- *Corresponding Author:
- Siva Hemalatha
Department of Pharmaceutical Engineering and Technology, Indian Institute of Technology (BHU), Varanasi, Uttar Pradesh-221005, India
E-mail: shemalatha.phe@iitbhu.ac.in
| Date of Received | 23 February 2022 |
| Date of Revision | 09 January 2023 |
| Date of Acceptance | 19 July 2023 |
| Indian J Pharm Sci 2023;85(4):987-996 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The current work aims to develop and evaluate hydrogel containing silver nanoparticles with Withania coagulans aqueous extract. The silver nanoparticles and silver nanoparticles with Withania coagulans were prepared by a green synthesis approach using 0.1 mM silver nitrate solution and a 1 % aqueous extract of Withania coagulans. The silver nanoparticles were used for characterization purposes, whereas the silver nanoparticles with Withania coagulans were incorporated in the hydrogel formulation containing carbopol 971P NF of different concentrations, propylene glycol, triethanolamine and distilled water of appropriate amount. The silver nanoparticles were evaluated for their particle size (Zavg), polydispersity index, surface morphology by scanning electron microscopy and high-resolution transmission electron microscopy. The prepared hydrogels were assessed for physical appearance, pH, viscosity, syneresis, spreadability, occlusivity, and drug-excipient compatibility. The synthesized silver nanoparticles were stable and spherical in shape with an average particle size of 174.9±0.062 nm, indicating the particles were in the nano range and the polydispersity index value of 0.174±0.054 showed the homogeneity of particle size distribution. The scanning electron microscopy and high-resolution transmission electron microscopy data reflected the spherical and smooth surface of silver nanoparticles. Energy-dispersive X-ray analysis spectra of silver nanoparticles showed a strong spectral signal of silver. The crystalline nature of the silver nanoparticles was confirmed from the spot pattern obtained by selected area electron diffraction. The silver nanoparticles with Withania coagulans loaded hydrogel formulations (F1, F2 and F3) were found to be yellowish-brown in color, homogeneous in nature, with pH of 6.24±0.047-6.78±0.082 and percentage syneresis from 0.46±0.074 to 1.47±0.12. The spreadability data (4.83±0.08-6.4±0.124 cm) indicated the easily spreadable nature of hydrogel and the viscosities in the range of 41840±257.07-87538±389.87 cp were found to be suitable for topical application. The percntage occlusiveness of the prepared hydrogel was found to be 54.43±1.24 to 74.82±2.48. The drug excipient compatibility by attenuated total reflectance-fourier transform infrared spectroscopy spectroscopy revealed the compatibility of the extract with the hydrogel formulation components.
Keywords
Formulation, silver nanoparticle, hydrogel, Withania coagulans, aqueous extract
Plant-based medicament has a long history of curing a variety of diseases. In the last few years, there has been a tremendous elevation in the use of herbal medicine. As per the World Health Organization (WHO) report, most of the world's population relies on drugs from natural origins for their primary health care needs[1]. Nanoparticles are colloidal particulate structures with sizes between 1 to 1000 nm in at least one dimension; however, the common size range of nanoparticles is 1 to 100 nm[2]. Among various metallic nanoparticles, silver and gold were considered valuable metals. Chemical reduction methods using dimethylformamide, hydrazine hydrate, ethylene glycol, etc., have been used previously for the preparation of metallic nanoparticles, which are toxic and hazardous in nature[3]. Thus, new approaches have to be taken to synthesize metallic nanoparticles without using any toxic chemicals as reductants. Preparation of silver nanoparticles (AgNPs) via biological method has been found to be safer, cost-effective as well as eco- friendly in nature[4]. Microbes and plant materials can be used instead of toxic reductants to synthesize metallic nanoparticles[5-7]. The use of plant parts in metallic nanoparticle synthesis is termed the “green synthesis” approach[8]. It has drawn great attention because of its eco-friendliness, rapid and facile synthesis, one-step, non-pathogenic, and easy scale- up ability.
AgNPs have the potential to facilitate wound healing by reducing inflammation through cytokine modulation[9]. Its antimicrobial effect is due to several mechanisms, including enzyme inactivation[10] and plasma membrane destabilization[11]. Different factors that affect the formation of AgNPs by plant extracts include concentration, pH, temperature, and time of reaction[12]. The most important characteristic of AgNPs is their antimicrobial activity against both Gram-positive as well as Gram- negative bacteria, fungi, and viruses[13]. The bactericidal activity of AgNPs depends upon dose, size, shape, concentration, etc.[14]. The plant extract-derived AgNPs facilitate wound healing through tensile strength enhancement, epithelization process, and induction of low inflammation[15].
Hydrogels have a great advantage for delivering herbal medicines due to their high biocompatibility as well as negligible toxicity, tunable release property, sustainability, and ability to load ingredients with various hydrophilicity[16]. Among various existing wound dressings, the hydrogel has the desirable properties and may be considered an ideal dressing formulation[17]. It can be used as a suitable carrier for nanoparticle loading and facilitate wound healing in all stages, i.e., vascular response (hemostasis), inflammatory response (inflammation), proliferative phase (granulation, epithelialization), and maturation phase (reconstruction phase)[18]. Metallic nanoparticles-incorporated hydrogel is the three- dimensional polymeric structure of a hydrophilic network containing metal nanoparticles, widely used in the field of pharmaceutical, biomedicine, catalysis, optics, etc.[19]. The AgNPs loaded hydrogel dressings have been widely studied[3,20]. Both silver and AgNPs are incorporated into topical gels and ointments to prevent infection against open wounds and burns[21]. AgNPs loaded hydrogel formulation is a promising approach for dermal delivery. Again, there is no requirement to use high pressure, energy, or temperature for nanoparticle synthesis. The nanoparticles improve the therapeutic efficiency due to their smaller size and surface properties.
