- *Corresponding Author:
- A. N. Bajaj
C. U. Shah College of Pharmacy, S.N.D.T. Women’s University, Juhu Campus, Santacruz (West), Mumbai - 400 049, India
E-mail: bajajamrita@rediffmail.com
| Date of Web Publication | 27 October, 2007 |
| Indian J Pharm Sci, 2007, 69 (5): 731-733 |
Abstract
Migraine headaches appear to be caused by complex interplay of neurologic and vascular changes and show waves of neurologic changes in brain. Essential oils like eucalyptus oil and peppermint oil have been used for aromatherapy for the treatment of migraine. The major active ingredient of eucalyptus oil is cineole (eucalyptol) that has soothing, stimulant and antidepressant effect. Microemulsions have a much greater solubilizing capacity for non-polar organic compounds than aqueous micellar solutions and hence attempts are made to develop microemulsion of eucalyptus oil for intranasal delivery.
Materials and Methods
Eucalyptus oil (Vedic Life Sciences, Mumbai), Tween80, Span80 and PEG400 (S. D. Fine Chemicals Ltd. and Merck Ltd.), nasal spray pumps (Valois Pvt. Ltd.)
Formulation development of eucalyptus oil microemulsions
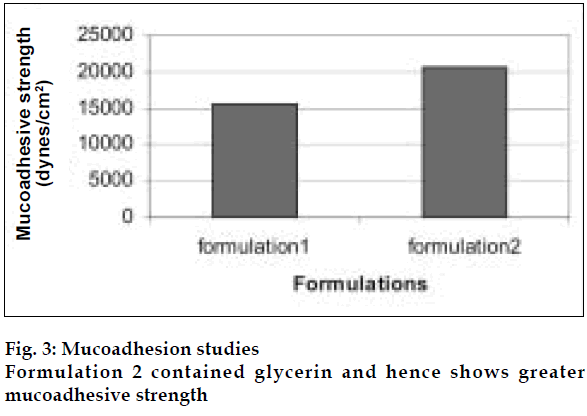
Eucalyptus oil microemulsions were prepared by combining hydrophilic and lipophilic surfactants namely Tween80, Span80 and Cosurfactant PEG400. Glycerin was added in the formulations as it acts as humectant for nasal formulations.
Phase solubility studies and microemulsion formulations
The pseudoternary phase diagrams of oil, surfactant, cosurfactant and water were constructed using water titration method. For solubility studies, the ratio of oil to the surfactant/cosurfactant mixture (Smix) was varied from 1:9-9:1 (w/w). Water was added drop wise to the oil-Smix mixture under vigorous stirring to form a clear and transparent microemulsion.
Evaluation of optimized microemulsion of eucalyptus oil
The optimized formulation was evaluated for parameters like clarity, viscosity, pH, particle size and stability. Eucalyptus oil content was determined by HPTLC method (Table 1).
| Parameters | Results |
|---|---|
| Clarity | Clear, transparent |
| pH | 5.60 |
| Viscosity (50 rpm) | 304.7 cps |
| Globule size | 355.8 nm |
| Stability on centrifugation at | Did not separate into two |
| 6000 rpm for 10 min | layers |
| Eucalyptus oil content | 97.47% w/w |
Table 1: Evaluation of Eucalyptus Oil Microemulsion
HPTLC method development for analysis of eucalyptus oil in formulations
Formulation equivalent to 25 mg of eucalyptus oil was accurately weighed and dissolved in 2.5 ml of methanol. The HPTLC scan of eucalyptus oil was developed on Camag Linomat IV using precoated silica gel G254 plates using benzene: ethylacetate (90:10) as mobile phase. The spots were developed with vanillin-conc.sulphuric acid as spraying agent and the plates were scanned at 540 nm. The method was validated for linearity, accuracy and reproducibility.
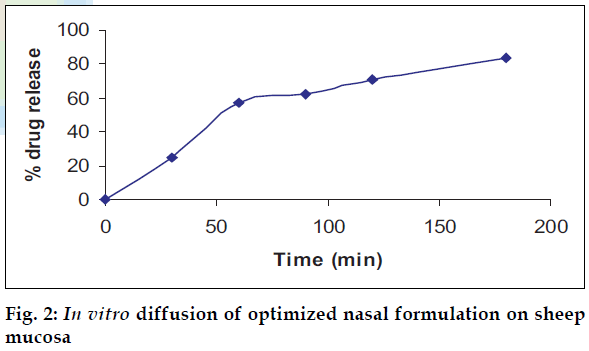
In vitro diffusion studies
The optimized formulations were subjected to release kinetic studies through sheep nasal mucosa in phosphate buffer: Tween80 (8:2) for a period of 4 h using Keshary-Chein diffusion apparatus.
Formulation and evaluation of nasal sprays
The developed formulations were filled in intranasal spray pumps. Three nasal pumps were used namely: VP7/100A CS20 AG+CB-18, VP7D/100+CB19 (Airless), VP6/100 CP20 AG+CB 20A. The nasal spray formulations of eucalyptus oil were evaluated for parameters like spray pattern, shot weight and content of eucalyptus oil per spray (Table 2, fig. 1)
| Parameters | Observations |
|---|---|
| Spray pattern | Spherically uniform |
| Shot weight | 110 mg |
| Uniformity of content per spray | 95-105%w/w |
Table 2: Evaluation of Nasal Spray
Assessment of effi cacy of the formulations
Blank and eucalyptus oil containing intranasal formulations were inhaled by a panel of human volunteers. The scores were allocated based on soothing, calming and comforting effects of the formulations.
Results and Discussion
The area of microemulsion existence obtained using Tween80/Span80/PEG400/Water system was used to determine the ratios of Smix and oil. Microemulsions developed were clear, with pH of 5.6 and particle size in the range of 300-500 nm and viscosity of 200 cps. The microemulsion was found to be stable on centrifugation at 6000 rpm for 10 min. During freeze thaw cycles no creaming and separation was observed. The in vitro diffusion studies on developed formulations showed a controlled release profile of 88% in 3 h (fig. 2). It was found that nasal spray pump VP6 with capacity of 100 µl gave constant shot weight of 110 mg with spherical and uniform spray pattern with content uniformity per spray to be 95%- 105%w/w. The solvent system comprising of benzene: ethylacetate (9:1) was found to give good separation and resolution by HPTLC method. Formulation 2 containing glycerin showed better mucoadhesive properties (fig. 3). The volunteers confirmed the soothing and calming effect of eucalyptus oil intranasal formulations as compared to the blank. Animal studies to quantify the sedative and calming effects of the intranasal formulations by aromatherapy are in progress. The intranasal spray of eucalyptus oil is a cost effective formulation as the excipients used are easily available. It is an efficient formulation, which provides rapid onset of action.
Acknowledgements
The authors wish to thank Exmore Chemicals Ltd., Valois India Pvt Ltd. and Vedic Lifesciences Pvt. Ltd for their support.