- *Corresponding Author:
- V.V. Nagathan
Department of Pharmaceutics, KLE College of Pharmacy, Hubballi, Karnataka 580031, India
E-mail: nagathanv@yahoo.com
| Date of Received | 24 October 2021 |
| Date of Revision | 14 May 2023 |
| Date of Acceptance | 04 October 2023 |
| Indian J Pharm Sci 2023;85(5):1436-1443 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of the present study was to develop film forming polymeric solution for the topical release of diclofenac sodium through the skin. A film forming polymeric solution of diclofenac sodium was prepared by using hydroxypropyl methylcellulose E5, hydroxypropyl methylcellulose E15 and hydroxypropyl methylcellulose E50 as a film forming polymer in different concentrations and dibutyl phthalate used as plasticizer. The prepared solution was tested for pH, viscosity, drying time, outward stickiness, thickness, weight uniformity, folding endurance, drug content uniformity followed by in vitro diffusion study. All the formulations showed the results within acceptable range for various tests. Formulations exhibited pH within the range of 5.7 to 6.1. Drying time of all the formulation ranged between 12±0.6 to 21±0.6 min. It was observed that formulation F1-F4 showed low outward stickiness, F5 and F6 showed medium, F7 to F9 showed high outward stickiness. The weight, thickness and folding endurance of the films were found to be consistent. Percentage cumulative drug release was found to be in the range of 72.05±0.4 to 93.35±13. Formulation F3 showed shortest drying time of 12 min, low outward stickiness, controlled drug release 88.14±2.46 % up to 6 h compared to the other formulations. Hence it was selected has optimized formulation. Kinetics study revealed that drug release from the polymeric solution followed non-Fickian. It can be concluded that topical film forming solution of diclofenac sodium formulated with hydroxypropyl methylcellulose E5, hydroxypropyl methylcellulose E15 and hydroxypropyl methylcellulose E50 as a film forming polymers, appears to be a promising option for the treatment of pain and inflammation.
Keywords
Diclofenac sodium, film-forming polymeric solution, hydroxypropyl methylcellulose, anti- inflammatory activity
Transdermal drug administration generally refers to topical application of agents to healthy intact skin either for localized treatment of tissues underlying the skin or for systemic therapy. It offers many advantages over conventional administration such as enhanced efficacy, increased safety and greater convenience and improved patient compliance[1]. Transdermal route permits the use of a relatively potent drug with minimal risk of system toxicity and avoids gastrointestinal degradation and hepatic first-pass metabolism. A good number of therapeutic agents, including anti-hypertensive, anti-anginal, anti-histaminic, anti-inflammatory, analgesic, antibiotic and anti-arthritic drugs, are being investigated and developed for the transdermal therapeutic system, all of which make it as a versatile platform for drug delivery[2].
The current dosage forms, i.e. patches, ointments, creams, etc., are associated with several limitations. Patches have various disadvantages, most commonly skin irritation, because of their occlusive properties causing obstruction of sweat ducts, which in turn prevents loss of water vapour from skin surface, difficulty in applying on the curved surfaces, pain while peeling off and poor aesthetic appeal. Semisolid preparations like creams and ointments overcome some of these drawbacks but have other limitations. These do not ensure persistent contact with the skin surface and can be easily wiped off by patient's clothes. Hence repeated application is required in case of chronic diseases like athlete's foot, ringworm and candidiasis. Also, these leave a sticky and greasy feel after application leading to poor patient compliance. Therefore, there is a need for development of a dosage form which permits less frequent dosing by maintaining a close contact with the skin for prolonged time period thereby improving the patient compliance.
Film Forming System (FFS) is a novel approach which can be used as an alternative to conventional topical and transdermal formulations. It is defined as non-solid dosage form that produces a film in situ, i.e., after application on the skin or any other body surface. Therefore, to overcome the drawbacks of the semisolid preparations and transdermal patches, the novel topical film forming solution has been developed.
The thin films can be perceived to be less obstructive and more acceptable by the patient[3]. Thin films may be useful for eliminating side effects of a drug and reducing extensive metabolism caused by proteolytic enzymes compared with existing traditional dosage forms, it stands to be superior in terms of enhanced bioavailability and high patient compliance[4].
The diclofenac sodium undergoes substantial hepatic first pass metabolism and only about 50 % of administered dose reaches to systemic circulation and its biological half-life is also very short[5]. Hence the objective of the present work is to develop topical film forming solution of diclofenac sodium to increase the patient compliance and bioavailability.