Diabetic wounds are mostly associated with a variety of connective tissue abnormalities, such as prolonged inflammation, impaired neovascularization, decreased synthesis of collagen, increased amount of proteases, and defective macrophage activity, which cause a delay in the wound healing processes[22]. The delayed wound healing in diabetes has become one of the major issues attributed to an imbalance between oxidant and antioxidant defense mechanisms, causing lipid peroxidation, DNA damage, enzyme inactivation, tissue damage, etc.
Withania coagulans Dunal. (Solanaceae), commonly- knownasacheesemaker, hasshowntopossessvarietyof pharmacological properties, such as hypoglycemic[23], wound healing[24,25], antibacterial[26], anthelmintic[27], antifungal[28], free radical scavenging, adaptogenic, anti-inflammatory[29,30], hepatoprotective[29], hypolipidemic[31], cardiovascular[32], antitumor and cytotoxic activities[33,34]. The fruits of W. coagulans are highly enriched with bioactive steroidal lactones (withanolides)[35]. Major withanolides in the plant include withanolide K, withanolide A, withaferin A, withacoagulin H, withanolide J, coagulanolide, and withanolide[35,36]. The present study describes the development and evaluation of hydrogel containing AgNPs with W. coagulans aqueous extract.
In our earlier study, the wound healing potential of W. coagulans in diabetic wound models has already been reported[24,25]. Based upon that, the prepared hydrogel containing AgNPs with W. coagulans aqueous extract can be used for diabetic wound dressing application.
Materials and Methods
Materials:
Silver nitrate (AgNO3) was obtained from S.D. Fine chemicals (Mumbai, India), Carbopol 971P NF obtained from Lubrizol Corporation (Wickliff, OH, USA). Propylene Glycol (PG) was purchased from Sisco Research Laboratory (SRL). Triethanolamine (TEA) was purchased from Sigma-Aldrich. All the aqueous solutions and reagents were prepared by using Millipore water.
Plant material collection and authentication:
The dried fruits of W. coagulans were purchased from the local herbal market (Varanasi, Uttar Pradesh, India) and authenticated by Prof. V. K. Joshi, Department of Dravyaguna, Institute of Medical Sciences, Banaras Hindu University. The voucher specimen (CHV/2017/01) was deposited in the Department of Pharmaceutical Engineering and Technology, IIT (BHU) Varanasi.
Preparation of plant extract:
W. coagulans fruits were crushed to obtain coarse powder and subjected to extraction by cold maceration method. Accurately 1 kg of crushed fruit was soaked with 2 l of water for 24 h and filtered with muslin cloth followed by Whatman No.1 filter paper (25 µm). The aqueous extract was stored at 4° for further use.
Synthesis of AgNPs and AgNPs with W. coagulans (WCAgNPs)
Different concentrations of aqueous plant extract (1 %, 2 %, 3 %, 4 % and 5 %) and aqueous AgNO3 solution of different molarity (0.1, 0.5, 1, and 2 mM) was selected for finding the best combination solution for synthesis of AgNPs. Initially, 10 ml of each concentration of AgNO3 solution was added to 500 μl aliquots of different concentrations of aqueous W. coagulans extracts. The reaction was allowed to take place in 25 ml volumetric flasks at room temperature. The solutions were placed on a mechanical shaker for 24 h and continuously observed for color changes, which is an indication of the synthesis of AgNPs. Simultaneously, aliquots were taken and analyzed for absorbance from 200 to 800 nm in a Ultraviolet (UV)-visible spectrophotometer (Shimadzu UV 1800, Japan). After the synthesis of AgNPs, the solution was centrifuged at 20 000 rpm (Remi centrifuge instrument, Mumbai) for 15 min, and the supernatants were discarded. Nanoparticles were washed with 10 ml of Millipore water, followed by centrifugation, decantation of supernatant, and further washing. Finally, AgNPs pellet was redispersed in 10 ml of Millipore water. The washing off of the extract was used only for obtaining purified AgNPs for their characterizations. In all other cases (formulation development of hydrogel), the washing step was avoided in order to prepare WCAgNPs that will retain the therapeutic effect of both AgNPs and aqueous W. coagulans extract.
Evaluation of AgNPs:
UV-visible spectral analysis: The absorption spectra were recorded within the wavelength range of 200- 800 nm utilizing UV-visible spectrophotometer (Shimadzu UV 1800, Japan) with 1 cm pathlength Quartz cuvette.
Particle size and polydispersity index: The particle size and polydispersity index of AgNPs were measured by a Dynamic Light Scattering (DLS) method (Malvern Zetasizer Nano S-90, Malvern Instruments, UK) at 25°. Samples were diluted ten times with Millipore water before analysis and filtered through a 0.2 µm syringe filter (Pall-Gelman Supor Acrodisc®). Each sample was analyzed in triplicate at a scattering angle of 173°[37].
