- *Corresponding Author:
- A. N. Sahu
Department of Pharmaceutical Engineering and Technology,
Phytomedicine Research Lab,
Indian Institute of Technology (Banaras Hindu University),
Varanasi,
Uttar Pradesh 221005,
India
E-mail: ansahu.phe@iitbhu.ac.in
| Date of Received | 12 December 2020 |
| Date of Revision | 20 September 2021 |
| Date of Acceptance | 08 July 2022 |
| Indian J Pharm Sci 2022;84(4):848-862 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Plant based therapeutics have been extensively used for wound healing due to their faster healing rate and lesser side effects. The combinatorial approach of traditional concept with modern hydrogel-based drug delivery system can able to heal the wound in an accelerated manner. The present study aimed to formulate and evaluate the wound healing potential of hydrogel containing standardized ethanolic extract of Lawsonia inermis Linn. Ethanolic extract of leaves was prepared, standardized with respect to lawsone by validated high-performance thin-layer chromatographic method. The hydrogel formulations were prepared using Carbopol 934 in different concentrations (0.5, 1.0 and 2.0 % w/w), assessed for cosmetic persona, pH, viscosity, spreadability, occlusion, syneresis, drug content, drug-excipient compatibility, skin irritation and for its wound healing activity. The hydrogel formulations (F1, F2 and F3) were found to be clear, homogeneous, compatible, nonirritant in nature, with pH (6.73±0.047 to 6.8±0.082), spreadability (6.81±0.09 to 8.13±0.08 cm), viscosities (39840±500.07 to 87538±389.87 cp), occlusion factor values (57.43±1.24 to 73.82±2.48 %), percent syneresis (2.696±0.368 to 5.5±0.226), and percent drug content (94.271±0.292 to 96.587±0.402) were found to be optimal for topical application. Hydrogel (F2) exhibited a significant healing response in the excision wound rat model. Wound healing was significantly improved among the test groups on the 4th, 8th and 12th d (p<0.001) in comparison to standard lawsone hydrogel and control group. The result showed that the studied hydrogel has the potential for wound dressing.
Keywords
Hydrogel, Lawsonia inermis, lawsone, high-performance thin-layer chromatography, wound healing
Wound healing is a complex and dynamic tissue regeneration process that repairs injured skin and other soft tissues. Usually, it involves three temporally overlapping phases inflammation, proliferation and remodeling[1]. The continuity of these three phases at a particular time for a specific duration at an optimum intensity in proper sequence is a bio-physiological function[2]. After an injury, an inflammatory response occurs and the cells under the dermis (the deepest layer of skin) begin to increase the production of collagen (connective tissue), later, the epithelial tissue (the outer layer of the skin) is regenerated[3].
Plants and their extracts have great potential in the administration and treatment of wounds. For fewer side effects and a quick healing rate, some herbal medicines have been widely used for the treatment of wounds since ancient times[4]. Lawsonia inermis (L. inermis) Lythraceae family, commonly known as henna or mehendi in Hindi. The leaves of L. inermis contain phenolic compounds such as coumarin, flavonoids, naphthalene, naphthoquinones (lawsone, 2-hydroxy- 1,4-naphthoquinone), lignans, alkylphenones; non- volatile terpenes such as lupeol, botulin, betulinic acid and 30-norlupan-3β-ol-20-one; oleamide; trace elements such as Ca, Na, Mg, P, K, and Se; fat; resin; gallic acid and mucilage[5,6]. The color matter is assigned to the quinone, lawsone, accountable for the dyeing principle of the plant and also related to many of the pharmacological activities. The plant has long been used for medicinal and cosmetic purposes, which are extremely connected to the old and modern cultures of Asia and North Africa[5]. Besides being used for personal adornment, Lawsonia played a crucial role in holistic medicine for some ancient cultures, offering medicinal and psychological benefits[5]. The folk remedy of Henna includes treatment of jaundice and digestive disorders, leprosy, headache, diabetic foot disorders, ulcers, applied to feet and hands to give protection against fungal microorganisms and to hair to rid lice and dandruff. Numerous pharmacological benefits have been reported for Lawsonia, including anthelmintic, anticancer, antitrypanosomal, hypotensive, sedative, astringent, anti-hemorrhagic activities as well as immune-modulatory, hepatoprotective, antioxidant, UV-protective, antifungal, virucidal, antiparasitic, anti-inflammatory, analgesic, wound healing and antimicrobial activity[3-5]. The wound healing activity of this miracle plant was most thoroughly investigated[3,4,7].
Hydrogel has a great advantage for delivering herbal medicines as it has high biocompatibility and compatibility with herbal components, shows negligible toxicity, tunable release sustainability, can load ingredients with various hydrophilicity and deliver multiple herbal components[8]. In addition, hydrogels can absorb moisture from an exudating wound and donate moisture to dehydrated tissue[9]. Hydrogels provide a moist atmosphere for wound healing which are non-toxic, non-adherent and non- particulate, smooth and flexible, high water content and well oxygenated. These properties are essential for providing a pain-free environment to the wound[9,10]. It can be described physically as a swellable three- dimensional hydrophilic polymers network that can absorb and retain a substantial amount of water when immersed in water or biological fluids, without dissolution[11]. In hydrogel, the polymeric structures are held together by ionic forces, hydrogen bonds, primary covalent cross-linking, affinity or “bio-recognition” interactions, hydrophobic interactions, polymer crystallites, physical entanglements of single polymer chains, or a combination of two or more of the above interactions[12]. Polymers are the major constituent of hydrogels, which can be natural or synthetic in origin. The promising potential of hydrogels has been well exploited for delivering herbal medicines in diverse areas, from product development to treatment of disease[8]. Furthermore, the use of hydrogels as a wound management product containing herbal extracts has been investigated. These include astragaloside IV-loaded nanoparticle-enriched hydrogel[13], ethyl acetate Salix alba leaves extract-loaded chitosan-based hydrogel film[14], topical application of an alcoholic extract of Terminalia arjuna bark in the form of a hydrogel[15], novel alginate-based hydrogel films containing Aloe vera[11] and asiaticoside-loaded alginate films capable of forming into hydrogel[16].
The present study aimed to formulate and evaluate standardized ethanolic extract of L. inermis loaded hydrogel for wound dressing application. The hydrogels were developed for application in soft tissues, in particular, topical skin applications and characterized through the evaluation of several parameters, such as cosmetic persona, pH, viscosity, spreadability, occlusion, syneresis, drug content, drug- excipient compatibility, skin irritation and also for its wound healing activity. In this work, the hydrogels were prepared using Carbopol 934 as a gallant due to its high-water absorption capacity, better gel consistency and good dispersion ability over natural hydrogel[17]. The formulated hydrogel allows the incorporation of standardized ethanolic extract of L. inermis, which are released into the wound to promote the healing process. The results showed a potential use of the formulated hydrogel by accelerating the wound healing process.
Materials and Methods
Chemicals:
Standard lawsone and triethanolamine was purchased from Sigma Aldrich (Sigma Aldrich, Germany). Ethanol (99 %) was obtained from Merck, Germany. Carbopol 934 was obtained from Lubrizol Corporation (Wickliff, OH, USA). Propylene Glycol (PG) was purchased from Sisco Research Laboratory (SRL). The pre-coated silica gel plates (Merck 60 F254 0.25 mm) were used in High- Performance Thin-Layer Chromatography (HPTLC) analysis. Millipore water was used throughout the experiment.
Plant material:
The leaf of the L. inermis was collected from the premises of the Department of Pharmaceutical Engineering and Technology, Indian Institute of Technology (IIT) (Banaras Hindu University (BHU)). The plant material was identified and authenticated by Prof. N. K. Dubey, Department of Botany, Faculty of Science, BHU, Varanasi, India. The voucher specimen was deposited in the herbarium of the Department of Botany, Faculty of Science, BHU, under the number Lythra 2017/1.
Preparation of extract:
The collected fresh leaves of henna were rinsed thoroughly with clean water and shaded dried at room temperature to avoid direct sunlight from preventing degradation of its phytoconstituents. After drying, leaves were coarsely powdered using a crude drug disintegrator and kept in a well-closed container. About 100 g of coarse powder of Henna was dispersed in 400 ml of ethanol and left for 48 hours at room temperature. The solution was filtered through a fine muslin cloth followed by filtration through Whatman 41 filter paper. The filtrate was concentrated in a rotary vacuum evaporator (IKA, RV 10 digital) at 45° with 60 RPM until complete removal of the solvent occurred. The dried extract was stored in a refrigerator until usage.
