- *Corresponding Author:
- Z Yu
Department of Pharmaceutics, School of Pharmacy, Shenyang, Liaoning 110016, PR China
E-mail: pharmzy@163.com
| Date of Received | 21 January 2022 |
| Date of Revision | 11 October 2022 |
| Date of Acceptance | 01 June 2023 |
| Indian J Pharm Sci 2023;85(3):829-834 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The objective of the present study was to develop a self-emulsifying drug delivery system of larotaxel and evaluate its in vitro and in vivo performance. The prepared larotaxel loaded self-emulsifying drug delivery system spontaneously formed microemulsion with a mean particle size of 115.4 nm when mixed with 100-fold water under gentle agitation. In vivo pharmacokinetics study of larotaxel loaded selfemulsifying drug delivery system demonstrated 5.19-fold enhancement in oral bioavailability compared to larotaxel-Sol. Furthermore, intestinal bio-distribution studies revealed that the intestinal residence time of larotaxel loaded self-emulsifying drug delivery system was dramatically extended in comparison to larotaxel-Sol. Lymphatic transport studies showed that cycloheximide, a chylomicron secretion blocker, would impede oral absorption of larotaxel loaded self-emulsifying drug delivery system, which confirmed that lymphatic route was involved in the absorption of larotaxel loaded self-emulsifying drug delivery system. What is more, in vivo antitumor experiment proved that the antitumor activity of oral larotaxel loaded self-emulsifying drug delivery system was significantly superior to oral larotaxel-Sol, which was close to intravenous larotaxel-Sol. In conclusion, self-emulsifying drug delivery system is a promising vehicle for the oral delivery of larotaxel.
Keywords
Larotaxel, self-emulsifying drug delivery system, oral bioavailability, antitumor
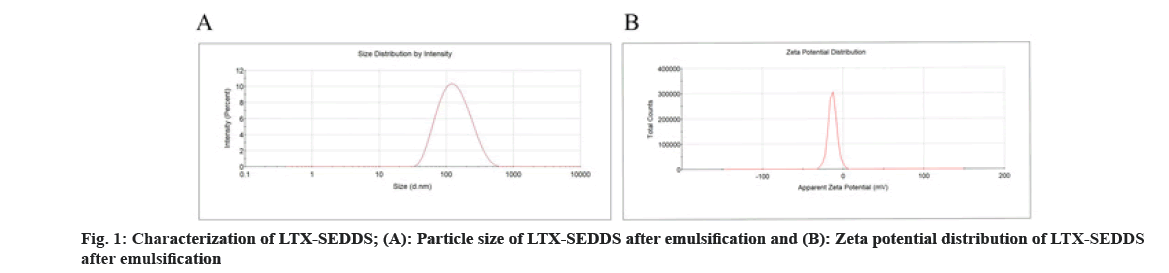
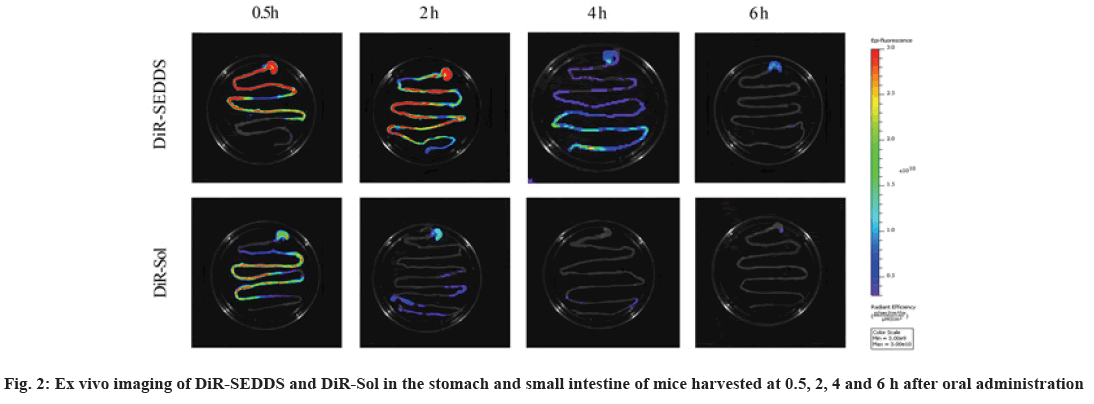
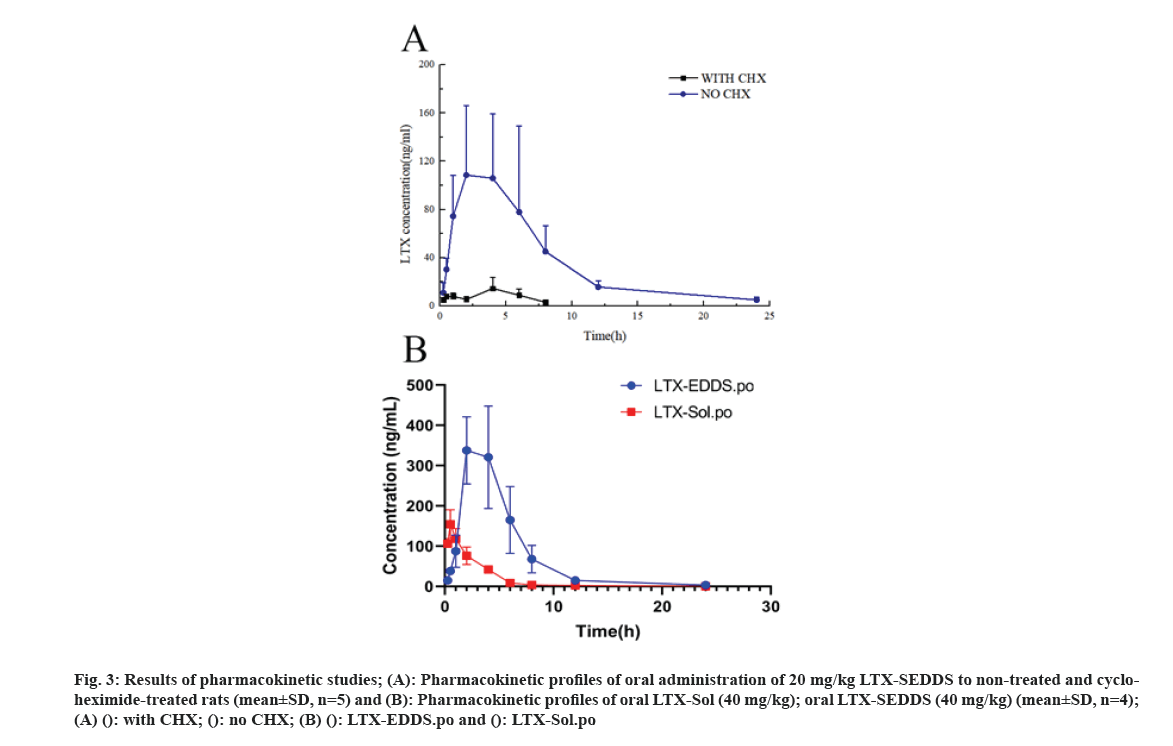
Cancer remains one of the most life-threatening diseases with significant increases in morbidity and mortality[1]. Currently, many treatment modalities have been applied for tumors, such as chemotherapy, surgery, laser therapy, molecular targeted therapy[2], photothermal therapy[3] and emerging immunotherapy[4]. Among these options, conventional chemotherapy still remains a vital treatment approach for most cancer cases due to its effective tumor killing effect[5-7]. Larotaxel (LTX) is a modified new- generation taxane compound, which exert their cytotoxic effect as the current taxanes by binding to tubulin to promote the formation of stable microtubules and prevent their depolymerization[8,9]. LTX has