Scanning Electron Microscopy (SEM): The surface morphology of AgNPs was visualized using a scanning electron microscope (Carl Zeiss EVO- 18) at 20 kV. The High-Resolution Transmission Electron Microscopy (HR-TEM) grid after HR-TEM analysis was subjected to SEM analysis. The film of the sample was prepared on a carbon-coated copper grid by dropping a small amount of diluted AgNPs solution and allowed to dry prior to measurements. The extra solution was removed using a blotting paper[38].
Energy-Dispersive X-ray Analysis (EDAX): In order to carry out EDAX analysis, the AgNPs were dried on a carbon-coated copper film and performed on a Carl Zeiss EVO-18 SEM instrument equipped with Thermo EDAX attachments[38].
HR-TEM: The prepared AgNPs were evaluated for surface morphology by HR-TEM using FEI instrument (FEI Company, TECNAI G2). AgNPs samples were prepared by diluting nanoparticles ten times with Millipore water, and one drop of the samples was placed on a carbon-coated copper grid. The excess water was removed with clean filter paper and dried to remove the trace amount of water. The images were captured with a HR-TEM instrument at 200 kV[37].
Preparation of topical hydrogel containing WCAgNPs:
The hydrogel containing WCAgNPs was prepared by aqueous dispersion method utilizing Carbopol 971 P NF of various concentrations, PG, TEA and Millipore water. The blank hydrogel formulations were prepared by dispersing the required quantity of Carbopol-971 P NF in 20 ml of Millipore water with continuous stirring and allowing hydration for 24 h. After that, 10 ml of WCAgNPs (prepared from 1 % aqueous extract and 0.1 mM AgNO3 without the washing off of extract) was added to each blank gel with continuous stirring till it dispersed in gel completely. Based upon the smaller particle size and low PDI value of AgNPs prepared from 1 % aqueous extract and 0.1 mM AgNO3, the corresponding WCAgNPs was selected for incorporation into the hydrogel. A required quantity of PG as a humectant was added to the hydrogel. Lastly, the TEA was added dropwise to adjust the pH of the gel formulation[39]. The prepared gels were packed in a wide-mouthed glass jar covered with the screw-capped plastic lid after covering the mouth with an aluminum foil and were stored in a dark and cool place. The composition of hydrogel prepared from WCAgNPs is presented in Table 1.
| Ingredients | F1 | F2 | F3 |
|---|---|---|---|
| Carbopol-971 P NF | 0.5 gm | 1 gm | 1.5 gm |
| Water up to (ml) | 20 ml | 20 ml | 20 ml |
| WCAgNPs | 10 ml | 10 ml | 10 ml |
| PG | 1 ml | 1 ml | 1 ml |
| Triethanolamine (TEA) | q.s | q.s | q.s |
Table 1: Composition of Hydrogel Formulations Containing WCAgNPs
Evaluation of WCAgNPs loaded hydrogel:
Physical appearance: The color, consistency, and homogeneity of prepared hydrogel formulations were observed visually.
pH: The pH of the formulated gel was determined using a digital pH meter (PC 700, Eutech Instrument, Singapore) which was previously calibrated using pH 4, pH 7, and pH 10 buffer solutions. 1 g of gel was accurately weighed, dispersed in 10 ml of Millipore water, evaluated for its pH in triplicates at ambient temperature, and average values were reported[40].
Viscosity: The spreadability and adhesiveness property of hydrogel depend upon the viscosity[41]. The viscosity of gel was measured by Brookfield viscometer using spindle S64 (Model-LVDVE, Brookfield Engineering Laboratories, MA, USA). The measurements were performed in triplicate at 10 rpm at room temperature.
Syneresis measurement: Syneresis is the shrinkage and contraction of the gel upon storing and separation of water from the gel[42]. In this test, the prepared hydrogel was kept in a cylindrical plastic centrifuge tube with perforations at the bottom and covered with filter paper (Whatman No. 41). Tubes were placed in a centrifuge and centrifuged for 15 min at 2000 rpm. The percentage of syneresis was evaluated as follows[43].
Percentage of syneresis=Weight of liquid separated from AgNPs/ Total weight of hydrogel before centrifugation×100
Spreadability measurement: About 0.5 g of each formulated gel and standard marketed gel (Silver X, Sun Pharma Laboratories Ltd.) was placed within a circle of 1 cm diameter pre-marked on a glass plate. A second plate is placed over it, and the weight of 500 gm is kept on it for 5 min. The minimum three diameters of each circle were measured in cm, and the average of three diameters was calculated. The increase in the diameter due to the spreading of hydrogel was observed and noted for all formulations separately in a triplicate manner[44,45].
Occlusion test: The occlusion test for prepared hydrogel was evaluated as per the method by Rai et al.[46]. In this method, three beakers of 100 ml volume were selected and each was filled with 50 ml of water. All the beakers were covered by cellulose acetate filter paper (90 mm thickness, cutoff size: 4-7 µm). A gel sample of 200 mg was spread with a stainless- steel spatula on the filter paper surface (19.63 cm2), leading to a 10.19 mg/cm2 applied amount. A visible thin film formed on the filter paper. The sample was preserved at skin temperature (32°) for a period of 48 h at 50-55 % Relative Humidity (RH). The sample weight was taken at 6, 24, and 48 h to evaluate the quantity of water loss due to evaporation (water loss through the filter paper). A beaker covered with filter paper without application of sample was taken as a reference. The experiment was conducted in triplicate (n=3). The occlusion factor F was calculated as:
F=A-B/A×100
where A is the amount of water loss without a sample (reference) and B is the amount of water loss with the sample. An occlusion factor of 0 indicates no occlusive effect as compared to the reference, and 100 is the maximum occlusion factor.