High-Performance Thin Layer Chromatography (HPTLC)
Preparation of standard solution and calibration plot:
A stock solution of lawsone (1000 μg/ml) was prepared by dissolving 10 mg of accurately weighed lawsone in 10 ml of ethanol. 1 ml of stock solution was diluted with 9 ml of ethanol to get the primary standard solution (100 μg/ml). The solution was kept protected from light in a refrigerator until further use. The calibration curve was constructed as per International Council for Harmonisation (ICH) guidelines. Six different volumes of the primary standard solutions were applied on 20×10 Thin Layer Chromatography (TLC) plate (TLC aluminium sheets silica gel 60 F 254, Merck, Darmstadt, Germany) to obtain the final range of 0.2 µg–1.2 µg/spot. The sample was applied (8 mm band length) by Linomat V sample applicator at an initial distance of 15 mm from the left side and 8 mm from the bottom of the plate. The dosage speed was 100 nl/s and the bands were developed in a twin trough glass chamber (CAMAG, 20×10 cm) up 80 mm distance using 20 ml of toluene-ethyl acetate-glacial acetic acid (16:2:2 v/v) as mobile phase. Deuterium lamp was used as sources of radiation. The slit dimension was kept at 6.00×0.45 mm micro and the scanning speed employed was 20 mm/s. After chromatographic development, the plate was dried by a hair drier and zones were scanned by a scanner (CAMAG TLC Scanner 4) equipped with WinCATS software (version 1.4.7.2018) in absorbance measurement mode at λmax 334 nm[18,19]. The calibration curve was prepared by plotting the peak area in y-axis against the amount of lawsone in µg/spot in x-axis. Three repetitive measurements were taken and the average of the area under the curve vs. sample amount (µg/spot) was considered for preparation of the calibration curve.
Method validation:
The above-mentioned method for quantification of lawsone was validated as per the ICH guidelines in terms of its system suitability, specificity, linearity, accuracy, precision (intra-day and inter-day), Limit of Detection (LOD) and Limit of Quantification (LOQ).
Lawsone content of ethanolic extract:
The dried extract was dissolved in ethanol (40 mg/ml), filtered through a 0.4 µm syringe filter (Pall-Gelman Supor Acrodisc®). Filtered solutions were applied to pre-coated TLC plate (TLC aluminium sheets silica gel 60 F254, Merck, Darmstadt, Germany). 5 µl of extract solution was applied as a band by Linomat V sample applicator in a triplicate manner at a speed of 100 nl/s. After application, the bands were developed to a distance of 80 mm using 20 ml of toluene:ethyl acetate:glacial acetic acid (16:2:2) as mobile phase in a twin trough chamber (CAMAG, 20×10 cm) after saturation with the same mobile phase. The developed layers were dried by a hair drier and the zones were scanned at λmax 334 nm. The lawsone content per spot was estimated from the extrapolation of the standard calibration curve. The amount present in the spot represents the amount of lawsone in the applied volume of the sample (5 µl). From that, the amount of lawsone present in the dry weight of extract was calculated.
Preparation of hydrogel:
Dried standardized ethanolic extract of L. inermis was used for the preparation of hydrogel. The hydrogel was prepared using Carbopol 934 of different concentrations (0.5, 1, 2 % w/w), PG, Tri-ethanolamine (TEA) and distilled water. The known amount of Carbopol 934 was slowly dispersed into the required quantity of water with continuous stirring to get uniform dispersion and allowed overnight for proper hydration. The accurately weighed quantity of standardized ethanolic extract along with other excipients was poured into the fixed amount of hydrated Carbopol 934 dispersion with constant mixing. TEA was used to adjust the pH of the gel formulation[20]. The composition of hydrogel prepared from standardized ethanolic extract of L. inermis is presented in Table 1.
| Ingredients | F1 | F2 | F3 |
|---|---|---|---|
| Carbopol 934 | 0.50 % | 1 % | 2 % |
| Water up to (ml) | 30 | 30 | 30 |
| Dried standardized L. inermis ethanolic extract | 1 g | 1 g | 1 g |
| PG | 2 ml | 2 ml | 2 ml |
| TEA | q.s | q.s | q.s |
Table 1: Composition of Hydrogel Formulations Containing Ethanolic Extract of L. inermis
Evaluation of hydrogel:
Preliminary physical evaluation of hydrogel: All formulated hydrogels were inspected for their cosmetic characteristics, which include color, appearance, texture, consistency and homogeneity.
pH determination: The pH of the formulation was measured at 25°±2° with a digital pH meter (Mettler- Toledo India Private Limited, Powai Mumbai, India) which was previously calibrated with buffered solutions of different pH. 1 g of hydrogel was precisely weighed and dispersed in 10 ml of distilled water. The estimation of pH of each hydrogel was done in a triplicate manner and the average was calculated[21].
Viscosity measurement of hydrogel: Viscosities of all formulated hydrogels were determined at 25°±2° by Brookfield cup and bob viscometer using spindle number S64 (model-LVDVE, Brookfield Engineering Laboratories, MA, USA). All measurements were done in triplicate at 10 RPM and the average was considered.
Spreadability: The spreading ability of hydrogel formulation was determined by placing 500 mg of hydrogel inside a pre-marked circle of 1 cm diameter on a 3 mm glass plate over which a second glass plate was kept[22]. Then a 500 g weight was assigned to place on the upper glass plate for 5 min. The increase in the diameter of the hydrogel due to spreading was observed and noted for all formulations separately. The results were considered from the average of three experiments.
Occlusion test of hydrogel: The occlusion test for prepared hydrogel was evaluated as per the method by de Vringer. In this method, three beakers of 100 ml volume were taken each of was filled with 50 ml of water[23]. All the beakers were subsequently covered by cellulose acetate filter paper (90 mm thickness, cutoff size 4-7 µm). A sample of 200 mg was spread on the filter paper surface (19.63 cm2), leading to an applied amount of 10.19 mg/cm2. A visible thin film of gel formed on the filter paper. The sample was preserved at skin temperature (32°) for 24 h at 50 %-55 % Relative Humidity (RH). Then the sample weight was taken to evaluate the amount of water loss due to evaporation (water flux through the filter paper). A beaker covered with filter paper (without application of sample) was taken as a reference. The experiment was conducted in triplicate and the average was calculated. The occlusion factor (F) was calculated by equation (1).
Occlusion factor (F)=((A-B)×100)/A
Where, A is the amount of loss of water without the sample (reference) and B is the amount of water loss with the sample. The zero value of F indicates no occlusive effect, whereas 100 is the maximum occlusion factor.
Syneresis:
It was evaluated as per the method[17] with some modifications. In this test, hydrogels were placed in a cylindrical plastic centrifuge tube and the initial weight was taken (M1). Tubes were kept in a centrifuge (REMI CM 12 Plus, India) and centrifuged for 15 min at 5000 RPM. After centrifugation, the released water was discarded and the gels were weighed (M2) again. Syneresis of hydrogel was calculated as equation (2). The data were reported as an average of 3 measurements.
% Syneresis=(M1-M2)/M1 (2)
Drug content:
The drug content of the hydrogel was estimated with respect to lawsone. The lawsone content of the hydrogel was determined by using the calibration curve of lawsone obtained from HPTLC analysis. Accurately 200 mg of each hydrogel formulation was weighed and dissolved in 1 ml of ethanol. The mixture was sonicated in a bath sonicator (Labman-Ultrasonic Cleaner LMUC-6, India) for 10 min for complete solubilization of lawsone in ethanol. After sonication, the mixture was filtered through a syringe filter (Pall- Gelman Supor Acrodisc®), and 5 µl sample was applied on the TLC plate in triplicate. Then the plate was developed to a distance of 80 mm using 20 ml of the same mobile phase as used in calibration curve preparation. The developed plate, after drying, was scanned at λmax 334 nm. The lawsone content/spot was estimated from extrapolation of the standard calibration curve and the amount present in the spot represents the amount of lawsone in the applied volume of the sample (5 µl). From that, the amount of lawsone present in the taken amount of hydrogel (200 mg) was calculated. The percentage drug content was calculated by equation (3) % Drug content=The obtained amount of lawsone in total hydrogel formulation/theoretical amount of lawsone present in formulation×100 (3)
Drug excipient compatibility study:
The physicochemical compatibility between L. inermis extract (mainly lawsone) and excipients used in the hydrogel formulation was investigated by using Fourier Transform Infrared spectroscopy (FTIR) (Alpha FTIR spectrometer, Bruker Co. Germany). The ATR-FTIR spectra were recorded in the wavenumber region between 4000 and 600 cm-1 with 4 cm-1 resolution. The spectra were recorded for the standardized ethanolic extract of L. inermis, three hydrogel formulations (F1, F2 and F3), physical mixture of formulation components and finally compared.