a much lower affinity for P-glycoprotein than docetaxel and it is more effective than paclitaxel in treatment of resistant cancers[10-13]. Nevertheless, clinical application of LTX is mainly hindered by its poor water solubility and there are urgent needs to develop a suitable drug delivery system for delivering LTX[14]. The oral drug delivery is one of the most acceptable routes of administration due to its safety, high compliance, and cost-effectiveness[15]. However, oral delivery of taxanes is hampered by its poor oral bioavailability owing to their practical insolubility in water and high affinity to drug efflux transporters in the gastrointestinal tract[16,17]. Self-Emulsifying Drug Delivery Systems (SEDDS) refers to a formulation consists of oil, surfactant and co-surfactant/co- solvent, which will disperse into oil-in water emulsion in the fluid of gastrointestinal tract upon oral administration. The excipients contained in the SEDDS tend to dissolve hydrophobic drugs and inhibit drug efflux mediated by P-glycoprotein expressed in the gut membrane and thus improve oral bioavailability[18,19]. SEDDS has exhibited significant potentials for improving oral delivery efficiency of different drugs[20,21]. In the present study, a novel LTX-SEDDS was prepared and systematically evaluated, including particle size, Zeta potential, emulsifying ability, oral bioavailability, intestinal biodistribution, absorption mechanism and in vivo anti-tumor efficacy of LTX-SEDDS. LTX was purchased from Yantai Badian Pharmacy Research Co., Ltd. Monoolein was supplied by Gattefossé. Tricaprylin was obtained from Sigma Chemical Co. Tween 80 was purchased from Shanghai Shenyu chemical Chemical Co. Ltd. Tert-Butyl methyl ether was obtained from Sinopharm Chemical Reagent Co. LTD. All other chemicals and reagents were of analytical or chromatographic grade. Sprague- Dawley rats (180-220 g) and Balb/c mice (18-22 g) were provided by the Animal Laboratory Center of Shenyang Pharmaceutical University. All of the animal experiments in this work were performed according to the Guidelines for the Care and Use of Laboratory Animal approved by the Ethics Committee of Animal Experimentation of Shenyang Pharmaceutical University. Statistical analysis of data was performed using GraphPad Prism 8. A difference of p<0.05 was considered to be statistically significant. The larotaxel self-emulsifying drug delivery systems was prepared as follows. 