Extract excipient compatibility study: The extract excipient interaction can be identified by Attenuated Total Reflectance- Fourier Transform Infrared Spectroscopy (ATR-FTIR, Alpha II, Bruker Co. Germany). The FTIR spectra of aqueous extract, WCAgNPs, and nano hydrogel formulations were recorded on a spectrometer in the frequency range 4000-500 wavenumbers (cm-1) with 50 number scans and 4 cm-1 spectral resolution[47].
Results and Discussion
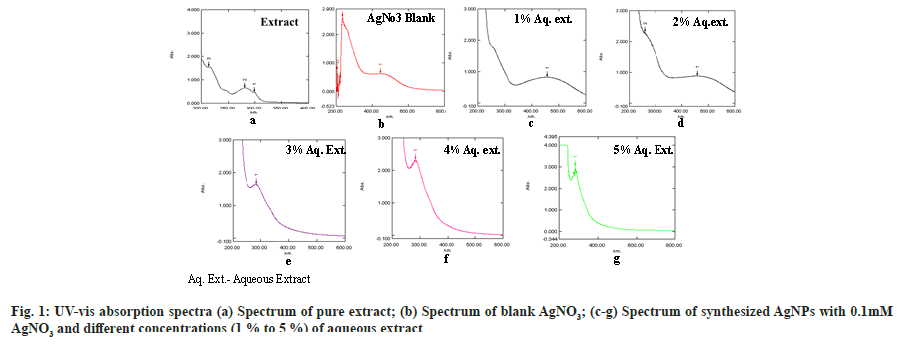
The solution of aqueous extract after suitable dilutions was scanned between 200-800 nm range, and λmax was found at 280 nm. Different peaks obtained from the scan of aqueous extract were given in Table 2 with respective absorbances. Different UV absorption peaks with 0.1 mM AgNO3 and various concentrations of plant extract (1 %-5 %) was shown in fig. 1 (a-g). On mixing plant extract with the silver nitrate solution, a change in the solution color from pale yellow to yellowish-brown was observed after 24 h which indicates the reduction of silver ions and the formation of AgNPs. The AgNPs solution showed yellowish-brown color due to excitation of surface plasmon vibrations[48]. The strong and broad surface plasmon peak was observed between 450-460 nm for AgNPs formulations containing 0.1 mM AgNO3 and 1 % or 2 % aqueous solution of plant extract (fig. 1c and fig. 1d).
| Peak No. | Wavelength (nm) | Absorbance |
|---|---|---|
| 1 | 299.41 | 0.478 |
| 2 | 280 | 0.659 |
| 3 | 213.7 | 1.589 |
Table 2: The Wavelength and Corresponding Uv Absorbances Recorded for the Aqueous Extract of W. coagulans
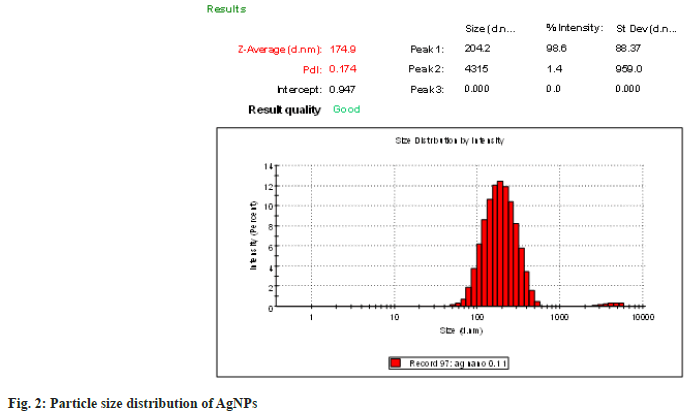
The average particle size and polydispersity index data are shown in fig. 2. The formulation with 0.1 mM AgNO3 and 1 % aqueous extract was found to have a minimum particle size of 174.9±0.062 nm among all AgNPs preparations. The PDI value of 0.174±0.054 reflected the homogenous population with a narrow size distribution.
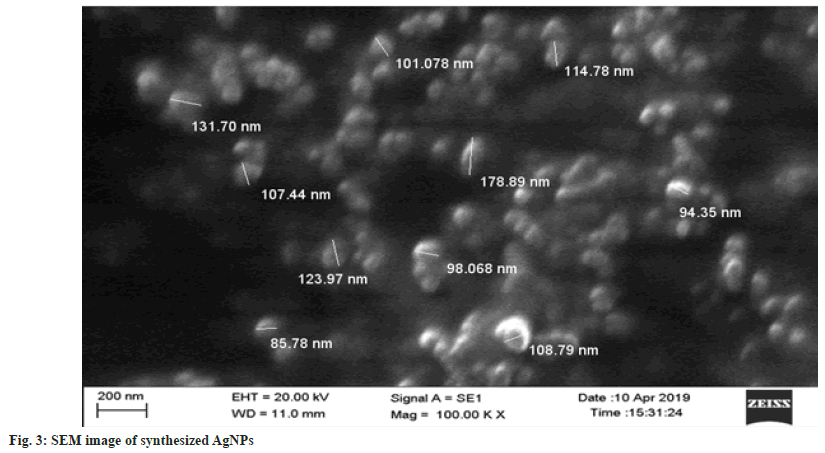
SEM analysis data of nanoparticles shows that synthesized WCAgNPs were spherical in shape and uniformly distributed (fig. 3). The image obtained from SEM analysis was processed through Image J software (version 1.41o, Java 1.6.0 10, Wayen Rasband, U.S. National Institutes of Health, Bethesda, MD, USA) for measuring the diameter of AgNPs. The particles were found to be within 85.78 to 178.89 nm (fig. 3). Some nanoparticles were bigger in size, which may be due to the overlapping of the particles on the grid during sampling.
