories) and sonicated in bath sonicator prior to use. The flow rate was set to be 1.3 ml/min at ambient temperature. The chromatographic data were acquired by a computerized integration and recording system. The column C18, Supelco, (250×4.6 mm i.e. rose safe, 5 μm particle size) was used, with injection volume of 20 μl, and elution was carried out at 405 nm. For plasma drug estimation internal standard (4 amino-4-nitronaphthalene) was used. Total chrome Navigator Version 6.2.0 software for chromatogram analysis was used. The method was validated for specificity, selectivity, linearity, precision and accuracy.
Entrapment efficiency
To the liposomal suspension, 250 μl was mixed with 1 ml distilled water and vortexed on vortexer (1 min). It was centrifuged at 4°, using centrifuge (Heraeus Biofuge, Thermo India Ltd.) 15,000 rpm for 20 min. The pellet was separated and dissolved in 1:1 dimethyl sulfoxide:methanol 1 ml solution by vortex mixing. The solution was suitably diluted and injected into HPLC for drug estimation. The drug peak area was used to calculate the drug concentration in the sample suspension.
Microscopic observation
A drop of the empty and drug loaded liposomes were placed on a clean glass slide covered with a cover slip and observed under high power (45˟) of the optical microscope for observation of shape of liposome aggregation, or any precipitation of drug, or any other component.
Determination of particle size
The particle size distributions of the liposomes were evaluated by dynamic light scattering using N5 Beckmann Coulter Submicron Particle Size Analyser. The liposomal suspension was diluted suitably with double distilled water and filtered through 0.45 μ filter. The intensity was set as per the manual instruction and the particle size distribution was estimated. The diameter of the particles was reported in nanometers (mean±SD) and polydispersity index was also recorded.
Transmission electron microscopy
Negative electron microscopy technique was used to study liposomal shape and lamellarity. A drop of appropriately diluted liposomal suspension was placed on a formvar coated 300 mesh size copper grid (M/s. SPI Suppliers, West Chester, PA, USA). The liposomes were allowed to adhere to grid for 5-6 min. The excess of liposomes were drawn off by touching the edge of the grid with a piece of tissue paper held at 90° to the plane of the grid. Without allowing the film on the grid to dry, a drop of 12% uranyl acetate stain was placed on it for 5 min. and again drop was drawn off as described earlier. The stained grid was air dried and observed. Image was visualized on the screen under the electron microscope and images were photographed.
Stability studies
Optimized conventional as well as pegylated (long circulating liposomal) formulations were stored at 5º and at 25° in amber colored sealed glass vials for 90 days. These samples were evaluated for particle size and drug entrapment at specified time intervals viz., 0, 1, 2, 3 months in triplicate. The liposomal suspensions were also observed visually for appearance, ease of redispersion, sedimentation.
Labelling procedure
Radiolabelling procedure was determined by minor modification of a reported method [16-18]. Preformed liposomes were radiolabeled by 99mTc after reduced by stannous chloride. To 0.5 ml liposomal preparation in a glass vial (FungisomeTM, conventional Amp B and long circulating liposomal preparation), 50 μl of freshly prepared stannous chloride solution in 0.1N HCl was added. This mixture was shaken vigorously for 2 min in a lead cell, to this mixture 4 mCi 99mTcO4 (technetium pertechnate) in normal saline was added. This mixture was then agitated on shaker in lead pot for 20 min.
The radiolabelled formulation was spotted on a precoated thin layer chromatography (TLC) plate at a distance of one cm from the lower end and acetone was used as mobile phase. The plate was removed, dried, and cut into lower 1/3rd and upper 2/3rd portions. The radioactivity in each part was determined under Gamma Camera fitted with pinhole collimator (General Electricals). The data acquisition from the gamma camera was performed with a computer ‘Acquisition System’. The data was acquired on GENIE acquisition station and then transferred to Entegra workstation for processing. The stability and reproducibility of radiolabelling was determined at 4, 12 and 24 h intervals for all three formulations.