10 mg LTX and 0.5 g tricaprylin were dissolved in adequate methylene chloride under ultrasound. Then, methylene chloride was evaporated under reduced pressure at 30° for 1 h. After that, 0.1 g monoolein and 0.4 g Tween 80 were added and dissolved thoroughly. The residual methylene chloride was removed completely by vacuum evaporation. The mean particle size of LTX-SEDDS after emulsification was 115.4 nm with small polydispersity index of 0.197 (fig. 1A). The Zeta potential of LTX-SEDDS after emulsification was around -13.0 mV (fig. 1B), which is beneficial to prevent particles aggregate and maintain the stability of LTX-SEDDS. Furthermore, LTX-SEDDS could completely emulsified and form stable microemulsion in different media (including distilled water, simulated gastric fluid and simulated intestinal fluid) within 3 min. It is speculated that LTX-SEDDS has high adhesiveness in the gastrointestinal tract[22], which is good for the absorption of drugs. To investigate the intestinal retention effect of LTX-SEDDS, the bio-distribution of the gastrointestinal tract was studied by ex vivo imaging using DiR as near infrared probe. SEDDS was labeled with DiR via hydrophobic interaction and was prepared according to the preparation method of LTX-SEDDS by replacing LTX with DiR. DiR-Sol of 2 mg/ml was set as the control group. Then, DiR labeled SEDDS and DiR-Sol was administered to Balb/c mice orally at DiR dose of 2 mg/kg, respectively. At 0.5 h, 2 h, 4 h, and 6 h after administration, the mice were sacrificed to collect stomach and small intestines for ex vivo imaging using excitation wavelength of 740 nm and emission wavelength at 790 nm. As shown in fig. 2, fluorescence intensity of DiR-Sol rapidly attenuated with only weak DiR fluorescence observed in the end of the ileum and colon at 2 h after administration. While DiR-SEDDS showed stronger fluorescence intensity than DiR-Sol throughout all segments of the intestine and still exhibited strong fluorescence at 4 h after administration. The results revealed that SEDDS could adhere to the gastrointestinal tract and prolong residence time of cargos in the intestine, which is beneficial to improve oral absorption of larotaxel. It is reported that self-micro emulsion drug delivery system could promote lymphatic transport to deliver therapeutic agent into systemic circulation[23,24]. This leads to avoidance of first-pass metabolism and enhancement of oral bioavailability of taxanes[25,26]. We studied the lymphatic transport of LTX-SEDDS by using cycloheximide to inhibit the secretion of chylomicrons. Cycloheximide is a protein synthesis inhibitor and does not affect other absorption pathways except for lymphatic transport. Sprague- Dawley rats (180-220 g) were divided randomly into two groups of five animals each, which were fasted overnight and had free access to water. Group I was intraperitoneally injected with high dose of cycloheximide (3 mg/kg) to prevent the secretion of chylomicrons from the enterocyte. 