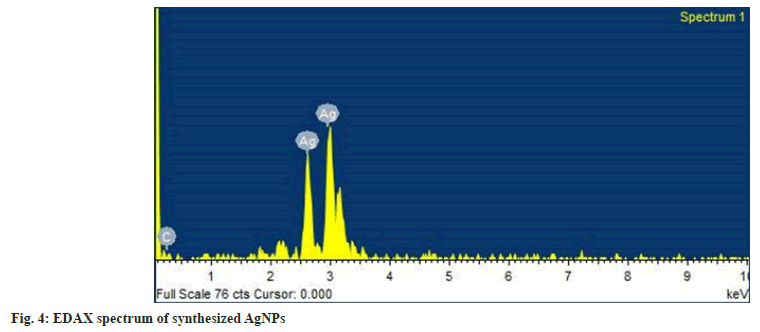
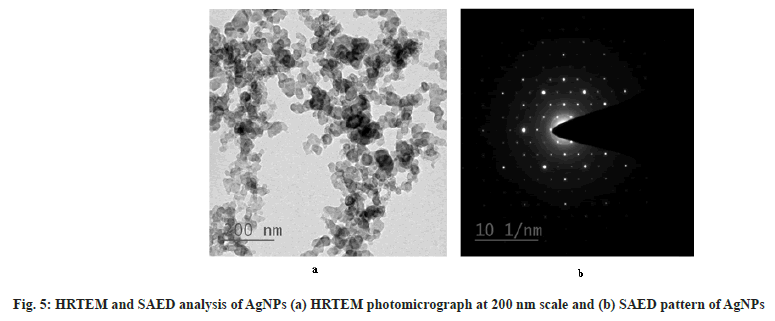
The EDAX spectra of AgNPs are shown in fig. 4. The spectra clearly showed a strong spectral signal due to silver at 3 keV due to surface plasmon resonance[49]. Other signal of carbon suggests the presence of phytochemicals adjacent to WCAgNPs. The HR- TEM photomicrograph of the AgNPs is shown in fig. 5a. The result clarified that synthesized AgNPs are spherical in shape and uniformly distributed with a smooth surface. The crystalline nature of the AgNPs was confirmed from the spot pattern obtained from SAED analysis (fig. 5b). Similar SAED results of AgNPs have been reported previously[50].
The results of the evaluation studies of the WCAgNPs loaded hydrogel are given in Table 3. From visual inspection, it was observed that all the prepared WCAgNPs hydrogel formulations were yellowish-brown in color, smooth, homogenous, and free from any lumps (Table 3). The pH of the topical formulations should be suitable for skin pH in order to avoid irritation or disruption of the skin[51]. The pH values of hydrogel formulation were found to be in a range of 6.24-6.78 (Table 3) after the addition of the required quantity of TEA to the prepared hydrogel. The pH of all the formulations was within the range of acceptable range for topical application.
| Formulations | F1 | F2 | F3 |
|---|---|---|---|
| Colour | Yellowish brown | Yellowish brown | Yellowish brown |
| Homoginity | Homogeneous | Homogeneous | Homogeneous |
| Constistancy | Excellent | Excellent | Excellent |
| pH | 6.24±0.047 | 6.43±0.067 | 6.78±0.082 |
| Viscosity (cp) | 41840±257.07 | 58786±210.83 | 87538±389.87 |
| Syneresis (%) | 1.47±0.12 | 0.82±0.058 | 0.46±0.074 |
| Spreadability (cm) | 6.4±0.124 | 5.4±0.081 | 4.83±0.08 |
| Occlusion test value | 54.43±1.24 | 67.67±2.37 | 74.82±2.48 |
Table 3: Pharmaceutical Characterization of Hydrogel Formulations
The results of viscosity studies of all formulations are shown in Table 3. The viscosity of F1 was found to be less in comparison to other formulations. An increase in the concentration of Carbopol-971 P NF shows an increase in viscosity. The results of viscosity indicated the hydrogel formulations were consistent, i.e., neither too thick nor too thin.
The minimum syneresis represents better hydrogel stability[52]. The percentage syneresis of formulations was found to range from 0.46±0.074 to 1.47±0.12. The results of the syneresis are shown in Table 3. This indicated that the F3 had the lowest syneresis in comparison to other formulations. The lower syneresis represented the good gelling stability of the hydrogel formulation.
The ease of application of the topical hydrogel- based product depends on its spreadability. From the patient compliance point of view, spreadability is an essential property of topical formulation. Good spreadability provides uniform application of gel to the skin. It mainly depends upon the concentration and type of gelling agent used in the formulation[51]. The results of spreadability shown in Table 3 are comparable and quite nearer to the marketed formulation (spreadability 5.87 cm) Silver X, Sun Pharma Laboratories Ltd., representing its suitability for topical application.