Materials and Methods
Materials:
Diclofenac sodium was obtained as gift sample from Shri Bhavani Pharmaceuticals, Hubballi. Hydroxypropyl Methylcellulose (HPMC) E5 LV, HPMC E15 LV, and HPMC E50 LV were purchased from Yarrow Chem. Product Ltd. Mumbai. Dibutyl phthalate and ethanol were procured from SD Fine Chem. Ltd. Mumbai, India.
Preformulation studies:
Determination of melting point of diclofenac sodium: Melting point of diclofenac sodium was
determined by digital melting point apparatus. In this method the capillary tube was sealed with gentle heating from one end, then the small quantity of pure drug diclofenac sodium was filled into the sealed capillary then capillary tube was placed in the melting point apparatus and temperature range at which the drug starts melting was noted[6].
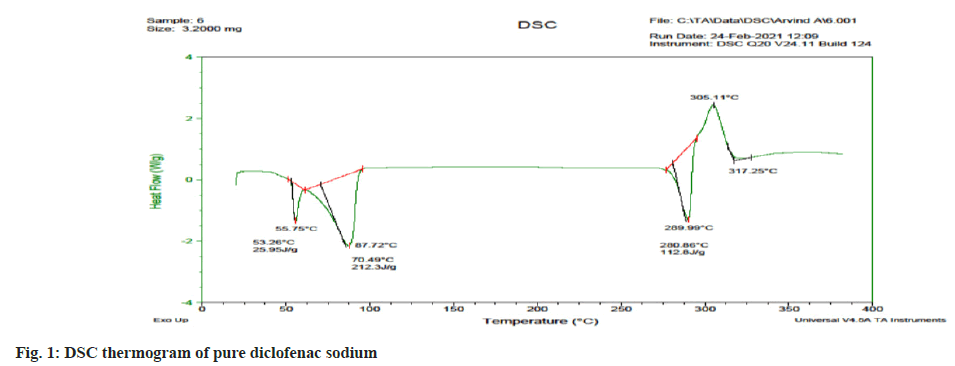
Differential Scanning Calorimetry (DSC): The DSC is performed to check the drug’s thermal behavior and its melting point. DSC was performed by using DSC Shimadzu-60. The samples were placed in aluminum pans and were crimped, followed by heating under nitrogen flow at a scanning rate of 10°/min from 50- 200°. Empty pan was used as reference. The heat flow as a function of temperature was measured[6].
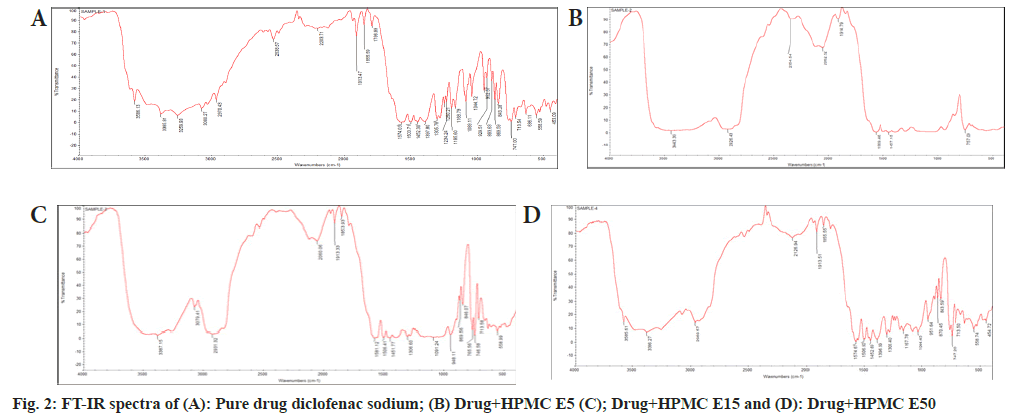
Fourier Transform Infrared (FT-IR) spectroscopy: The drug-excipients interaction studies were carried out to check the physical and chemical interaction of materials that used in the formulation. The drug excipients interaction was studied by FT- IR spectroscopy (Shimadzu FT-IR) by KBr pellet method. Sample for analysis and KBr were taken in 1:100 ratio and ground in motor for even distribution of sample in KBr. The pellet was prepared in the form of disk by applying pressure of 5 tons for 5 min using hydraulic press and subjected to FT- IR. The wave number range of 4000-400 cm-1[7].