Skin irritation study:
The skin irritation potential of hydrogel formulation was evaluated by Draize patch test[24]. The study was performed using three healthy white albino rabbits weighted between 2-3 kg in a group. Two such groups were selected, group 1 served as placebo (group topically applied with the hydrogel without ethanolic extract), group 2 received F2 formulation. Each rabbit was kept individually in the cage and supplied an adequate amount of food and water in-between the study. 24 h prior to the test, hair from the backside area of each rabbit was removed by hair clippers (area of 4 cm×4 cm) and washed with distilled water. Accurately 0.5 g of hydrogel formulation was applied within the area of 4 cm2. The skin surface was examined for the sign of erythema and edema at 24, 48, and 72 hours after application. The response was evaluated as reactions on the skin and given the scores according to the grading values for skin responses described in Table 2[25]. The Primary Irritation Index (PII) was calculated and verified with the response category table (Table 3) [26].
| Skin responses | Score of PII |
|---|---|
| Erythema and eschar formation | |
| No erythema | 0 |
| Very slight erythema (barely perceptible) | 1 |
| Well-defined erythema | 2 |
| Moderate to severe erythema | 3 |
| Severe erythema (beet-redness) to slight eschar formation (injuries in depth) | 4 |
| Edema formation | |
| No edema | 0 |
| Very slight edema (barely perceptible) | 1 |
| Slight edema (edges of area well-defined by definite raising) | 2 |
| Moderate edema (raised approximately 1.0 mm) | 3 |
| Severe edema (raised more than 1.0 mm and extending beyond the area of exposure) | 4 |
| Total possible score for irritation | 8 |
Table 2: Grading Values for the Primary Skin Irritation Test
| Category | PII |
|---|---|
| Negligible | 0–0.4 |
| Slight irritation | 0.5–1.9 |
| Moderate irritation | 2.0–4.9 |
| Severe irritation | 5.0–8.0 |
Table 3: Response Categories and PII
Evaluation of wound healing activity:
Animals: Adult male Wistar albino rats (150-180 g, n=18) were procured from Central Animal House of Institute of Medical Sciences, BHU, Varanasi. The rats were individually housed in standard environmental conditions, 12 h light-dark cycle with adequate ventilation, fed with standard food and water ad libitum during the whole experimental period. The permission for the use of animals and experimental protocols were approved by the Institutional Animal Ethics Committee (No. Dean/2017/CAEC/705 Dated 30-03- 2017).
Wound healing study: The wound healing activity of hydrogel containing standardized ethanolic extract of L. inermis was evaluated by using the excision wound model. The animals were acclimatized for 1 w before the study. The animals were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg/kg). The dorsal skin of the rat was shaved with scissors and a surgical blade, cleaned with 70 % ethanol, a round section of full-thickness skin wound (25 mm diameter and 2 mm depth) was created in all the rats with the aid of sterilized toothed forceps, surgical scissors and blade under semi-aseptic condition. Rats were homed individually in disinfected cages after recovery from anesthesia with unrestricted access to water and food and divided into three groups equally. The progression of the healing process was investigated by macroscopic photographs of the wounds.
Initially, the amount of hydrogel that can spread evenly to the entire wound area was determined by spreading the preweighed amount of prepared hydrogel uniformly. Accurately, 400 mg of extract-loaded hydrogel was found to spread evenly throughout the wound area as a very thin layer without any spillage to the adjacent area. Hence, 400 mg of standardized L. inermis extract loaded hydrogel and 400 mg of hydrogel containing pure lawsone (equivalent to the amount of lawsone present in the extract loaded hydrogel) were applied topically for wound healing study.
Treatment groups:
Wistar albino rats were divided into three groups consisting of six in each and were labeled as group Ӏ to group ӀӀӀ.
Group-I: Immediately after creating the wound, the wound area of animals was left as such (untreated), served as the control group (for comparison) and allowed the natural process of wound healing.
Group-II: Hydrogel of pure lawsone (placebo F2 hydrogel containing pure lawsone) promoting wound healing was selected as the standard group for the comparison of wound healing actions in experimental animals. The quantity of lawsone used for the standard hydrogel preparation was chosen as per the amount of lawsone present in 1 g of standardized ethanolic extract (estimated by HPTLC densitometry method) to make each formulation uniform with respect to lawsone content. Accurately weighed (400 mg) hydrogel containing pure lawsone was applied once in 24 h for 12 d.
Group-III: Based upon the optimum viscosity, medium occlusivity, spreadability and consistency nature of formulation F2, it was selected for wound healing activity evaluation. Formulation F2 (400 mg) containing standardized ethanolic extract of leaf of L. inermis was applied at once daily for 12 d, which is served as a test group for evaluation of wound healing potential of the hydrogel.
Wound photography and measurement of percentage wound reduction:
The photographs of the wound of all animals were taken separately on 0, 4, 8 and 12 d. The post-excision wound area was measured at a predetermined time interval on above mentioned days of all group animals. During the healing process, the wound had not perfect circular shapes. Therefore, the areas of the wounds were traced on transparent paper by using a fine tip marker. The wound areas within the traced boundaries were calculated using a millimeter scale graph paper[25]. The results of wound healing on various days were expressed as percentage wound contraction calculated by Wilson’s formula[1,27] as in equation (4).
% Wound contraction=0 d wound area-wound area on said day/0 d wound area×100 (4)
Statistical analysis:
The mean and Standard Deviation (SD) for all values were calculated. All data are expressed in terms of mean±SD. Statistical analysis was performed by one- way ANOVA followed by Tukey’s test or students t-test using at p<0.05 using Origin 2019b software (Trial Version Software, Northampton, MA, USA).