1 h after intraperitoneal injection, all rats in Group I and Group II were given LTX-SEDDS (20 mg/kg) via oral gavage. Blood samples were collected from orbital vein at 15 min, 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, 12 h and 24 h post-administration into heparinized tubes. Plasma was separated and stored at -20° until Ultra Performance Liquid Chromatography- tandem Mass Spectrometry (UPLC-MS/MS). The typical pharmacokinetic parameters were calculated by DAS 2.0 software. As shown in fig. 3A and Table 1, after oral administration of LTX-SEDDS, a distinct absorption phase was observed with the plasma AUC 0-24 h of 892.429±279.785 μg/l.h. However, the absorption of LTX in the LTX-SEDDS group with intraperitoneal injection of cycloheximide (3 mg/kg) was significantly declined with the plasma AUC 0-24 h of 64.759±19.951 μg/l.h. These results indicated that LTX-SEDDS could promote oral absorption of LTX through lymphatic transport pathway, and oral absorption of LTX-SEDDS decreased significantly when lymphatic pathway was blocked. In order to systematically evaluate the oral bioavailability, the pharmacokinetic behaviors of LTX-SEDDS were assessed in SD rats, and LTX-Sol was set as control group. LTX-SEDDS and LTX-Sol both equivalent to 40 mg/kg of LTX were given separately to two groups of rats (n=4) by intragastric administration. About 0.3 ml blood samples were taken from orbital vein at predetermined time points of 15 min, 30 min,1 h, 2h, 4 h, 6 h, 8 h, 12 h and 24 h post- administration. Plasma samples were extracted with tert-Butyl methyl ether and the concentrations of drugs were determined by a validated UPLC-MS/MS method. The mean larotaxel plasma concentration- time profiles and main pharmacokinetic parameters are presented in fig. 3B and Table 2, respectively. AUC 0-24 h (1896.517±485.276 μg/l.h) of LTX- SEDDS was 3.4-fold higher than that of LTX-Sol and the relative bioavailability of LTX-SEDDS was 519.32 %. The results of pharmacokinetic study proved the advantageous of LTX loaded SEDDS in promoting intestinal absorption of hydrophobic LTX.
Fig 3: Results of pharmacokinetic studies; (A): Pharmacokinetic profiles of oral administration of 20 mg/kg LTX-SEDDS to non-treated and cycloheximide- treated rats (mean±SD, n=5) and (B): Pharmacokinetic profiles of oral LTX-Sol (40 mg/kg); oral LTX-SEDDS (40 mg/kg) (mean±SD, n=4); (A) (): with CHX; (): no CHX; (B) (): LTX-EDDS.po and (): LTX-Sol.po
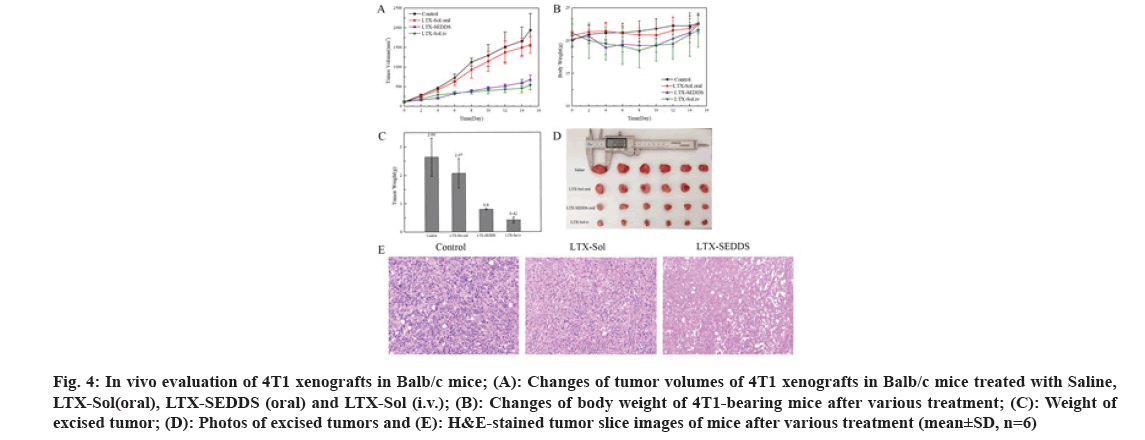