Analytical method:
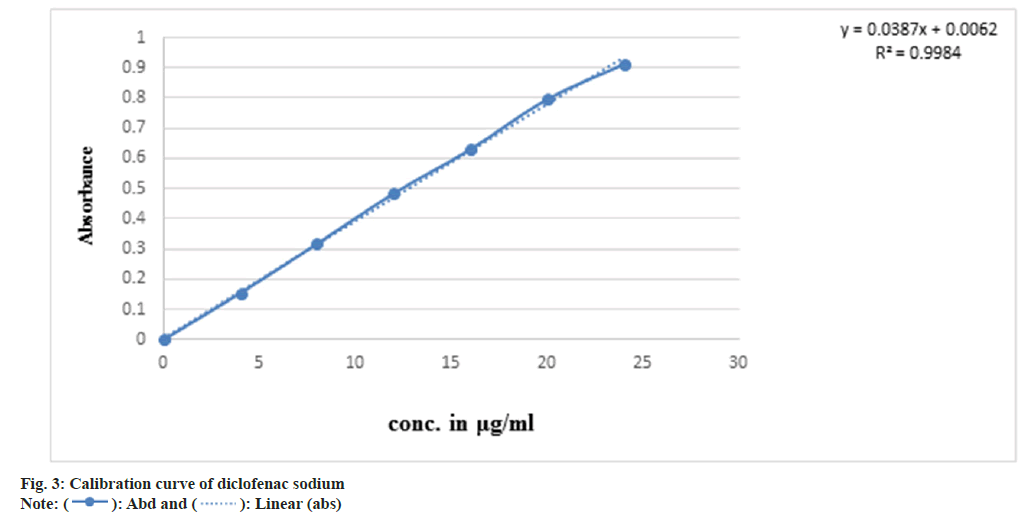
Determination of absorption maximum (λmax) of diclofenac sodium: A standard stock of diclofenac sodium was prepared by dissolving accurately weighed 10 mg of diclofenac sodium in 100 ml volumetric flask and the volume was made up to 100 ml with phosphate buffer pH 6.8 to obtain concentration of 100 µg/ml. The resulting solution was scanned between 200-400 nm using Ultra-Violet (UV) spectrophotometer to get absorption maxima (λmax)[8].
Calibration curve of diclofenac sodium in phosphate buffer: From the standard stock solution, 0.4 ml, 0.8 ml, 1.2 ml, 1.6 ml, 2 ml, 2.4 ml was pipetted out and transfer into 10 ml of volumetric flasks and volume was made up to the mark by using phosphate buffer in 10 ml of volumetric flasks with a beers range of 2-40 µg/ml to get the final concentration of 4, 8, 12, 16, 20 and 24 µg/ml. these dilutions were analyzed for absorbance at 276 nm in UV spectrophotometer and calibration curve was plotted against concentration vs. absorbance[8].
Preparation of film forming polymeric solutions: The film forming polymeric solutions of HPMC E5, HPMC E15 and HPMC E50 were prepared in ethanol using dispersion method. HPMC E5, HPMC E15 and HPMC E50 were sprinkled over of ethanol and solutions were allowed to stir to produce clear solutions. Accurately weighed quantity (10 mg) of diclofenac sodium was dissolved in the above polymeric solution containing dibutylphthalate as plasticizer. The drug polymeric dispersion was mixed properly with continuous stirring and volume was made up to the mark using ethanol[9]. The compositions of all the formulations are presented in the Table 1.
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Diclofenac sodium (mg) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| HPMC E5 LV (mg) | 300 | 400 | 500 | - | - | - | - | - | - |
| HPMC E15 LV (mg) | - | - | - | 300 | 400 | 500 | - | - | - |
| HPMC E50 LV (mg) | - | - | - | - | - | - | 300 | 400 | 500 |
| Dibutyl phthalate (ml) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Ethanol (95 %) up to (ml) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
Table 1: Formulation Design of Film Forming Polymeric Solution
Evaluation parameters:
pH: The pH of the prepared film forming solutions was measured at room temperature using digital pH meter. Ideally, topical film forming solutions should possess pH in the range of 5-6, so as to minimize discomfort or irritation due to acidic pH and microbial growth due to basic pH[10].
Viscosity: The viscosity of the solution was evaluated visually and rated as low (water-like), medium (glycerol-like) or high (syrup-like)[11].