Results and Discussion
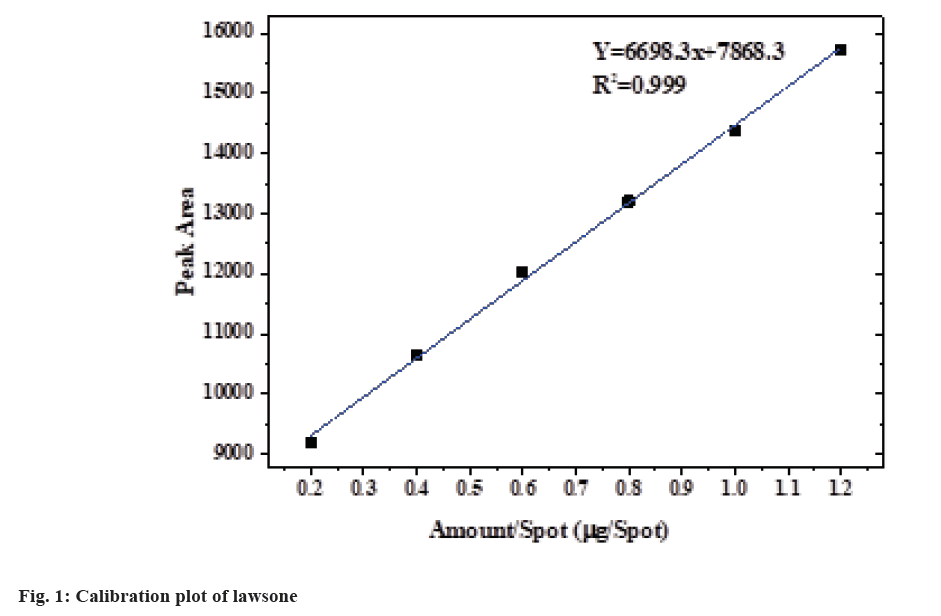
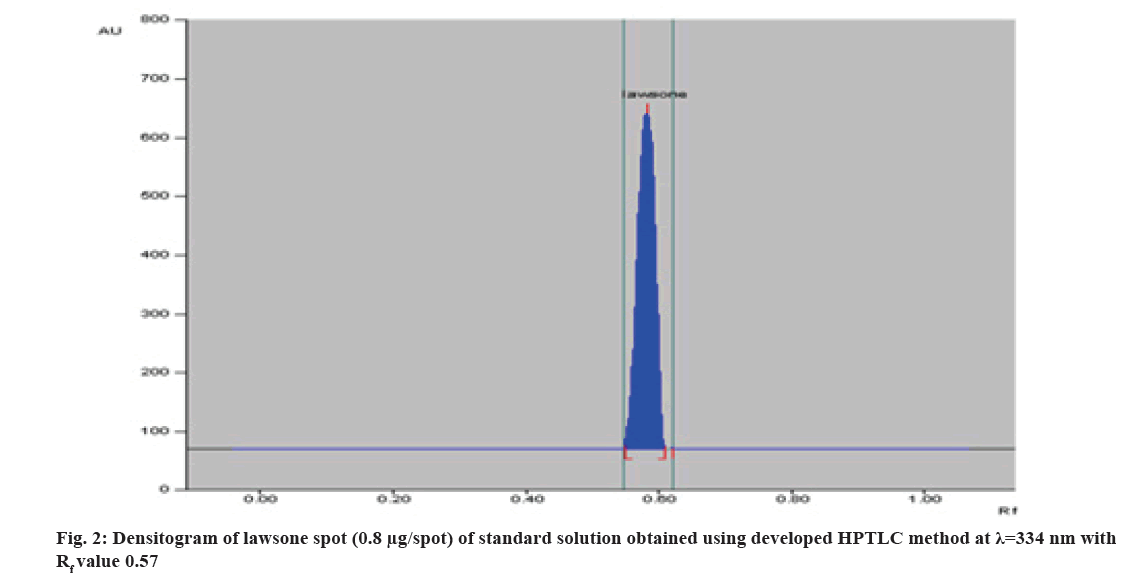
Different mobile phase was tried preliminarily and good separation of standard lawsone and extract was achieved with toluene:ethyl acetate:glacial acetic acid (16:2:2 v/v/v). Lawsone was detected at 0.57 Retardation Factor ( Rf) value at 334 nm by absorbance mode. The average of the area obtained from 3 repetitive measurements was plotted against amount/spot (fig. 1). The above mobile phase gave a compact spot as well as a symmetrical peak with a Rf value at 0.57 (±0.03) shown in fig. 2.
The system suitability was checked by applying six replicate spots (6 μl) of primary standard solution (100 μg/ml) and analyzing the percentage Relative Standard Deviation (RSD) of peak areas. The percentage RSD of peak areas was found to be 0.9994, which is <2 % showing no significant variations among the peak area, which indicates the suitability of the developed method for doing further validation (Table 4).
| S. No | Amount/spot (µg/spot) | Peak area | Average | SD | % RSD |
|---|---|---|---|---|---|
| 1 | 0.6 | 12091.5 | 12110.39 | 121.0376 | 0.9994 |
| 2 | 0.6 | 11991.7 | |||
| 3 | 0.6 | 12012.4 | |||
| 4 | 0.6 | 12046.8 | |||
| 5 | 0.6 | 12245.67 | |||
| 6 | 0.6 | 12274.28 |
Table 4: System Suitability (RSD)
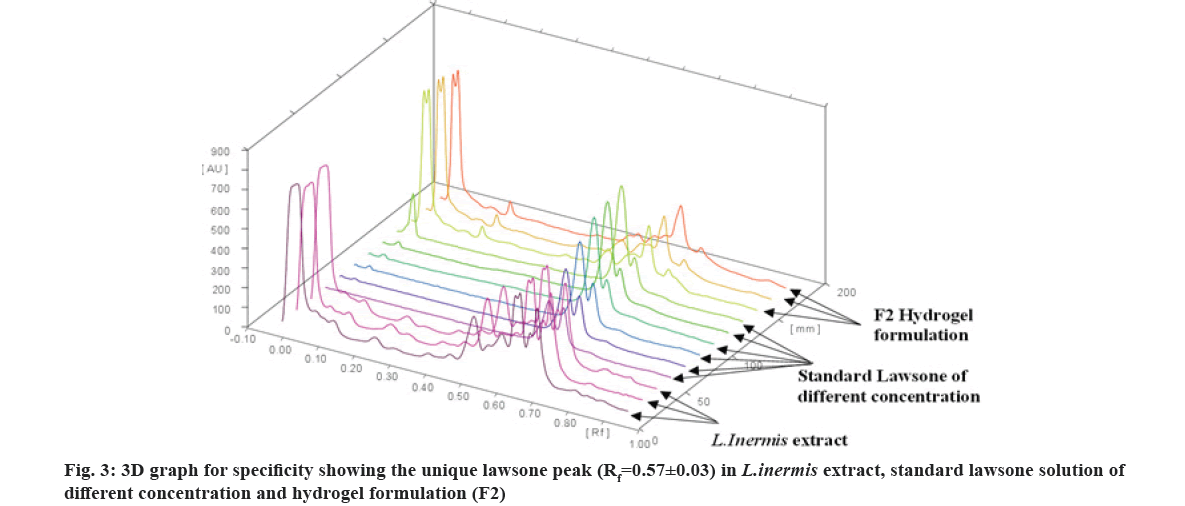
The specificity of the method was verified by comparing the Rf value and the spectra of the ethanolic extract of L. inermis, standard lawsone and formulation (F2). Fig. 3 showed a good agreement of Rf value (0.57±0.03) of the lawsone in ethanolic extract and formulation (F2). On the obtained densitogram of pure lawsone, no additional peaks were found. The densitogram of extract and F2 formulation represent multiple peaks with the easily identifying unique lawsone peak ( Rf value 0.57±0.03). Hence, the developed HPTLC method is specific for the determination of lawsone.
From the calibration plot (fig. 1), the obtained linear equation y=6698.3x+7868.3 (n=3). Regression analysis of data was considered to obtain a linear regression model. A good linear relationship was obtained in the range of applied amount (0.2-1.2 µg/spot) of lawsone. The correlation coefficient of the fitted model (0.999) indicates a relatively strong relationship among variables. The level of significance (p<0.001) reflects the statistically significant correlation between the peak area and the amount of lawsone at each level. Thus, the method is in accordance with the guidelines of the ICH essential for the quantitative analysis of an active ingredient (lawsone).
The accuracy of this method was ascertained based on the percentage recovery by standard addition method at three levels (50 %, 100 % and 150 %). The recovery was found to be within the range of 97.5 %-99.33 % (Table 5), which is within the acceptance range as per the ICH guidelines.
| Initial quantity of lawsone (µg/µl) | Percent of standard lawsone added | Theoretical quantity of lawsone in sample (µg/spot) | Average quantity of recovered lawsone (mean±SD) | Recovery (%) |
|---|---|---|---|---|
| 0.1 | 50 | 0.9 | 0.884±0.005 | 98.22 |
| 0.1 | 100 | 1.2 | 1.17±0.015 | 97.5 |
| 0.1 | 150 | 1.5 | 1.497±0.005 | 99.33 |
Note: Results are shown as mean±SD
Table 5: Accuracy Data of the HPTLC-Densitometric Method for Lawsone (n=3)
Table 6 displays the results of precision studies (expressed as % RSD) of the measured peak area of lawsone in the samples at three different amounts (0.2, 0.6 and 1.2 µg/spot). The % RSD values of intra- and inter-day precision were <2 % in all cases, which confirmed that the proposed method is capable of producing reproducible results. Therefore, the HPTLC method is considered to be precise.
| Amount of lawsone (µg/spot) | Peak area (mean±SD) | % RSD of peak area |
|---|---|---|
| Intra-day precision (repeatability, n=3) | ||
| 0.2 | 9194.3±164.62 | 1.79 |
| 0.6 | 12036.36±51.059 | 0.424 |
| 1.2 | 15842.334±85.602 | 0.54 |
| Inter-day precision (repeatability, n=3) | ||
| 0.2 | 9062.9±115.310 | 1.272 |
| 0.6 | 12316.914±224.900 | 1.825 |
| 1.2 | 15825.137±58.096 | 0.367 |
Note: Results are shown as mean±SD
Table 6: Precision of Proposed HPTLC-Densitometric Method for Lawsone
The LOD and LOQ values were found to be 0.0701 µg/spot and 0.212 µg/spot respectivelsy which confirm that the proposed method is sensitive enough for the estimation of lawsone.