The occlusive effects of the tested hydrogels are given in Table 3. The occluding nature of the topical gel favors the permeation of the AgNPs to the deeper site of the skin. This is due to hydration of the stratum corneum in which water associates with the polar head groups of the lipid bilayers present in the intercellular spaces through hydrogen bonding. The formation of a hydration shell loosens the lipid packing, reduces the intermolecular forces, and increases the fluidity of the bilayer region[53].
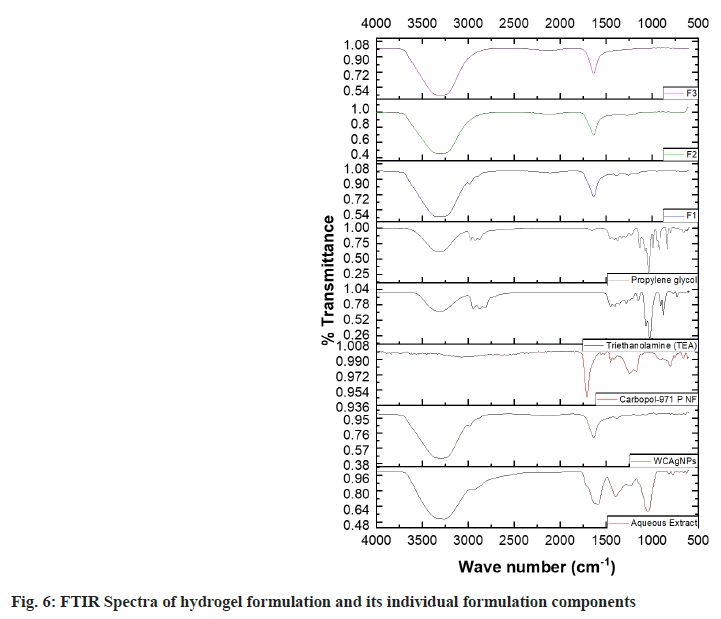
The ATR-FTIR spectra of formulation components and the hydrogel formulations (F1, F2, and F3) are shown in fig. 6. The FTIR spectrum of the pure aqueous extract showed the characteristic bands at 3424.99, 1708.44, and 1640.86 cm-1 ascribed to -OH, α, β-unsaturated δ-lactone, and carbonyl groups, respectively. A similar result was previously found by Youn et al.[54]. The spectra of WCAgNPs showed the -OH group at 3300.83 cm-1 and the carbonyl group at 1612.04 cm-1, reflecting the integrity of the chemical groups in WCAgNPs. However, the intensity of FTIR peak due to α, βunsaturated δ-lactone at 1725.24 cm-1 was diminished in the WCAgNPs formulation, which might be due to the lower concentration of extract in the WCAgNPs. In all WCAgNPs hydrogel formulations (F1, F2 and F3), the presence of all the prominent peaks of extract represents its chemical integrity in the hydrogel formulations. So, all the formulation components used in the formulations are compatible with each other.
The green synthesis approach to nanoparticle production has gained growing demand. Among the different metallic nanoparticles, AgNPs are regarded as excellent antibacterial agents due to their non- toxic effect on human cells. Due to the presence of numerous bioactive phytoconstituents in medicinal plants, they have been used as a ho`me remedy since ancient times. The phytoconstituents in plants have the tendency to reduce the silver ions and facilitate the synthesis of AgNPs from plant extracts without using harmful, toxic, and hazardous chemicals. Again, these AgNPs have a strong binding tendency with many functional groups of the plant extracts. The present study revealed a simple, rapid, economic, eco-friendly method to synthesize AgNPs utilizing aqueous fruit extract of W. coagulans. The synthesized WCAgNPs were incorporated into a gelling agent for ease of application and better retention at the site of application. The formulated WCAgNPs topical gels were transparent, non-greasy, and pleasant in appearance. The diabetic wound healing activity of W. coagulans has already been reported earlier. The activity is ascribed to the presence of withaniolides in the fruits. The hydrogel containing AgNPs with W. coagulans aqueous extract will retain the therapeutic property of bpth AgNPs and aqueous extract, thus may produce a synergistic effect. Hence WCAgNPs in hydrogel base may be used as a suitable formulation for the healing of the diabetic wound.
Acknowledgments:
The financial assistance provided to Mr. Chethan HV as Teaching Assistantship by the Ministry of Human Resource Development (MHRD), Government of India, is greatly acknowledged.
Conflict of interest:
The authors declare no conflicts of interest.
References
- Byeon JC, Ahn JB, Jang WS, Lee SE, Choi JS, Park JS. Recent formulation approaches to oral delivery of herbal medicines. J Pharm investigation. 2019 Jan 15;49:17-26.
- Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 2018;9(1):1050-74.
[Crossref] [Google Scholar] [PubMed]
- Juby KA, Dwivedi C, Kumar M, Kota S, Misra HS, Bajaj PN. Silver nanoparticle-loaded PVA/gum acacia hydrogel: Synthesis, characterization and antibacterial study. Carbohydr Polym 2012;89(3):906-13.
[Crossref] [Google Scholar] [PubMed]
- Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces 2008;65(1):150-3.
[Crossref] [Google Scholar] [PubMed]
- Narayanan KB, Sakthivel N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater Lett 2008;62(30):4588-90.
- Kasthuri J, Veerapandian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B Biointerfaces 2009;68(1):55-60.