Drying time: Formulations were cast onto a plane slide to determine drying time. After a set time span, another glass slide was mounted on to the film without pressure. If no remains of liquid are visible on the glass slide after removal, the film is considered dry. If remains of liquid are visible on the glass slide the experiment is repeated with a rise in drying time[12].
Outward stickiness: Cotton wool was pressed onto the dried film under low pressure to assess the stickiness of the outer surface. Depending on the quantity of cotton fibers that are retained by the film the stickiness is rated as high (dense accumulation of fibers on the film), medium (thin fiber layer on the film) or low (occasional or no adherence of fibers)[12].
Physical appearance: All the films were visually inspected for color, clarity, flexibility and smoothness[11,13,14].
Thickness: At five different places, the thickness of the film was measured using a digital micrometer screw gauge. For each batch of the drug loaded film, the average standard deviations of five readings were calculated[15].
Weight uniformity: Each batch of the films was randomly selected, and 1 cm2 films was cut at five different places in the cast film and weighed separately on an electronic balance. Mean weight of each film was recorded[16].
Folding endurance: The folding endurance of the film was determined by repeatedly folding a small strip of the film (about 2×2 cm) at the same location until it broke. The number of times film could be folded at the same place, without breaking gives the value of folding endurance[17].
Drug content uniformity: The films of known weight (dimension 1×1 cm2) were dissolved in small quantity of ethanol. The solution was suitably diluted and absorbance was measured at 276 nm using UV-spectrophotometer[14].
In vitro drug release study (diffusion study): Franz diffusion cell was used to determine the release profile of the drug from the film forming system. The cell was made up of two compartments, the donor and the receiver compartment between which the diffusion membrane was placed which was previously soaked in phosphate buffer pH 7.4 for 24 h. The donor compartment was exposed to the atmosphere and the receptor compartment contains the diffusion medium. Phosphate buffer pH 7.4 was utilized as the diffusion medium. The sampling arm in the receptor compartment allows for sampling. The assembly was kept at 37±0.5° and constantly stirred at 50 rpm. About 0.5 ml of the drug containing film forming formulation was placed on the donor compartment.
About 1 ml of sample was taken at predetermined intervals and drug content was estimated using a UV spectrophotometer. After withdrawal of each sample, the receptor compartment was replaced with an equivalent amount of phosphate buffer[18].
In vitro release kinetics: To examine the drug release kinetics, the release data of all the formulations were fitted to different models such as zero order, first order, Higuchi’s square root of time kinetics and Korsemeyer Peppas. (R2) values were calculated from the plots of % Cumulative Drug Release (CDR) vs. t for zero order, log % CDR remaining vs. t for first order and % CDR vs. t1/2 for Higuchi model, where % CDR is the amount of drug released at time t, log % CDR is the amount of drug remaining after time t. The best fit kinetic model was determined from R2 values[19].
Results and Discussion
The melting point of diclofenac sodium was found to be 289.86° which is within the reported value of 279° to 289°, indicating the purity of drug sample. In DSC the pure drug diclofenac sodium showed endothermic peak at 289.99° which is shown in fig. 1. The peak value matches with literature value this confirms the purity in the drug sample. The compatibility of diclofenac sodium with polymer was tested using FT-IR spectroscopy. The individual drug and drug with polymers were scanned separately. Both the spectra were compared for confirmation of common peaks. Diclofenac sodium with polymers showed no significant difference in height, intensity and position of peaks, when compared with pure drug peak, suggesting that drug and excipients were compatible. FT-IR spectra of drug and polymer mixture are illustrated in fig. 2. From the UV scanning of the drug, it was seen that the drug has absorption maxima (λmax) of 276 nm.The calibration curve obtained for diclofenac sodium showed good linearity with regression coefficient (R2) value 0.998. The graph shows that absorbance value increased with increase in the concentration. Thus, the standard calibration curve obeys the Beer-Lambert’s Law and calibration curve is depicted in fig. 3.