The results of the robustness analysis are shown in Table 7. The data indicate no marked change in results. The values of percent RSD (<2 %) of peak area for all parameters of robustness indicating the robustness of the developed HPTLC method.
| Variations of chromatographic conditions | % RSD of peak area |
|---|---|
| Volume of mobile phase (±0.8 ml) | 1.76 |
| Time of chromatographic plates activation (±5 min) | 1.23 |
| Saturation time of developing chamber (±5 min) | 0.85 |
| Development distance (±10 mm) | 1.31 |
Table 7: Robustness Data of HPTLC Method for Lawsone Quantification (n=3)
The lawsone content of the extract was estimated using a linear equation, y=6698.3x+7868.3 (n=3), obtained from the calibration curve of standard lawsone. 1 g of dried ethanolic extract of L. inermis was found to possess 5.450±0.0352 mg of lawsone.
All the formulated hydrogels were light brown in color, having an acceptable smooth homogeneous appearance and texture, free from aggregates or absence of lumps.
In order to avoid irritation or disruption of skin, pH of the topical formulations should be compatible with pH of the skin[28]. The pH of all the formulations (Table 8), are in the acceptable range for topical administration, permitting the use of the prepared formulations[21].
| Formulations | F1 | F2 | F3 |
|---|---|---|---|
| pH | 6.73±0.047 | 6.53±0.067 | 6.8±0.082 |
| Viscosity (cp) | 39840±500.07 | 58786±610.83 | 87538±389.87 |
| Spreadability (cm) | 8.87±0.07 | 8.13±0.08 | 6.81±0.09 |
| Occlusion test value | 57.43±1.24 | 64.67±2.37 | 73.82±2.48 |
| Syneresis (%) | 5.5±0.226 | 3.243±0.202 | 2.696±0.368 |
Note: Results are shown as mean±SD
Table 8: pH, Viscosity, Spreadability, Occlusion and Syneresis Values of Hydrogel Formulations
Spreadability, adhesiveness, drug release and subsequent penetration may greatly be influenced by the rheological properties of topical hydrogel formulations while delivering the drug molecules onto or across the skin[29]. The viscosity results of formulation (Table 8) indicate that the developed hydrogels possess adequate consistency to retain on the skin. The results indicated the viscosities of hydrogel formulations were consistent, neither too thick nor too thin.
Spreadability results of prepared hydrogels were presented in Table 8. The ability of a gel to spread over an area on application to the skin surface is expressed in terms of spreadability. This is an important characteristic of topical formulation for better patient compliance and uniform application of gel to the skin surface. More viscous formulation possesses poor spreadability, which results in difficulty during application. It is influenced by the type and concentration of gelling agent used in the formulation[28]. All formulations showing good spreadability indicate uniform spreading and take less time to spread at the application site.
The occlusive effects of the tested hydrogels are shown in Table 8. The hydrogels form an adhesive layer that occludes the skin surface. The occluding property of the topical gel allows the permeation of the extract to the deeper site of the skin. This is due to hydration of the stratum corneum in which water associates with the polar head groups of the lipid bilayers through hydrogen bonding. The hydrated cell loosens the lipid bilayers, decreases intermolecular forces and increases the fluidity of the bilayer region[30].
Syneresis of the gel system is a natural phenomenon during which gel shrinks a bit and little amount of unbound excess water is pressed out from it upon standing. It is an undesirable property that can be controlled by proper choice of gallant concentration and suitable additives. The results of syneresis are shown in Table 8. The decrease in the syneresis was obtained with an increase in the Carbopol 934 concentration for all hydrogels.
The drug content with respect to lawsone was calculated for all formulations and from that, the percent drug content was estimated. The F1, F2 and F3 formulation was found to contain (94.271±0.292) %, (96.587±0.402) %, (95.287±0.17) % respectively. The slight decrease in content in all formulations may be due to the uneven distribution of extract in the blank hydrogel during formulation.
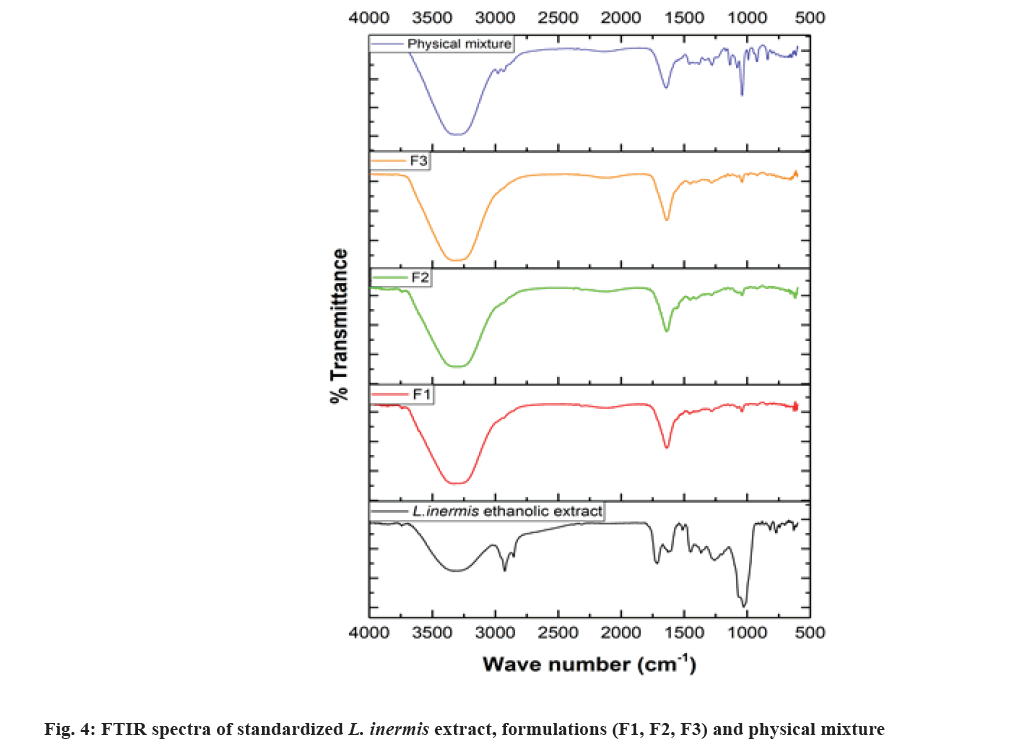
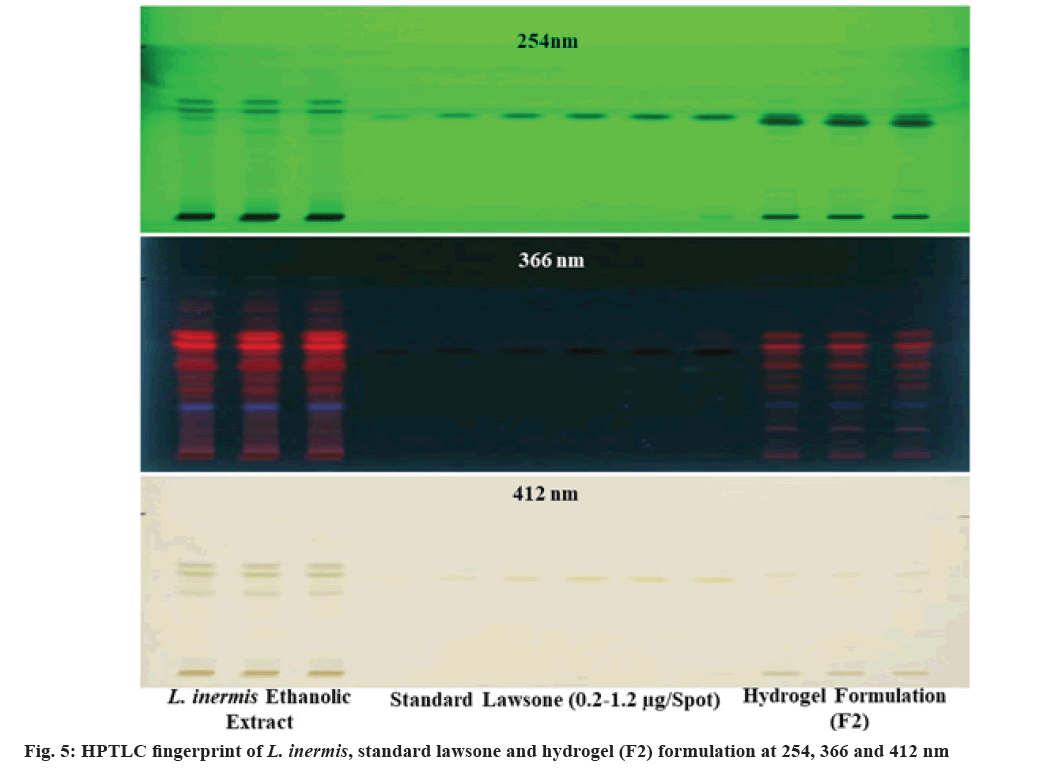