Biodistribution studies
Biodistribution studies were carried out in healthy BALB/c mice of either sex, of 12-20 weeks old, weighing 20±2 g. All experiments were approved by the institutional animal ethics committee of the Bombay Veterinary College, Parel, Mumbai. The mice were fasted overnight before experiment. The animals were anaesthetized by injecting xylazine as preanaesthetic and ketamine as anesthetic agent as per body weight.
Radiolabelled Amp B was administered and static and dynamic images were acquired using a Millenium MPS Acquisition System, (Multipurpose Single Head Square Detector) Gamma Camera Fitted with Low Energy General Purpose pinhole collimeter. Dynamic images were acquired in 64×64 matrix for 30 min and static images were acquired in 256×256 matrix by setting energy level at 140 keV and 20% window. 99mTc-Amp B labeled liposomes (FungisomeTM, conventional Amp B and long circulating liposomal Amp B preparation) were injected intravenously through tail vein as single bolus at 0.5 mg/kg. For the purpose of determining total injected dose the radioactivity of the full and empty syringe static images were acquired for one minute.
The counts of 99mTc-Amp B labeled liposomes (FungisomeTM), conventional Amp B and long circulating liposomal Amp B) in the body were determined at various time points (0.5, 4, 12 and 24 h static images of one min each). The percentage uptake of the radiolabelled formulations at 0.5, 4, 12 and 24 h was estimated in various organs of interest like heart, liver and kidney were counted by background subtraction and decay correction.
Additionally, Amp B liposomes at 0.5 mg/ kg (FungisomeTM, conventional Amp B and long circulating liposomal Amp B preparation) was administered to BALB/c mice as a single intravenous bolus and drug levels in plasma were quantified. Two mice were used for each time point. The blood samples were collected at 2, 6, 12, 18 and 24 h post i.v. injection. The blood was collected by heart puncture, in heparinized syringe. Plasma was separated by using centrifuge at 4°, at 15,000 rpm for 20 min. The plasma was stored at -80°, until analysis using High Performance Liquid Chromatography (HPLC). The separated plasma was then processed and the supernatant was used for estimation of Amp B concentrations.
Results and Discussion
The formulations were optimized using 23 factorial designs for entrapment efficiency and particle size of liposomes containing Amp B (data not shown). The prepared liposomal dispersions were observed under compound microscope (45˟). The optimized batch of liposomal preparation was found to be spherical in shape without any aggregation under 45˟ magnification. The multilamellar liposomes were produced.
Formulation AMP-A5 prepared with soyaphosphotidylcholine: Cholesterol ratio of (2:1) with 10 ml aqueous phase and sonication time of 15 s showed better drug entrapment i.e. 96.04%. Entrapment as low as 67.48% was observed for formulation AMP-A8 prepared with lipid cholesterol composition of 1:1 with 5 ml hydration buffer and sonication time of 45 s (data not shown).
The particle size varied from 393 nm for formulation AMP –A4 with phospholipid: Cholesterol ratio of 2:1, 5 ml hydration buffer and 15 s sonication time to as high as 857 nm with formulation AMP –A1 with phospholipid: Cholesterol ratio of 1:1 with 10 ml hydration buffer and 15 s sonication time. The study indicated that incorporation of drug in liposomes with varying amount of hydration medium influenced size of liposomes. It was expected that increasing sonication time would reduce liposome size but with 10 ml hydration buffer larger liposomes size were obtained, however contrary to what was anticipated sonication did not have direct effect on the size. As evidenced by formulation AMP-A2, with 5 ml hydration buffer and 45 s sonication time, however particle size was 770.67 nm, same was the case with formulation AMP-A6, with 10 ml buffer and 45 s sonication time. In a study reported by Sezer et al. [19] who noted no significant effect of hydration medium on the size of liposomes. However, the above mentioned study did not include influence of sonication time on the size of liposomes. The same optimized formulation was subjected to prepare long circulating liposomes incorporating 5 mol % of MPEG 2000-DSPE which resulted in 94.55% entrapment efficiency and a particle size of 247.33 nm.