The pH of all formulations ranged between 5.7±0.6 and 6.1±0.6 and the results are shown in the Table 2, which is in accordance with the pH of the skin indicating compatibility with the skin’s natural pH. The viscosity of all the formulations were inspected visually and it is shown in Table 2. The HPMC E5 based formulations showed low viscosity and the HPMC E15 based formulations showed medium viscosity and the HPMC E50 based formulations showed high viscosity. Drying time of formulations varied from 12 to 21 min. With an increase in concentration of polymer, increase in drying was observed. The batch F1 showed the lowest drying time i.e. 12 min and formulation F9 showed highest drying time i.e. 21 min. The variation in the drying time due to the viscosity of the polymers. The results of drying time all formulations are represented in Table 2. Results from outward stickiness clears that the films prepared with HPMC E50 had high outward stickiness; this is due to high viscosity of the polymer. Whereas HPMC E5 had low outward stickiness and this is due to low viscosity of the polymer. Results of the outward stickiness of all formulations are represented in Table 2.
| Formulation code | pH mean±SD | Viscosity | Drying time (min) mean±SD | Outward stickiness |
|---|---|---|---|---|
| F1 | 5.8±0.3 | Low | 12±0.070 | Low |
| F2 | 6.0±0.2 | Low | 13±0.072 | Low |
| F3 | 5.9±0.4 | Low | 14±0.075 | Low |
| F4 | 5.7±0.6 | Medium | 15±0.072 | Low |
| F5 | 5.8±0.5 | Medium | 16±0.075 | Medium |
| F6 | 5.9±0.4 | Medium | 17±0.077 | Medium |
| F7 | 6.1±0.6 | High | 18±0.072 | High |
| F8 | 6.0±0.3 | High | 19±0.077 | High |
| F9 | 5.8±0.5 | High | 21±0.079 | High |
Note: Standard deviation (±), each value is the mean of three observations
Table 2: Results for ph, Viscosity, Drying Time, Outward Stickiness of F1 To F9 Formulations
Films of all the formulations were transparent, having good and visually smooth surface. The drug and polymer distribution were uniform. The mean value of thickness for HPMC E5 films varied from 0.034±0.025 to 0.051±0.046 mm, HPMC E15 films varied from 0.042±0.010 to 0.042±0.048 mm and HPMC E50 films varied from 0.045±0.039 to 0.071±0.065 mm respectively. All formulations prepared by the solvent evaporation method showed uniform thickness with low standard deviation values. Thickness of the films was increased with increase in polymer concentration. Thickness of all the films is shown in Table 3. The mean value of weight uniformity for HPMC E5 films varied from 0.037±0.0063 to 0.051±0.0046 mg, HPMC E15 films varied from 0.042±0.0048 to 0.063±0.0087 mg and HPMC E50 films varied from 0.054±0.0039 to 0.071±0.0065 mg respectively. The weight of film was increased with increase in polymer concentration. Weight uniformity values are shown in Table 3. The folding endurance HPMC E5 films varied from 51±2.34 to 61±1.46, HPMC E15 films varied from 53±1.78 to 50±2.44 and HPMC E50 52±1.68 to 46±1.46 respectively. The results showed that the folding endurance was found to be satisfactory. Folding endurance of all films is shown in Table 3. In order to make sure about the uniform dispersion of drug in the films, content uniformity test was carried out. The drug content for all formulations is found to be within the range of 95.26±0.12 % to 98.37±0.62 % as shown in Table 3. This indicates that the formed film had a uniform distribution of drug. From the in vitro drug diffusion study, it was observed that the drug diffusion was found to be faster from films containing HPMC E5 as a polymer than HPMC E15 based films, whereas the diffusion of drug was not proper from HPMC E50 films because of formation of fakes in the films. The data revealed that the drug release rate was greatly influenced by the film former. The drug release was found to be in the following order; F1>F2>F3.