The information of chemical composition and compatibility of the drug with the additives can be obtained by adopting FTIR analysis[31]. The FTIR spectra of standardized ethanolic extract of L. inermis, standardized extract containing formulations (F1, F2 and F3) and the physical mixture of formulation components were illustrated in fig. 4. The peak at 1451 cm-1, 1452 cm-1, 1454 cm-1, 1456 cm-1 correspond to -C=C- (aromatic) stretching band, peak in between 1630 cm-1 to 1641 cm-1 correspond to >C=O stretching band and the phenolic O-H stretching having a broad peak at around 3335 cm-1 for all formulation analyzed. This is in accordance with the FTIR signature of lawsone as reported previously[32-34]. The physical mixture of formulation components contains all the prominent peaks without any shifting or disappearance, which eliminates the possibility of incompatibility among them. The spectrum of formulations (F1, F2 and F3) and the standardized ethanolic extract of L. inermis has shown all the dominant absorption peaks almost at the same position indicating the retention of the chemical identity of lawsone. However, the decreased intensity of the peaks in the formulation was observed, which is due to the low quantity of extract in the hydrogel formulation. There is no disappearance of any important functional group positions. These findings suggested the compatibility of the extract (mainly lawsone) with additives in the prepared hydrogel. In addition to the FTIR results, the compatibility of lawsone with the formulation excipients was also revealed from the integrity of lawsone peak ( Rf=0.57±0.03) in the HPTLC chromatogram (fig. 3). The band of lawsone is also available in the HPTLC fingerprint captured at 254, 366 and 412 nm (fig. 5).
Skin irritation study measures the safety of polymeric hydrogel containing the active ingredient on rabbit skin[24]. A topical formulation with high acidic pH can cause skin irritation[25]. All the prepared formulations have pH towards neutrality, hence has very less chance of skin irritation due to acidity. The erythema and edema were observed on applied areas of all three rabbits of each group after 24 h, 48 h and 72 h applied with hydrogel F2 as well as placebo gel. Two rabbits showed very slight erythema, which disappeared after 72 h which received a placebo gel. No erythema and edema were observed on formulation F2. The results were presented in Table 9, which represents the formulated hydrogel of ethanolic extract of L. inermis has no skin irritation tendency.
| Formulation | Response | After a specific time interval (h) | Rabbit | PII score | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Placebo gel | Erythema | 24 | 1 | 0 | 0 | 0.33 |
| 48 | 0 | 1 | 0 | 0.33 | ||
| 72 | 0 | 0 | 0 | 0 | ||
| Edema | 24 | 0 | 0 | 0 | 0 | |
| 48 | 0 | 0 | 0 | 0 | ||
| 72 | 0 | 0 | 0 | 0 | ||
| F2 | Erythema | 24 | 0 | 0 | 0 | 0 |
| 48 | 0 | 0 | 0 | 0 | ||
| 72 | 0 | 0 | 0 | 0 | ||
| Edema | 24 | 0 | 0 | 0 | 0 | |
| 48 | 0 | 0 | 0 | 0 | ||
| 72 | 0 | 0 | 0 | 0 | ||
Table 9: Reaction on Skin and Score of Ethanolic Extract of L. inermis Hydrogels
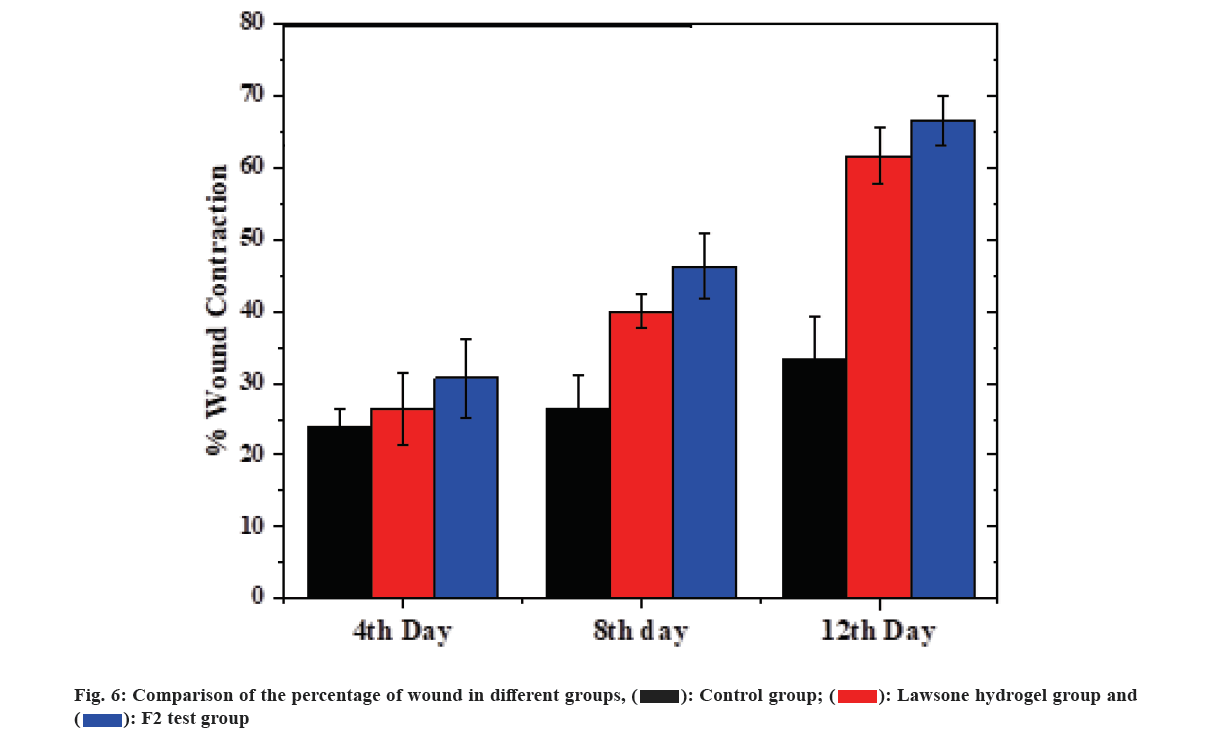
The full excision wound model in rats was utilized to find the ability of hydrogel containing standardized ethanolic leaf extract in promoting wound healing. In the protocol, an untreated (control group), neat lawsone containing hydrogel (standard group) and the standardized ethanolic leaf extract hydrogel F2 (test group) were applied over the wounds, and their ability to reduce wound area was determined. The percentage of wound area was investigated at different time points up to 12 d period as shown in fig. 6. It was observed that there is a significant (p<0.001) decrease in the wound area in the rat treated with formulation F2 compared to the control group. In the control group, animals had a large difference in wound area contraction (33 % in 12 d) compared to the test group (66.66 % in 12 d) found because of a slow natural wound healing process of the body in untreated rats[35]. The closure rate in the standard group is nearer to the test group as the standard group contains lawsone, the important phenolic principle present in henna leaf contribute to its wound healing activity[7]. Standardized ethanolic extract of L. inermis gel (F2) showed a significant increase in the rate of wound contraction. The increased activity of F2 than the standard group is due to the additive effect of phytoconstituents present in L. inermis extract[3]. To conclude the wound healing study, the healing process with F2 hydrogel was faster than other groups. Presence of flavonoids, the bioactive constituents in henna can promote fibroblast proliferation and collagen secretion crucial for the healing of wounds[7,36]. In addition to flavonoids, various phenolic compounds in L. inermis extracts could combine with the bacterial cell wall and inactive their function[7,37,38] and accelerate healing of wounds.