Fig 4: In vivo evaluation of 4T1 xenografts in Balb/c mice; (A): Changes of tumor volumes of 4T1 xenografts in Balb/c mice treated with Saline, LTX-Sol(oral), LTX-SEDDS (oral) and LTX-Sol (i.v.); (B): Changes of body weight of 4T1-bearing mice after various treatment; (C): Weight of excised tumor; (D): Photos of excised tumors and (E): H&E-stained tumor slice images of mice after various treatment (mean±SD, n=6)
| Parameters | LTX-SEDDS | LTX-SEDDS with cycloheximide |
|---|---|---|
| AUC (0-24) (μg/l.h) | 892.429±279.785 | 64.759±19.951 |
| AUC (0-∞) (μg/l.h) | 908.87±261.143 | 76.181±19.04 |
| Cmax (μg/l) | 143.682±59.898 | 13.805±5.5 |
| Tmax (h) | 3.4±1.949 | 3.25±1.5 |
| T1/2 z (h) | 3.808±2.34 | 2.628±1.035 |
| Vz(l/kg) | 302.872±277.135 | 2217.069±1348.061 |
| MRT(0-t) (h) | 6.243±0.879 | 3.838±0.478 |
Table 1: Pharmacokinetic Parameters of Oral Administration of 20 mg/kg LTX-SEDDS in Non-Treated Rats and Cycloheximide-Treated RATS, Mean±SD, n=5
| Parameters | LTX-SEDDS.po | LTX-Sol.po |
|---|---|---|
| AUC (0-24 h) (μg/l.h) | 1896.517±485.276 | 347.973±30.968 |
| Cmax (μg/l) | 409.725±73.499 | 119.14±24.376 |
| Tmax (h) | 2.5±1 | 1.25±0.5 |
| T1/2 z (h) | 3.154±0.522 | 1.731±0.418 |
| MRT(0-t) (h) | 4.668±0.765 | 2.639±0.163 |
| Frbs | 539.12 % | - |
Table 2: Pharmacokinetic Parameters of Oral LTX-SOL (40 mg/kg) and Oral LTX-SEDDS (40 mg/kg), Mean±SD, n=4
The significantly improved oral bioavailability might be ascribed to the following aspects. The excipients in SEDDS increased the solubility of LTX. SEDDS could circumvent P-glycoprotein mediated efflux to improve absorption of LTX in intestinal. SEDDS could effectively adhere to the intestinal, which was confirmed in the intestinal retention experiment. SEDDS could promote lymphatic transport to deliver LTX into systemic circulation and avoid first-pass hepatic metabolism to increase oral bioavailability. Inspired by the results of pharmacokinetics study, it could be speculated that oral LTX-SEDDS might possess superior in vivo antitumor ability. The 4T1- tumor-bearing mice were selected to investigate the in vivo anti-tumor potential of LTX-SEDDS. Twenty four 4T1-tumor-bearing mice were randomly divided into four groups(n=6), and then treated with Saline, intravenous injection of LTX-Sol (5 mg/kg), oral administration of LTX-Sol (20 mg/kg) and LTX- SEDDS (20 mg/kg), respectively. All the mice were treated on d 0, d 2, d 4, d 6 and d 8 and the tumor volume was measured every other day. As shown in fig. 4A, the tumor volume of the mice treated with Saline and oral administration of LTX-Sol grew quickly, demonstrating oral administration of LTX- Sol could not inhibit the growth of tumors.