[Crossref] [Google Scholar] [PubMed]
- Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: Technological concepts and future applications. J Nanopart Res 2008;10:507-17.
- Jha AK, Prasad K, Prasad K, Kulkarni AR. Plant system: Nature's nanofactory. Colloids Surf B Biointerfaces 2009;73(2):219-23.
[Crossref] [Google Scholar] [PubMed]
- Tian J, Wong KK, Ho CM, Lok CN, Yu WY, Che CM, et al. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2007;2(1):129-36.
[Crossref] [Google Scholar] [PubMed]
- Yang W, Shen C, Ji Q, An H, Wang J, Liu Q, et al. Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology 2009;20(8):085102.
[Crossref] [Google Scholar] [PubMed]
- Varaprasad K, Mohan YM, Ravindra S, Reddy NN, Vimala K, Monika K, et al. Hydrogel–silver nanoparticle composites: A new generation of antimicrobials. J Appl Polymer Sci 2010;115(2):1199-207.
- 12. Khan M, Shaik MR, Adil SF, Khan ST, Al-Warthan A, Siddiqui MR, et al . Plant extracts as green reductants for the synthesis of silver nanoparticles: Lessons from chemical synthesis. Dalton Transact 2018;47(35):11988-2010.
- Marin S, Mihail Vlasceanu G, Elena Tiplea R, Raluca Bucur I, Lemnaru M, Minodora Marin M, et al. Applications and toxicity of silver nanoparticles: a recent review. Curr Top Med Chem 2015;15(16):1596-604.
[Crossref] [Google Scholar] [PubMed]
- Rai MK, Deshmukh SD, Ingle AP, Gade AK. Silver nanoparticles: The powerful nanoweapon against multidrug‐resistant bacteria. J Appl Microbiol 2012;112(5):841-52.
[Crossref] [Google Scholar] [PubMed]
- Ovais M, Ahmad I, Khalil AT, Mukherjee S, Javed R, Ayaz M, et al. Wound healing applications of biogenic colloidal silver and gold nanoparticles: recent trends and future prospects. Appl Microbiol Biotechno 2018;102:4305-18.
[Crossref] [Google Scholar] [PubMed]
- Lai WF, Rogach AL. Hydrogel-based materials for delivery of herbal medicines. ACS Applied Mater Interfaces 2017;9(13):11309-20.
- Morgan DA. Wound management products in the drug tariff. Pharmaceutical J 1999;263(7072):820-5.
- Morgan D. Wounds: what dressings should a formulary include. Hosp Pharm. 2002;9:261-6.
- Jovanović Ž, Krklješ A, Stojkovska J, Tomić S, Obradović B, Mišković-Stanković V, et al. Synthesis and characterization of silver/poly (N-vinyl-2-pyrrolidone) hydrogel nanocomposite obtained by in situ radiolytic method. Radiation Physics Chem 2011;80(11):1208-15.
- Park S, Murthy PK, Park S, Mohan YM, Koh WG. Preparation of silver nanoparticle-containing semi-interpenetrating network hydrogels composed of pluronic and poly (acrylamide) with antibacterial property. J Industrial Eng Chem 2011;17(2):293-7.
- Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 2009;32:79-84.
[Crossref] [Google Scholar] [PubMed]
- Gupta A, Upadhyay NK, Sawhney RC, Kumar R. A poly‐herbal formulation accelerates normal and impaired diabetic wound healing. Wound Repair Regenerat 2008;16(6):784-90.
- Hemalatha S, Wahi A, Singh P, et al. Hypoglycemic activity of Withania coagulans Dunal in streptozotocin induced diabetic rats. J Ethnopharmacol. 2004;93(2-3):261-264. [Crossref] [Google Scholar] [PubMed]
- Hemalatha S, Mishra N, Kumar M, Singh PN, Chansouria JP, Mandal V. Wound healing activity of Withania coagulans fruits. In: 12th Annual National Convention of Indian Society of Pharmacognosy at Moga, Punjab: OP 2008.
- Prasad SK, Kumar R, Patel DK, Hemalatha S. Wound healing activity of Withania coagulans in streptozotocin-induced diabetic rats. Pharm Biol 2010;48(12):1397-404.
[Crossref] [Google Scholar] [PubMed]
- Khan MT, Ashraf M, Tehniyat S, Bukhtair MK. Antibacterial activity of Withania coagulants. Fitoterapia-Milano 1993;64:367.
- Gaind KN, Budhiraja RD. Antibacterial and anthelmintic activity of Withania coagulans Dunal. Indian J Pharm 1967;29:185-6.
- Choudhary MI, Parveen Z, Jabbar A, Ali I. Antifungal steroidal lactones from Withania coagulance. Phytochemistry 1995;40(4):1243-6.
[Crossref] [Google Scholar] [PubMed]
- Budhiraja RD, Sudhir S, Garg KN. Antiinflammatory activity of 3 β-Hydroxy-2, 3-dihydro-withanolide F. Planta Med 1984;50(02):134-6.
[Crossref] [Google Scholar] [PubMed]
- Rajurkar SM, Thakre PN, Waddukar SG. Phytochemical and pharmacological screening of Withania coagulans berries as anti-inflammatory. In: Proceedings of the 53rd Indian Pharmaceutical Congress (IPC’01) 2001.
- Hemalatha S, Wahi AK, Singh PN, Chansouria JP. Hypolipidemic activity of aqueous extract of Withania coagulans Dunal in albino rats. Phytother Res 2006;20(7):614-7.