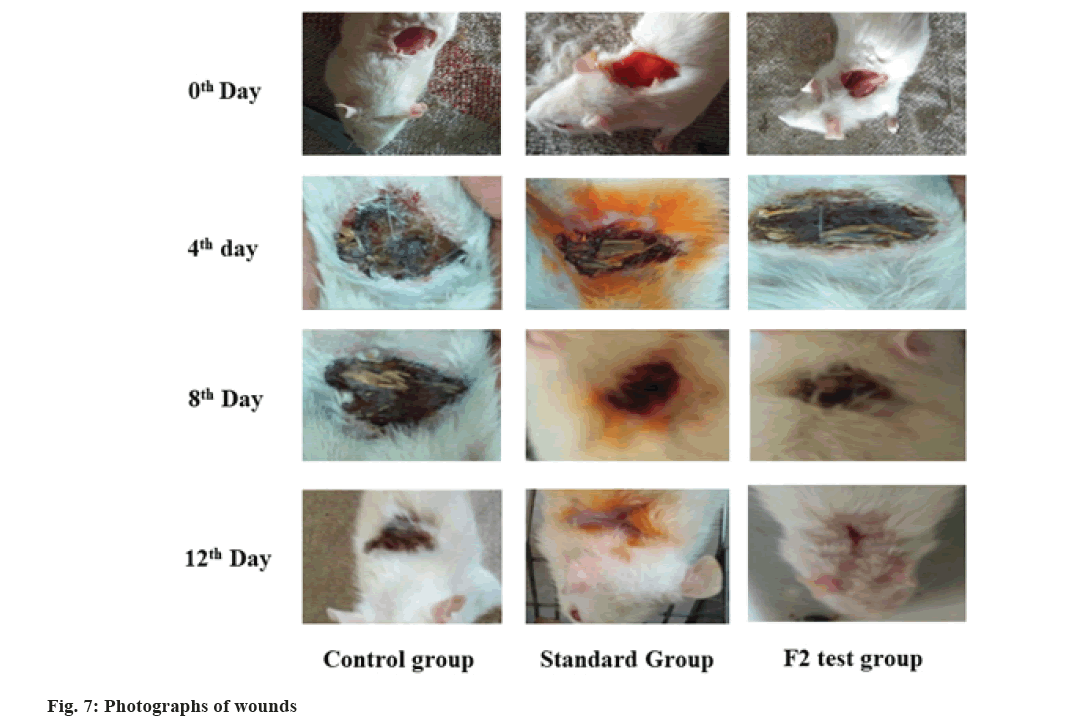
The 0th, 4th, 8th and 12th d representative images of the control, standard and test group were shown in fig. 7. After the 4th d, no remarkable change in wound appearance was visible for all groups. After the 8th d, the wound closure area was more in standard and test compared to the control group. After 12th d more than 50 % of the wound in the test and standard were noticeably closed and scabs had fallen off while the controls had wounds covered with scabs. The greatest wound healing rate of the test group (formulation F2) than other groups happened for several reasons, L. inermis extracts promotes wound healing activity[3]; L. inermis extracts prevents antibacterial infection[7,37,38]; henna extract has the potential to suppress the formation of superoxide anion and release of neutrophil elastase, thereby enhances anti-inflammatory activity. Hence the anti-inflammatory response protects the fibroblasts from oxidative stress and increases fibroblast proliferation and deposition of collagen at wound area[6]; the hydrogel usually extracts moisture from the wound, pulling the skin together which gradually closing the wound and reducing wound area, thereby falling the risk of infection[39].
Nature has had a significant role in offering medicinal values for thousands of years in the treatment and prevention of various ailments. The existence of different life-sustaining chemical moieties in plants has urged scientists to work on these plants to identify their potential therapeutic activity. The wound healing potential of various plants has been well explored. The activity is attributed to their anti- microbial, anti-inflammatory, healing, haemostatic, analgesic, suppression of superoxide anion and immuno-modulating activities. The extract-based multicomponent system will be able to treat the wound in a multi-dimensional way.
Compared to the traditional dressing, hydrogel, a recent formulation, offers several advantages, such as smoothness and ease of applicability, better patient compliance, spreading over a larger area, safety, ease of removal due to non-adhesive properties, etc. Furthermore, it has the tendency of moisture balance by absorbing physiological exudates from wounds, which facilitate the wound healing process.
The work offers the development of herbal wound healing hydrogel containing standardized ethanolic extract of L. inermis for topical wounds. The ethanolic leaf extract of henna was obtained from fresh leaves. The standardization of ethanolic extract with respect to lawsone was done by HPTLC densitometric method. The hydrogel containing an equal amount of extract was formulated with different concentrations of Carbopol 934. All the formulations showed acceptable pH, viscosity and spreadability, occlusion, syneresis, drug content, drug-excipient compatibility. No formulation containing the extract was able to cause any skin irritation. The presence of lawsone in the formulation was confirmed by FTIR spectra. The wound area contraction of formulation F2 was faster than the standard and control group after 12 d of treatment. It is concluded that the formulated hydrogel would have promising application in topical wound healing. The plant-based hydrogel formulation will provide safe, efficacious, patient compliance and cost- effective medication for the treatment of wounds, with consideration of the traditional claim of plant-based therapy and newer dosage form approach.
Acknowledgements:
The authors are thankful to the Department of Pharmaceutical Engineering and Technology and Central Instrumental Facility-IIT (BHU), Varanasi, India, for providing necessary infrastructural and instrumental facilities. We also acknowledge Prof. N. K. Dubey, Department of Botany, Banaras Hindu University, for helping in the authentication of L. inermis Linn. Author Alakh N. Sahu is thankful to the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, New Delhi, India, for providing the funding [Sanction order No: BT/PR25498/NER/95/1223/2017] for exploring Phytochemical and pharmacological evaluations of bioactivity guided fractions of medicinal plants of Tripura. Also, the financial support for this work provided as a scholarship to Kohina Dixit and Debadatta Mohapatra by the Ministry of Human Resource Development (MHRD), Government of India, is highly acknowledged.
Conflict of interests:
The authors declared no conflict of interests.
References
- Kant V, Gopal A, Kumar D, Gopalkrishnan A, Pathak NN, Kurade NP, et al. Topical pluronic F-127 gel application enhances cutaneous wound healing in rats. Acta Histochem 2014;116(1):5-13.
[Crossref] [Google Scholar] [Pub Med]
- Mathieu D, Linke JC, Wattel F. Handbook on hyperbaric medicine. New York: Springer; 2006. p. 401-28.
- Nayak BS, Isitor G, Davis EM, Pillai GK. The evidence based wound healing activity of Lawsonia inermis Linn. Phytother Res 2007;21(9):827-31.
[Crossref] [Google Scholar] [Pub Med]
- Vakilian S, Norouzi M, Soufi-Zomorrod M, Shabani I, Hosseinzadeh S, Soleimani M. Lawsonia inermis-loaded nanofibrous scaffolds for wound dressing applications. Tissue Cell 2018;51:32-8.
[Crossref] [Google Scholar] [Pub Med]
- Semwal RB, Semwal DK, Combrinck S, Cartwright-Jones C, Viljoen A. Lawsonia inermisL. (henna): Ethnobotanical, phytochemical and pharmacological aspects. J Ethnopharmacol 2014;155(1):80-103.
[Crossref] [Google Scholar] [Pub Med]
- Liou JR, El-Shazly M, Du YC, Tseng CN, Hwang TL, Chuang YL, et al. 1, 5-Diphenylpent-3-en-1-ynes and methyl naphthalene carboxylates from Lawsonia inermis and their anti-inflammatory activity. Phytochemistry 2013;88:67-73.