| Formulation code | Thickness mean±SD | Weight uniformity mean±SD | Folding endurance mean±SD | Drug content uniformity mean±SD |
|---|---|---|---|---|
| F1 | 0.034±0.025 | 0.037±0.0063 | 55±2.34 | 95.26±0.12 % |
| F2 | 0.040±0.032 | 0.045±0.0075 | 58±1.34 | 96.45±0.27 % |
| F3 | 0.052±0.076 | 0.051±0.0046 | 61±1.46 | 98.37±0.62 % |
| F4 | 0.041±0.087 | 0.042±0.0048 | 53±1.78 | 97.12±0.57 % |
| F5 | 0.053±0.035 | 0.059±0.0063 | 51±2.65 | 96.76±0.21 % |
| F6 | 0.060±0.066 | 0.063±0.0087 | 50±2.44 | 94.49±0.74 % |
| F7 | 0.045±0.083 | 0.054±0.0039 | 52±1.68 | 96.85±0.58 % |
| F8 | 0.052±0.024 | 0.067±0.0053 | 48±2.26 | 95.63±0.63 % |
| F9 | 0.063±0.081 | 0.071±0.0065 | 46±1.46 | 97.75±0.18 % |
Note: Standard deviation (±), each value is the mean of three observations
Table 3: Results for Thickness, Weight Uniformity, Folding Endurance, Drug Content Uniformity of Polymeric Films F1 to F9
Among the three formulations (F1, F2 and F3) formulation F3 was found to be the best formulation in terms of drug content, drying time and in vitro drug release. Hence F1 was selected as optimized formulation. The values of the diffusion study are shown in Table 4. From the kinetic modeling of drug release it was found that the formulations showed zero order release kinetics. This suggests that the release rate was independent of the concentration of dissolved species. Korsemeyere Peppas equation indicates that the mechanism of drug release from the polymeric solution followed non-Fickian. The results of the release kinetic study of all formulation are presented in Table 5.
| Cumulative Drug Release (%) (± SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 20.51±0.75 | 15.66±0.152 | 14.53±0.216 | 17.11±1.489 | 16.79±1.426 | 14.53±2.14 | 11.30±2.741 | 10.31±0.987 | 8.33±2.751 |
| 60 | 37.24±0.92 | 28.52± 0.214 | 26.64±0.126 | 30.32±2.158 | 25.71±2.325 | 2.80±2.18 | 22.44±2.85 | 29.68±2.165 | 24.58±3.156 |
| 120 | 50.19±0.152 | 42.44± 0.123 | 39.30±0.256 | 44.12±0.156 | 41.79±2.214 | 39.43±2.46 | 33.49±3.789 | 43.95±3.124 | 37.79±1.489 |
| 180 | 64.05± 0.65 | 56.46±0.246 | 52.48±0.217 | 58.46±0.246 | 55.03±1.365 | 52.45±2.96 | 46.89±2.456 | 54.97±2.167 | 48.86±2.126 |
| 240 | 78.35±0.71 | 70.89±0.95 | 66.50±1.25 | 72.67±0.245 | 69.57±2.146 | 65.85±1.25 | 59.98±1.269 | 68.70±2.158 | 63.11±0.124 |
| 300 | 84.98±126 | 86.04±0.185 | 79.68±2.53 | 83.65±324 | 81.68±1.32 | 80.78±3.124 | 73.35±0.125 | 77.16±2.156 | 77.45±0.149 |
| 360 | 93.35±136 | 91.63±0.251 | 88.14±2.46 | 85.08±2.452 | 82.28±2.45 | 80.79±2.78 | 77.89±2.216 | 75.27±1.452 | 72.05±0.425 |
Note: Standard deviation (±), each value is the mean of three observations
Table 4: In Vitro Drug Release Studies of F1-F9 formulations
| Formulation | Zero Order | First Order | Higuchi | Korsmeyers-Peppas | |
|---|---|---|---|---|---|
| Code | (R2) | (R2) | (R2) | N | (R2) |
| F-1 | 0.995 | 0.893 | 0.974 | 0.76 | 0.99 |
| F-2 | 0.993 | 0.931 | 0.968 | 0.75 | 0.995 |
| F-3 | 0.99 | 0.942 | 0.957 | 0.75 | 0.997 |
| F-4 | 0.989 | 0.905 | 0.962 | 0.77 | 0.999 |
| F-5 | 0.986 | 0.908 | 0.958 | 0.75 | 0.997 |
| F-6 | 0.984 | 0.985 | 0.982 | 0.75 | 0.998 |
| F-7 | 0.979 | 0.955 | 0.972 | 0.75 | 0.998 |
| F-8 | 0.982 | 0.926 | 0.971 | 0.76 | 0.998 |
| F-9 | 0.981 | 0.906 | 0.974 | 0.76 | 0.998 |
Table 5: Kinetic Data of Various Models for Release Study
A film forming polymeric solution of diclofenac sodium was prepared. Among all formulations F1 showed shortest drying time and low outward stickiness and better drug release up to 6 h. Therefore, it can be chosen as an optimized formulation. The prepared film forming solution was effective, improve the patient compliance. Hence this novel dosage form will improve the bioavailability and can be alternative to conventional topical application.
Acknowledgements:
The authors are grateful to Shri Bhavani Pharmaceuticals Hubballi Karnataka for providing gift sample of drug for carrying out project work.