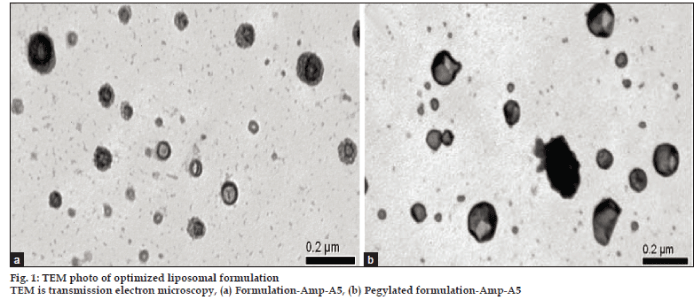
The drug loaded conventional and pegylated (long circulating) liposomes appeared spherical and multilamellar. The thick walls of the liposomes structure indicate stacks of multiple layers, and they are dark in appearance on the micrograph showing high electron density at the outer surface indicating the building up of the negative stain there. The liposomes show faint appearance in the centre indicating absence of the bilayer there. The outer layers shows granular texture indicating the presence of a beaded particulate structure.This is attributed to the pegylation (figs. 1a and b).
It is well documented in the literature that certain artifacts do arise in sample preparation for transmission electron microscopy (TEM) of liposomes [20,21]. However, we have carefully avoided this by using negative staining with 12% uranyl acetate and taking care that the samples were prepared well below the transition temperature. This negative staining procedure gave rise to reliable TEM photomicrographs. High electron densities are seen in area where there is greater stain uptake, for example in between and outside of the membrane of bilayer and in between the particles of the pegylated layer. This established the relation between the electron density staining method and morphology.
The mean particle size obtained from TEM photomicrographs was in the range of 0.2 to 1 μm for empty (picture not shown) and drug loaded liposomes for both conventional and pegylated liposomes. These values correlated well with the results from particle size analysis. This indicated that the entrapment of drug in both pegylated and conventional liposomes did not alter the size of liposomes significantly.
The effect of PEG on bilayer structure has been studied in great details. It is reported that at PEG concentrations greater that 20 mol% there is a transition from lamellar to micellar structure. Hence in the present study the DSPE PEG 2000 concentration was limited to 5 mol%. Indeed, by TEM measurements reported as passive forms that the liposomes were essentially spherical, hollow, and appeared like powder puff structures.
Conventional and pegylated liposomal formulations were stored at 5 and 25° for three months period and evaluated periodically for change in colour, appearance, particle size, entrapment efficiency etc. The freshly prepared AMP A-5 conventional liposomal formulation contained 96.05% of Amp B (Table 1). When stored at 5° and 25° the entrapment reduced to 74.20 and 62.47% in three month’s time, respectively. The particle size was not significantly reduced even when stored at varying temperature for 3 months. During the stability studies there was considerably high leaching of the drug from conventional liposomes indicating further processing like lyophilization would be required for limiting release of entrapped drug during storage.
| Formulation code | Duration (month) | 5° | 25° | ||
|---|---|---|---|---|---|
| Entrapment efficiency (%, mean±SD, n=3) (*F 96.04±3.57) | Particle size (nm, mean±SD, n=3) (**F700± 255.50) | Entrapment efficiency (%, mean±SD, n=3) (*F 96.04±3.57) | |||
| Particle size (nm,mean±SD, n=3)(F 247±9.60) | |||||
| AMP B A5 | 1 | 95.46±8.99 | 219±2.08 | 83.91±8.55 | 231±12.7 |
| 2 | 84.53±1.35 | 222±10.4 | 63.52±3.04 | 338±41.3 | |
| 3 | 74.20±3.80 | 218±10.1 | 62.47±1.95 | 298±13.5 | |
| AMP B A5 PEG | 1 | 93.55±3.66 | 259±4.59 | 84.61±1.81 | 224±16.2 |
| 2 | 91.67±7.06 | 326±44.6 | 79.83±4.01 | 211±24.7 | |
| 3 | 88.72±5.80 | 265±11.0 | 72.48±3.13 | 218±10.7 | |
SD- Standard deviation, n=3, AMP B PEG- amphotericin B polyethylene glycol AMP B A5- Conventional amphotericin B, *F– entrapment efficiency when freshly prepared, **F particle size when freshly prepared
Table 1: Stability Study; Effect Of Temperature On Entrapment Efficiency And Particle Size.