[Crossref] [Google Scholar] [Pub Med]
- Hadisi Z, Nourmohammadi J, Nassiri SM. The antibacterial and anti-inflammatory investigation of Lawsonia inermis-gelatin-starch nano-fibrous dressing in burn wound. Int J Biol Macromol 2018;107:2008-19.
[Crossref] [Google Scholar] [Pub Med]
- Lai WF, Rogach AL. Hydrogel-based materials for delivery of herbal medicines. ACS Appl Mater Interfaces 2017;9(13):11309-20.
- Thomas S, Hay P. Fluid handling properties of hydrogel dressings. Ostomy Wound Manage 1995;41(3):54-6.
- Corkhill PH, Hamilton CJ, Tighe BJ. Synthetic hydrogels VI: Hydrogel composites as wound dressings and implant materials. Biomaterials 1989;10(1):3-10.
[Crossref] [Google Scholar] [Pub Med]
- Pereira R, Carvalho A, Vaz DC, Gil MH, Mendes A, Bártolo P. Development of novel alginate based hydrogel films for wound healing applications. Int J Biol Macromol 2013;52:221-30.
[Crossref] [Google Scholar] [Pub Med]
- Alexander A, Patel RJ, Saraf S, Saraf S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J Control Release 2016;241:110-24.
[Crossref] [Google Scholar] [Pub Med]
- Chen X, Peng LH, Shan YH, Li N, Wei W, Yu L, et al. Astragaloside IV-loaded nanoparticle-enriched hydrogel induces wound healing and anti-scar activity through topical delivery. Int J Pharm 2013;447(1-2):171-81.
[Crossref] [Google Scholar] [Pub Med]
- Qureshi MA, Khatoon F, Rizvi MA, Zafaryab M. Ethyl acetate Salix alba leaves extract-loaded chitosan-based hydrogel film for wound dressing applications. J Biomater Sci Polym Ed 2015;26(18):1452-64.
[Crossref] [Google Scholar] [Pub Med]
- Rane MM, Mengi SA. Comparative effect of oral administration and topical application of alcoholic extract of Terminalia arjuna bark on incision and excision wounds in rats. Fitoterapia 2003;74(6):553-8.
[Crossref] [Google Scholar] [Pub Med]
- Sikareepaisan P, Ruktanonchai U, Supaphol P. Preparation and characterization of asiaticoside-loaded alginate films and their potential for use as effectual wound dressings. Carbohydr Polym 2011;83(4):1457-69.
- Banerjee S, Bhattacharya S. Compressive textural attributes, opacity and syneresis of gels prepared from gellan, agar and their mixtures. J Food Eng 2011;102(3):287-92.
- Thomson RH. Naturally occurring quinones. 2nd ed. New York: Elsevier; 2012.
- El-Shaer NS, Badr JM, Aboul-Ela MA, Gohar YM. Determination of lawsone in henna powders by high performance thin layer chromatography. J Sep Sci 2007;30(18):3311-5.
[Crossref] [Google Scholar] [Pub Med]
- Chaudhary H, Rohilla A, Rathee P, Kumar V. Optimization and formulation design of Carbopol loaded piroxicam gel using novel penetration enhancers. Int J Biol Macromol 2013;55:246-53.
[Crossref] [Google Scholar] [Pub Med]
- Pillai AB, Nair JV, Gupta NK, Gupta S. Microemulsion-loaded hydrogel formulation of butenafine hydrochloride for improved topical delivery. Arch Dermatol Res 2015;307(7):625-33.
[Crossref] [Google Scholar] [Pub Med]
- Bachhav YG, Patravale VB. Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation. Int J Pharm 2009;365(1-2):175-9.
[Crossref] [Google Scholar] [Pub Med]
- Bhaskar K, Mohan CK, Lingam M, Mohan SJ, Venkateswarlu V, Rao YM, et al. Development of SLN and NLC enriched hydrogels for transdermal delivery of nitrendipine: In vitro and in vivo characteristics. Drug Dev Ind Pharm 2009;35(1):98-113.
[Crossref] [Google Scholar] [Pub Med]
- Ahmad S, Minhas MU, Ahmad M, Sohail M, Abdullah O, Badshah SF. Preparation and evaluation of skin wound healing chitosan-based hydrogel membranes. AAPS PharmSciTech 2018;19(7):3199-209.
[Crossref] [Google Scholar] [Pub Med]
- Amin MA, Abdel-Raheem IT. Accelerated wound healing and anti-inflammatory effects of physically cross linked polyvinyl alcohol–chitosan hydrogel containing honey bee venom in diabetic rats. Arch Pharm Res 2014;37(8):1016-31.
[Crossref] [Google Scholar] [Pub Med]
- Sun F, Sui C, Zhou Y, Liu X, Shi Y, Wu Y, et al. Preparation, characterization and pharmacological evaluation of tolterodine hydrogels for the treatment of overactive bladder. Int J Pharm 2013;454(1):532-8.
[Crossref] [Google Scholar] [Pub Med]
- Jangde R, Srivastava S, Singh MR, Singh D. In vitro and in vivo characterization of quercetin loaded multiphase hydrogel for wound healing application. Int J Biol Macromol 2018;115:1211-7.
[Crossref] [Google Scholar] [Pub Med]
- Nikumbh KV, Sevankar SG, Patil MP. Formulation development, in vitro and in vivoevaluation of microemulsion-based gel loaded with ketoprofen. Drug Deliv 2015;22(4):509-15.
[Crossref] [Google Scholar] [Pub Med]
- Guan Y, Zuo T, Chang M, Zhang F, Wei T, Shao W, et al. Propranolol hydrochloride-loaded liposomal gel for transdermal delivery: Characterization and in vivo evaluation. Int J Pharm 2015;487(1-2):135-41.
[Crossref] [Google Scholar] [Pub Med]
- Barry BW. Action of skin penetration enhancers-The lipid protein partitioning theory. Int J Cosmet Sci 1988;10(6):281-93.
[Crossref] [Google Scholar] [Pub Med]
- Pachauri M, Gupta ED, Ghosh PC. Piperine loaded PEG-PLGA nanoparticles: Preparation, characterization and targeted delivery for adjuvant breast cancer chemotherapy. J Drug Deliv Sci Techno 2015;29:269-82.
- Rostkowska H, Nowak MJ, Lapinski L, Adamowicz L. Molecular structure and infrared spectra of 2-hydroxy-1,4-naphthoquinone; experimental matrix isolation and theoretical Hartree–Fock and post Hartree–Fock study. Spectrochim Acta Mol Biomol Spectrosc 1998;54(8):1091-103.
- Sangeetha J, Philip J. Synthesis, characterization and antimicrobial property of Fe3O4-Cys-HNQ nanocomplex, with l-cysteine molecule as a linker. RSC Adv 2013;3(21):8047-57.
- Yousefi I, Pakravan M, Rahimi H, Bahador A, Farshadzadeh Z, Haririan I. An investigation of electrospun henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater Sci Eng C 2017;75:433-44.
[Crossref] [Google Scholar] [Pub Med]
- Yasasvini S, Anusa RS, VedhaHari BN, Prabhu PC, Ramya Devi D. Topical hydrogel matrix loaded with Simvastatin microparticles for enhanced wound healing activity. Mater Sci Eng C 2017;72:160-7.
[Crossref] [Google Scholar] [Pub Med]
- Mahmoud NN, Hikmat S, Ghith DA, Hajeer M, Hamadneh L, Qattan D, et al. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: Effect of nanoparticles’ shape and surface modification. Int J Pharm 2019;565:174-86.
[Crossref] [Google Scholar] [Pub Med]
- Dev VG, Venugopal J, Sudha S, Deepika G, Ramakrishna S. Dyeing and antimicrobial characteristics of chitosan treated wool fabrics with henna dye. Carbohydr Polym 2009;75(4):646-50.
- Zhang W, Ronca S, Mele E. Electrospun nanofibres containing antimicrobial plant extracts. Nanomaterials 2017;7(2):42.
[Crossref] [Google Scholar] [Pub Med]
- Wang Z, Wang Y, Peng X, He Y, Wei L, Su W, et al. Photocatalytic antibacterial agent incorporated double-network hydrogel for wound healing. Colloids Surf B Biointerfaces 2019;180:237-44.
[Crossref] [Google Scholar] [Pub Med]