Conflict of interests:
The authors declare that they have no competing interest.
References
- Jain A, Goswami RB, Goswami N. Formulation and evaluation of anti-dandruff shampoo of fluconazole and ketoconazole. World J Pharm Res 2021;2(5):1685-703.
- Nimase SA, Patil PB, Saudagar RB. A recent review on film forming topical formulation. J Drug Deliv Ther 2019;9(3-s):1041-5. [Crossref] [Google Scholar] [PubMed]
- Kathe K, Kathpalia H. Film forming systems for topical and transdermal drug delivery. Asian J Pharm Sci 2017;12(6):487-97.
[Crossref] [Google Scholar] [PubMed]
- Umar AK, Butarbutar M, Sriwidodo S, Wathoni N. Film-forming sprays for topical drug delivery. Drug Des Dev Ther 2020:2909-25.
[Crossref] [Google Scholar] [PubMed]
- Zawar LR, Bhandari GS, Bari SB. Formulation and evaluation of transdermal films of Lovastatin. Res J Pharm Biol Chem Sci 2011;2(4):575.
- Giordano F, Rossi A, Pasquali I, Bettini R, Frigo E, Gazzaniga A, et al. Thermal degradation and melting point determination of diclofenac. J Therm Anal Calorim 2003;73(2):509-18.
- Siozou E, Sakkas V, Kourkoumelis N. Quantification and classification of diclofenac sodium content in dispersed commercially available tablets by attenuated total reflection infrared spectroscopy and multivariate data analysis. Pharmaceuticals 2021;14(5):440.
[Crossref] [Google Scholar] [PubMed]
- Talele S, Nikam P, Ghosh B, Deore C, Jaybhave A, Jadhav A. A research article on nanogel as topical promising drug delivery for diclofenac sodium. Indian J Pharm Edu Res 2017;51(4S):S580-587.
- Ammar HO, Ghorab M, Mahmoud AA, Makram TS, Ghoneim AM. Rapid pain relief using transdermal film forming polymeric solution of ketorolac. Pharm Dev Technol 2013;18(5):1005-16.
[Crossref] [Google Scholar] [PubMed]
- A Rajab N. Preparation and evaluation of ketoprofen as dermal spray film. Karbala J Pharm Sci 2013;4(6):1-8.
- Schroeder IZ, Franke P, Schaefer UF, Lehr CM. Development and characterization of film forming polymeric solutions for skin drug delivery. Eur J Pharm Biopharm 2007;65(1):111-21.
[Crossref] [Google Scholar] [PubMed]
- Harak PD, Zalte AG, Gulecha VS. Formulation and evaluation of film forming solution of tavaborole for treatment of skin infections. Res J Pharm Technol 2023;16(3):1342-6.
- Shaharpitkumar P, Patel MR, Patel KR, Patel NM. Formulation and evaluation of fast dispersible tablet of fexofenadine hydrochloride. Int J Pharm Res Bio Sci 2012:7(3):238-267.
- Pandya SJ, Pasha TY, Bhandari A, Patel JK, Trivedi N, Trivedi U. Design and optimization of taste masked fexofenadine hydrochloride resinate by ion exchange resin. Int J Drug Formul Res 2011;2:134-47.
- Panchaxari DM, Pampana S, Pal T, Devabhaktuni B, Aravapalli AK. Design and characterization of diclofenac diethylamine transdermal patch using silicone and acrylic adhesives combination. Daru J Pharma Sci 2013;21:1-4.
[Crossref] [Google Scholar] [PubMed]
- Uppala A, Swapna NV, Devi NR, Sheren GG, Kumar KB. Formulation and evaluation of mucoadhesive buccal films of diclofenac potassium. Res J Pharm Technol 2015;8(9):1269-75.
- Dey BK, Kar PK, Nath LK. Formulation design, preparation and in vitro–in vivo evaluation of propranolol hydrochloride transdermal patches using hydrophilic and hydrophobic polymer complex. Res J Pharm Technol 2009;2(1):155-60.
- Kathe K, Kathpalia H. Film forming systems for topical and transdermal drug delivery. Asian J Pharma Sci 2017;12(6):487-97.
[Crossref] [Google Scholar] [PubMed]
- Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm 2010;67(3):217-23.
[Google Scholar] [PubMed]
 : Abd and
: Abd and  : Linear (abs)
: Linear (abs)