In case of pegylated liposomal formulation the entrapment reduced from initial of 94.55% to 88.72 and 72.48 % at 5 and 25°, respectively. The pegylated formulation comparatively rated better than conventional liposomal preparation suggesting better stability of the formulation. However, further refinement of the formulation is warranted to get better stability. The particle size did not change significantly during the accelerated storage suggesting the absence of aggregation. Both formulations appeared similar during storage, except that conventional formulation appeared little turbid. Both, conventional and pegylated formulation exhibited same pattern of sedimentation and redispersion during stability studies.
The drug was directly radiolabelled using the well known stannous chloride method and radiolabelling above 95% could be achieved during in vitro studies using precoated TLC plates. Stability of the radiopharmaceutical was established for 24 h period.
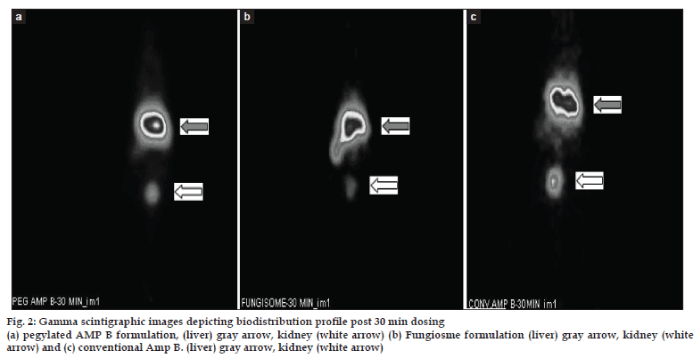
In case of 99mTc labelled pegylated formulation the % uptake by liver was observed to be ranging from 67.07% ( 0.5 h dynamic scan), to 72.66% at the end of 24 h post injection (fig. 2 and Tables 2 and 3). This high drug amount in the liver at the end of 24 h was in compliance with the hypothesis which was proposed stating that the long circulating formulation of Amp B will have better residence in the blood. These finding suggests that pegylated liposomes circulated in blood for long. This could be due to reduced uptake by the reticuloendothelial system of the liposomes due to the electrostatic sheath produced by polymer pegylation of the liposomes thus preventing the interaction with the blood components especially albumin like proteins. The findings are in compliance with those reported by Etten et al., who showed the long residence time in blood of pegylated Amp B over 24 h time at two different doses of 0.5 and 5 mg of the drug formulation. This long residence time would assist in improving the therapeutic efficacy of Amp B in blood. The % uptake by the kidney was found to be ranging from 8.20% to 0.41% over 24 h. This indicates that the drug kidney burden decreased over period of time and indicated that the clearance was consistent without damaging the kidneys.
| Formulation | 0.5 h (±SD) | 4 h (±SD) | 12 h (±SD) | 24 h (±SD) |
|---|---|---|---|---|
| PEG AMP B | 67.07(8.92) | 72.35(1.55) | 79.88(9.35) | 72.66(8.42) |
| Fungiosme | 80.62(11.29) | 46.35(24.26) | 22.21(23.93) | 11.71(2.87) |
| Conv. AMP B | 76.41(9.89) | 60.76(14.60) | 58.89(12.03) | 32.11(10.61) |
SD- Standard deviation, n=3, PEG AMP B- Polyethylene glycol amphotericin B, Conv. AMP B- Conventional amphotericin B
Table 2: 99mtc Labelled Drug Percentage Uptake By Liver
| Formulation | 0.5 h (±SD) | 4 h (±SD) | 12 h (±SD) | 24 h (±SD) |
|---|---|---|---|---|
| PEG -AMP B | 8.20(5.14) | 2.49(1.58) | 2.78(2.38) | 0.41(0.70) |
| Fungisome | 5.67(0.50) | 5.71(0.57) | 2.98(2.80) | 0.86(0.57) |
| Conv. AMP B | 1.05(0.96) | 1.36(0.85) | 0.85(0.56) | 6.48(1.22) |
SD- Standard deviation, n=3, PEG AMP B- Polyethylene glycol amphotericin B, Conv. AMP B- Conventional amphotericin B
Table 3: 99mtc Labelled Drug Percentage Uptake By Kidney
With 99mTc labelled Fungisome group the percentage uptake by liver was found to be ranging between 80.62–11.27% from time of injection (0.5 h dynamic scan) to 24 h (p.i.) suggesting that the drug remained into the circulation for 4 h in significant concentration and levels reduced thereafter (Tables 3 and 4). The % drug uptake by kidney was higher in the initial phase i.e. 5.67% however it reduced with time to 0.86% in kidney at the end of 24 h suggesting that the excretion of drug was normal from body.