[Crossref] [Google Scholar] [PubMed]
- Budhiraja RD, Sudhir S, Garg KN. Cardiovascular effects of a withanolide from Withania coagulans, dunal fruits. Indian J Physiol Pharmacol 1983;27(2):129-34.
[Google Scholar] [PubMed]
- Budhiraja RD, Sudhir S. Review of biological activity of withanolides. J Sci Indust Res 1987.
- Chattopadhyay P, Mahaur K, Saha SK, Singh L, Shukla G, Wahi AK. Effect of aqueous extract of fruits of Withania coagulans on cytotoxicity and tumor necrosis factor α-production in chicken lymphocytes. Indian J Nat Prod 2007;23:8-12.
- Das S, Das J, Samadder A, Bhattacharyya SS, Das D, Khuda-Bukhsh AR. Biosynthesized silver nanoparticles by ethanolic extracts of Phytolacca decandra, Gelsemium sempervirens, Hydrastis canadensis and Thuja occidentalis induce differential cytotoxicity through G2/M arrest in A375 cells. Colloid Surf B Biointerfaces 2013;101:325-36.
[Crossref] [Google Scholar] [PubMed]
- Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochimica Acta Mol Biomol Spectroscopy 2011;79(3):594-8.
- Chaudhary H, Rohilla A, Rathee P, Kumar V. Optimization and formulation design of carbopol loaded Piroxicam gel using novel penetration enhancers. Int J Biol Macromol 2013;55:246-53.
[Crossref] [Google Scholar] [PubMed]
- Pillai AB, Nair JV, Gupta NK, Gupta S. Microemulsion-loaded hydrogel formulation of butenafine hydrochloride for improved topical delivery. Arch Dermatol Res 2015;307:625-33.
[Crossref] [Google Scholar] [PubMed]
- Guan Y, Zuo T, Chang M, Zhang F, Wei T, Shao W, et al. Propranolol hydrochloride-loaded liposomal gel for transdermal delivery: Characterization and in vivo evaluation. Int J Pharm 2015;487(1-2):135-41.
[Crossref] [Google Scholar] [PubMed]
- Mohapatra A, Parikh RK, Gohel MC. Formulation, development and evaluation of patient friendly dosage forms of metformin, Part-II: Oral soft gel. Asian J Pharm 2008;2(3).
- Ashara KC, Paun JS, Soniwala MM, Chavda JR, Mendapara VP, Mori NM. Microemulgel: An overwhelming approach to improve therapeutic action of drug moiety. Saudi Pharm J 2016;24(4):452-7.
[Crossref] [Google Scholar] [PubMed]
- Bachhav YG, Patravale VB. Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation. Int J Pharm 2009;365(1-2):175-9.
[Crossref] [Google Scholar] [PubMed]
- Khan AW, Kotta S, Ansari SH, Sharma RK, Kumar A, Ali J. Formulation development, optimization and evaluation of aloe vera gel for wound healing. Pharmacogn Mag 2013;9(Suppl 1):S6-10.
[Crossref] [Google Scholar] [PubMed]
- 44. Bhaskar K, Mohan CK, Lingam M, Mohan SJ, Venkateswarlu V, Rao YM, et al. Development of SLN and NLC enriched hydrogels for transdermal delivery of nitrendipine: in vitro and in vivo characteristics. Drug Dev Ind Pharm 2009;35(1):98-113.
[Crossref] [Google Scholar] [PubMed]
- Pramod K, Suneesh CV, Shanavas S, Ansari SH, Ali J. Unveiling the compatibility of eugenol with formulation excipients by systematic drug-excipient compatibility studies. J Anal Technol 2015;6(1):1-4.
- Rai A, Singh A, Ahmad A, et al. Role of halide ions and temperature on the morphology of biologically synthesized gold nanotriangles. Langmuir 2006;22(2):736-741. [Google Scholar]
[PubMed]
- Magudapathy P, Gangopadhyay P, Panigrahi BK, Nair KG, Dhara S. Electrical transport studies of Ag nanoclusters embedded in glass matrix. Physica B: Condensed Matter 2001;299(1-2):142-6.
- Nagar N, Devra V. A kinetic study on the degradation and biodegradability of silver nanoparticles catalyzed Methyl Orange and textile effluents. Heliyon 2019;5(3):e01356.
[Crossref] [Google Scholar] [PubMed]
- Nikumbh KV, Sevankar SG, Patil MP. Formulation development, in vitro and in vivo evaluation of microemulsion-based gel loaded with ketoprofen. Drug deliv 2015;22(4):509-15.
[Crossref] [Google Scholar] [PubMed]
- Jagdale S, Brahmane S, Chabukswar A. Optimization of microemulgel for tizanidine hydrochloride. Anti-Inflamm Anti-Allergy Agents Med Chem 2020;19(2):158-79.
[Crossref] [Google Scholar] [PubMed]
- Barry BW. Action of skin penetration enhancers—the lipid protein partitioning theory. Int J Cosmet Sci 1988;10(6):281-93.
[Crossref] [Google Scholar] [PubMed]
- Youn UJ, Chai X, Park EJ, Kondratyuk TP, Simmons CJ, Borris RP, et al. Biologically active withanolides from Withania coagulans. J Nat Prod 2013;76(1):22-8.
[Crossref] [Google Scholar] [PubMed]