By contrast, the oral LTX-SEDDS group presented remarkable inhibition efficacy toward tumor growth. At the end of the treatment, mice were sacrificed and tumors were dissected and weighted. Tumor weight and the photographs of the tumors were shown in fig. 4C and fig. 4D, respectively. Both oral LTX-SEDDS and intravenous LTX-Sol exhibited effective tumor inhibition. What is more, the Tumor Inhibition Rates (TIRs) of the oral LTX-SEDDS group was 69.69 %, which is 3.2-fold higher than that of oral LTX- Sol group with 21.59 %. Furthermore, histological examination of hematoxylin and eosin staining tumor tissue section indicated that the tumors treated with oral LTX-SEDDS exhibited obviously enhanced cell death compared with oral LTX-Sol (fig. 4E). In addition, oral LTX-SEDDS group exhibited slight body weight loss during treatment as intravenous injection LTX-Sol (fig. 4B), which was attributed to the systemic toxicity caused by the high bioavailability of LTX-SEDDS, and the body weight of mice returned to original level after the last dose. In conclusion, the LTX loaded SEDDS was successfully prepared, and it exhibited excellent in vivo performance in terms of oral delivery. SEDDS could prolong the residence time of LTX in the gastrointestinal tract and facilitate lymphatic transport compared with LTX-Sol. Notably, LTX-SEDDS exhibited a 5.19- fold higher oral bioavailability than that of LTX-Sol. It is worth noting that oral administration of LTX- SEDDS presented remarkable in vivo therapeutic efficacy with tumor inhibition rates of 69.69 %. LTX-SEDDS have good clinical application prospects for maintenance therapy of cancers due to the convenience of oral administration, which is beneficial to increase the compliance of patients and ease the pressure of medical institutions during the COVID-19 pandemic.
Conflict of interests:
The authors declared no conflict of interests.
References
- Chen W. Cancer statistics: Updated cancer burden in China. Chin J Cancer Res 2015;27(1):1.
[Crossref] [Google Scholar] [PubMed]
- Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol 2018;834:188-96.
[Crossref] [Google Scholar] [PubMed]
- Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol 2020;17(11):657-74.
[Crossref] [Google Scholar] [PubMed]
- Yu LY, Tang J, Zhang CM, Zeng WJ, Yan H, Li MP, et al. New immunotherapy strategies in breast cancer. Int J Environ Res Public Health 2017;14(1):68.
[Crossref] [Google Scholar] [PubMed]
- Lutfi W, Talamonti MS, Kantor O, Wang CH, Liederbach E, Stocker SJ, et al. Perioperative chemotherapy is associated with a survival advantage in early stage adenocarcinoma of the pancreatic head. Surgery 2016;160(3):714-24.
[Crossref] [Google Scholar] [PubMed]
- Ba-Sang DZ, Long ZW, Teng H, Zhao XP, Qiu J, Li MS. A network meta-analysis on the efficacy of sixteen targeted drugs in combination with chemotherapy for treatment of advanced/metastatic colorectal cancer. Oncotarget 2016;7(51):84468.
[Crossref] [Google Scholar] [PubMed]
- Yu XF, Wang C, Chen B, Liang CL, Chen DB, Yu Y, et al. The effect of adjuvant chemotherapy in male breast cancer: 134 cases from a retrospective study. ESMO Open 2017;2(2):e000134.
[Crossref] [Google Scholar] [PubMed]
- Hamel E. Antimitotic natural products and their interactions with tubulin. Med Res Rev 1996;16(2):207-31.
[Crossref] [Google Scholar] [PubMed]
- Woods CM, Zhu J, McQueney PA, Bollag D, Lazarides E. Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol Med 1995;1(5):506-26.
[Crossref] [Google Scholar] [PubMed]
- Kurata T, Shimada Y, Tamura T, Yamamoto N, Hyodo I, Saeki T, et al. Phase I and pharmacokinetic study of a new taxoid, RPR 109881A, given as a 1-hour intravenous infusion in patients with advanced solid tumors. J Clin Oncol 2000;18(17):3164-71.
[Crossref] [Google Scholar] [PubMed]
- Rowinsky, MD EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med 1997;48(1):353-74.