| Formulation | 0.5 h | 4 h | 12h | 24 h |
|---|---|---|---|---|
| PEG- AMP B | 24.04 | 32.89 | 59.20 | 35.20 |
| Fungisome | 53.05 | 9.27 | 10.05 | 75.80 |
| Conv. AMP | 25.18 | 65.40 | 74.50 | 78.90 |
PEG AMP B- Polyethylene glycol amphotericin B, Conv. AMP B- Conventional amphotericin B
Table 4: 99m Tc Labelled Drug Liver To Kidney Percentage Uptake Ratio
With 99mTc labelled conventional AMP B solution we found that the % uptake by liver ranged from 76.41 to 32.11% up to 24 h post injection. However the % uptake by kidney ranged 1.05 to 6.48% (Table 2 and 3). Initially, the burden on kidney was less in 12 h of the study but it increased after 12 h post injection in one of the animals. This indicated that the drug was not sufficiently cleared form the kidney and also got localized in the liver with respect to time. This is in line with reported information that the conventional AMP B is nephrotoxic in nature.
We also calculated ratio of % uptake liver to kidney for the 99mTc labeled amphotericin B in all the three groups (Table 4). This ratio was calculated on the basis of counts/sec in liver and kidney at specific time. It was observed that the rate of clearance with pegylated and Fungisome groups was almost similar (not significant difference) suggesting that excretory system was not overburdened. In the conventional AMP B group this ratio was found to be almost 62% higher that the above mentioned two groups indicating that there is no satisfactory renal clearance and retention of AMP B in the kidneys. These finding gave the evidence of nephrotoxicity by conventional AMP B.
Therefore, it can be said that the hepatic cumulation was high and kidney burden was less indicating the safe and sustained action of the newly formulated long circulating formulation of AMP B in healthy BALB/C mice.
After drug administration the levels at 2 h post injection were found to be 27.12, 24.78 and 15.24 ng/ml in all three formulations (Table 5). However, the levels dropped to 37.66 and 14.54 ng/ml at 12 and 24 h with pegylated group. The drug blood levels with the Fungisome group were 28.84, and 11.74 ng/ml at 12 and 24 h post injection indicating that the drug release was sustained for a long period. In the conventional Amp B group the drug concentration decreased with time from 9.31 to 0.33 ng/ml suggesting the drug was excreted from the body quickly. These findings were correlating with the observations of the gamma scintigraphy study, which showed the sustained and prolonged release of the drug from pegylated liposomal and the conventional liposomal (Fungisome) formulation as against the conventional AMP B.
| Formulation | 2 h | 6 h | 12 h | 18 h | 24 h |
|---|---|---|---|---|---|
| PEG AMP B | 27.12 | 45.41 | 37.66 | 16.66 | 14.54 |
| Fungisome | 24.78 | 41.98 | 28.88 | 22.80 | 11.74 |
| Conv. AMP B | 15.25 | 18.5 | 9.31 | 1.44 | 0.33 |
Concentration in ng/ml, n= 2, PEG AMP B- Polyethylene glycol amphotericin B, Conv. AMP B- Conventional amphotericin B
Table 5: Plasma Drug Levels –Time Profile For All 3 Formulations In Healthy Balb/C Mice
Pegylated liposomal formulation showed favorable results with reference to particle size, entrapment efficiency, and stability studies. When subjected to gamma scintigraphy kinetic tracer studies the formulation showed longer residence time in the blood in BALB/C mice. The pharmacokinetic studies also supported the long residence time of amphotericin B in blood. In conclusion, the laboratory studies with long circulating liposomes for amphotericin B have shown promising and encouraging results. This liposomal drug delivery system can further be extensively investigated with better refinements and properties for the treatment of systemic fungal infections.
Acknowledgements
We are thankful to the Electron Microscopy Department, Jaslok Hospital, Mumbai for providing Electron Microscopy facility.
References
- Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Rev Infect Dis 1990;12:308-29.
- Lyman CA, Walsh TJ. Systemically administered antifungal agents?a review of their clinical pharmacology and therapeutic applications. Drugs 1992;44:9-35.
- Marie De, Janknegt SR, Bakker-Woudenberg IA. Clinical use of liposomal and lipid-complexed amphotericin B. J AntimicrobChemother 1994;33:907-16.
- Gates C, Pinney RJ. Amphotericin B and its delivery by liposomal and lipid formulations. J Clin Pharm Ther 1993;18:147-53.
- Schmitt HJ. New methods of delivery of amphotericin B. Clin InfectDis 1993;17:S501-6.
- Van Etten EW, Otte-Lambillion M, van Vianen W, ten Ket MT, Bakker-Woudenberg IA. Biodistribution of liposomal Amphotericin B (Ambisome) and Amphotericin B-deoxycholate (Fungizone) in uninfected immunocompitent mice and leucopenic mice infected with Candida albicans. J AntimicrobChemother 1995;35:509-19.
- Wassan KM, Vadiel K, Lopez?Berestein G, Luke DR. Pharmacokinetics, tissue distribution, and toxicity of free and liposomal amphotericin B in diabetic rats. J Infect Dis 1990;161:562-6.
- Bakker-Woudenberg IA, Lokerse AF, ten Kate MT, Mouton JW, Woodle MC, Storm G. Liposomes with prolongedblood circulation and selective localization in Klebsiellapneumoniae-infectedlung tissue. J Infect Dis 1993;168:164-71.
- Blume G, Cevc G. Liposomes for the sustained drug release in vivo. BiochimBiophys Acta 1990;1029:91-7.
- Klibanov AL, Huang L. Long circulating liposomes: Development and perspectives. J Liposome Res 1992;2:321-34.
- Klibanov, AL, Maruyama K, Torchillin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett 1990;268:23537.
- Celia C, Calvagno MG, Paolino D, Bulotta S, Ventura CA, Russo D, et al. Improved in vitro anti-tumoral activity, intracellular uptake and apoptotic induction of gemcitabine-loaded pegylatedunilamellar liposomes. J NanosciNanotechnol 2008;8:2102-13.
- Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J MolBiol 1965;13: 238-52.
- Dos Santos N, Allen C, Doppen AM, Anantha M, Cox KA, Gallagher RC, et al. Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: Relating plasma circulation lifetimes to protein binding. BiochimBiophys Acta 2007;1768:1367-77.
- Eldem YM, Bunty KL, Yankamotu KY. Development and method validation for amphotericin B using solid phase extraction. J Pharm Res 2001;87:345-51.
- Morgan JR, Williams KE, Davies KE, Leach K, Thompson M, Williams LA. Localization of experimental Staphylococcus abscesses by 99mTc-technetium labeled liposome. J Med Microbiol 1981;14:213-7.
- Reddy LH, Sharma RK, Chuttani K, Mishra AK, Murthy RR. Etoposide-incorporated tripalmitin nanoparticles with different surface charge: Formulation, characterization, radiolabeling, and biodistribution studies. AAPS J 2004;6:55-64.
- Arulsudar N, Subramanian N, Mishra P, Sharma RK, Murthy RS. Preparation, characterization and biodistribution of 99mTc-labeled liposome encapsulated cyclosporine. J Drug Target 2003;11:187-96.
- Seizer AD, Bas LS, Akbauga J. Encapsulation of enrofloxacine in liposomes I: Preparation and in vitro characterization of LUV. J Lipo Res 2004;14:77-86.
- Chetanachan P, Akarachalanon P, Worawirunwong D, Dararutana P, Bangtrakulnonth A, Bunjop M, et al. Ultrastructural characterization of liposomes using tranmission electron microscope. Adv Mat Res 2008;55-57:709-11.
- Foldvari M, Gesztes A, Mezei M. Dermal drug delivery by liposome encapsulation: Clinical and electron microscopic studies. J Microencapsul 1990;7:479-89.