[Crossref] [Google Scholar] [PubMed]
- Gelmon KA, Latreille J, Tolcher A, Génier L, Fisher B, Forand D, et al. Phase I dose-finding study of a new taxane, RPR 109881A, administered as a one-hour intravenous infusion days 1 and 8 to patients with advanced solid tumors. J Clin Oncol 2000;18(24):4098-108.
[Crossref] [Google Scholar] [PubMed]
- Metzger-Filho O, Moulin C, de Azambuja E, Ahmad A. Larotaxel: broadening the road with new taxanes. Expert opinion on investigational drugs. 2009 Aug 1;18(8):1183-9.
[Crossref] [Google Scholar] [PubMed]
- Li X, Li J, Xu J, Chen K, Zhang Z, Duan J, et al. Nanostructure of functional larotaxel liposomes decorated with guanine‐rich quadruplex nucleotide–lipid derivative for treatment of resistant breast cancer. Small 2021;17(13):2007391.
[Crossref] [Google Scholar] [PubMed]
- Fox CB, Kim J, Le LV, Nemeth CL, Chirra HD, Desai TA. Micro/nanofabricated platforms for oral drug delivery. J Control Release 2015;219:431-44.
[Crossref] [Google Scholar] [PubMed]
- Flores JP, Saif MW. Novel oral taxane therapies: Recent Phase I results. Clin Investig 2013;3(4):333-41.
[Crossref] [Google Scholar] [PubMed]
- Schellens JH, Malingré MM, Kruijtzer CM, Bardelmeijer HA, van Tellingen O, Schinkel AH, et al. Modulation of oral bioavailability of anticancer drugs: From mouse to man. Eur J Pharm Sci 2000;12(2):103-10.
[Crossref] [Google Scholar] [PubMed]
- Rani S, Rana R, Saraogi GK, Kumar V, Gupta U. Self-emulsifying oral lipid drug delivery systems: advances and challenges. AAPS PharmSciTech 2019;20:129.
[Crossref] [Google Scholar] [PubMed]
- Mittal K, C Mashru R, R Thakkar A. Challenges in oral delivery: role of P-gp efflux pump. Curr Drug Ther 2014;9(1):47-55.
- Kamal MM, Nazzal S. Novel sulforaphane-enabled self-microemulsifying delivery systems (SFN-SMEDDS) of taxanes: Formulation development and in vitro cytotoxicity against breast cancer cells. Int J Pharm 2018;536(1):187-98.
[Crossref] [Google Scholar] [PubMed]
- Beg S, Kaur R, Khurana RK, Rana V, Sharma T, Singh B. QbD-based development of cationic self-nanoemulsifying drug delivery systems of paclitaxel with improved biopharmaceutical attributes. AAPS PharmSciTech 2019;20:1-3.
[Crossref] [Google Scholar] [PubMed]
- Hong JW, Lee IH, Kwak YH, Park YT, Sung HC, Kwon IC, et al. Efficacy and tissue distribution of DHP107, an oral paclitaxel formulation. Mol Cancer Ther 2007;6(12):3239-47.
[Crossref] [Google Scholar] [PubMed]
- Cui W, Zhang S, Zhao H, Luo C, Sun B, Li Z, et al. Formulating a single thioether-bridged oleate prodrug into a self-nanoemulsifying drug delivery system to facilitate oral absorption of docetaxel. Biomater Sci 2019;7(3):1117-31.
- Attili-Qadri S, Karra N, Nemirovski A, Schwob O, Talmon Y, Nassar T, et al. Oral delivery system prolongs blood circulation of docetaxel nanocapsules via lymphatic absorption. Proc Natl Acad Sci USA 2013;110(43):17498-503.
[Crossref] [Google Scholar] [PubMed]
- Meerum Terwogt JM, Malingré MM, Beijnen JH, ten Bokkel Huinink WW, Rosing H, Koopman FJ, et al. Coadministration of oral cyclosporin A enables oral therapy with paclitaxel. Clin Cancer Res 1999;5(11):3379-84.
[Google Scholar] [PubMed]
- Joerger M. Metabolism of the taxanes including nab-paclitaxel. Expert Opin Drug Metab Toxicol 2015;11(5):691-702.
[Crossref] [Google Scholar] [PubMed]