- *Corresponding Author:
- P. P. Warrier
Department of Chemistry, Ramnarain Ruia Autonomous College, Mumbai, Maharashtra 400019, India
E-mail: pravithwarrier@gmail.com
| Date of Received | 02 June 2022 |
| Date of Revision | 17 March 2023 |
| Date of Acceptance | 20 November 2023 |
| Indian J Pharm Sci 2023;85(6):1773-1780 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The herbal species of Curcuma caesia Roxb. belongs to Zingiberaceae family. The goal of the present research is to examine the phytochemical components present within Curcuma caesia rhizomes and this information is crucial for comprehending the use of the plant as medicine. In the current work, the rhizome powder is extracted in ethanol by different techniques, such as Soxhlet extraction and ultrasound-assisted extraction. The gas chromatography-mass spectrometric analysis of the extracts gives detailed information about the phytoconstituents present and this study lists the common phytoconstituents that are present by extraction of the rhizome by both extraction techniques. This information can assist in future phytoconstituent isolation and bioactivity evaluation. The information offered in this paper will also confirm the use of Curcuma caesia rhizomes for therapeutic reasons, opening new avenues for future research into other methods of plant extraction.
Keywords
Curcuma caesia Roxb., rhizome, Soxhlet, ultrasound assisted extraction, gas chromatography- mass spectrometry
Since ancient times, natural herbs have been frequently used to treat and prevent an assortment of ailments. Herbs contain a wide range of active phytoconstituents with varying pharmacology, each with their own metabolism and binding properties[1]. Curcuma caesia Roxb. (C. caesia) is an herb inherent to India, principally the Northeast, scattering up to Chhattisgarh’s Bastar area. The rhizome is greyish black or deep bluish black in colour. It is used in traditional medicine[2]. C. caesia is a rare and endangered herb, well-preserved by indigenous communities in rural areas due to its remedial properties[3]. The therapeutic qualities of C. caesia are well established. This plant is therefore regarded as an extremely beneficial medicinal plant[4].
Plant parts containing phytoconstituents with biological properties have traditionally been used as medicines. The study of medicinal plants begins with the extraction of bioactive phytoconstituents, which are very critical in the study of plant materials. Traditionally, Soxhlet extraction and maceration are typically employed to extract plant-based constituents from medicinal herbs. Contemporary approaches such as Microwave- Assisted Extraction (MAE), Ultrasound-Assisted Extraction (UAE) and Supercritical Fluid Extraction (SFE) have made important strides in the processing of medicinal plants with the intention of raising yield while lowering cost[5].
Quite a lot of research experiments have been reported for C. caesia rhizomes extracted in diverse solvents by various extraction techniques. Soxhlet extraction of C. caesia rhizomes sequentially with solvents such as hexane, ethyl acetate, methanol and water was performed and hexane extract data with Gas Chromatography-Mass Spectrometric (GC-MS) analysis has already been reported[6].
C. caesia rhizome extract prepared using cold percolation methods for methanol, acetone, and ethyl acetate solvents are studied for its bioactive metabolites by GC-MS[7]. The phytoconstituents profiling of petroleum ether and chloroform extracts of C. caesia rhizome by GC-MS is documented by Atom et al.[8]. GC-MS analysis of methanolic extract of C. caesia rhizomes by Soxhlet extraction is reported by Pakkirisamy et al.[9]. Several GC-MS studies have been performed for essential oil extracted from the rhizomes and leaves of C. caesia by different distillation techniques[4,10-12]. Some studies are also reported for GC-MS analysis of hydrosol of C. caesia prepared by hydro distillation[13].
The current work imparts information on the phytoconstituents present in the ethanol extract of C. caesia rhizomes extracted by Soxhlet and UAE. Soxhlet extraction of C. caesia rhizomes was performed using n-hexane, ethyl acetate and ethanol solvents successively and UAE of rhizomes was done in ethanol. This study focuses on the ethanol extracts acquired by Soxhlet and UAE of C. caesia rhizomes and lists and compares the phytoconstituents detected in the GC-MS analysis of both extracts. This study also provides information on the structure and molecular weight of the phytoconstituents present in both extracts. No research findings have been reported regarding the utilisation of UAE with ethanol solvent for the extraction of C. caesia rhizomes, and to the extent that we know, this is the first research study to be reported for UAE extraction of C. caesia rhizomes. This study will pave the way for exploring different approaches to extraction for plants in the future, and the data presented in this work will reiterate the usage of C. caesia rhizomes for medicinal purposes.
Materials and Methods
Plant materials and chemicals:
C. caesia rhizome was acquired from Indian Agricultural Research Institute-Indian Institute of Spices Research (IISR), Kozhikode, Kerala, in the month of March in 2020 and 2021 and was authenticated and assigned accession number Acc. 292 (IC 349014). The studies were conducted using analytical grade of absolute ethanol and the additional solvents. For extract filtration, Whatman Filter paper 41 and MDI 0.45 micron nylon syringe filters were used.
Sample preparation:
The rhizomes were washed with running water, cut into pieces, dried for 5 d under shade, milled in a mixer grinder and sieved to obtain a fine powder. The powdered rhizomes were kept in sealed containers and used for further testing.
GC-MS analysis:
For Soxhlet extraction, a weighed portion of rhizome powder (about 5 g) was taken in a thimble and extracted using a Soxhlet apparatus with 125 ml of n-hexane, ethyl acetate and ethanol successively and the extraction was continued till nine cycles were completed. Vacuum distillation was used to concentrate the ethanol extract.
For UAE, a weighed portion of rhizome powder (about 1 g) added in a volumetric flask, with 25 ml of ethanol transferred into the flask, then sonicated for 30 min in a sonicator water bath. The extraction was done in a pulsed manner with breaks; initial sonication for 15 min, then a break of 2-3 min, then again sonicated for 6 min with a break of 2-3 min, and finally sonicated for 9 min. The temperature was maintained between 25°-30°. Both the samples obtained were passed across Whatman Paper 41 and 0.45 micron nylon membranes consecutively and inserted to the GC-MS.
Extractive value:
The extracts from the three distinct Soxhlet extractions in n-hexane, ethyl acetate and ethanol were used to figure out the extractive value in each solvent. An established size of extract was transferred into a previously weighed porcelain dish and evaporated in a water bath. The lasting residue was weighed, and the extractive values were calculated.
Instrumentation:
Sonicator: The sonicator used had an ultrasonic frequency of 36±3 kHz, an ultrasonic power of 200 W, and a tank capacity of 6.5 l Stainless steel tank. The make was Dakshin, 200H, with an electric supply of 230 V A.C., 50 Hz, and a maximum temperature capacity of 60°.
GC-MS: The GC-MS instrument utilised was a Shimadzu GCMS-QP2010 Plus model coupled with a GCMS-QP2010 Ultra Mass Spectrometer with a Restek Rtx-5MS 30 m×0.25 mm internal diameter, 0.25 µm capillary column.
GC Condition: Helium gas was used to carry samples for the study due to its better GC-MS sensitivity than nitrogen and hydrogen. The oven temperature was held at 80° for 5 min, and augmented with a heating rate of 10° per min, first to 150°, then to 220° with being steady for 2 min at each temperature, subsequently amplified to 290° and kept 5 min unchanged. Total run time of temperature programme was for 35 min[14]. The injector port temperature was fixed at 250° with constant pressure as the flow control mode, set at 83.5 kPa. The injection volume was 1 µl for Soxhlet extract and 2 µl for UAE extract. The split ratio was set to 90.0.
MS Condition: The temperatures of the ion Source and the Interface were static at 220° and 260° respectively. The detector gain was 1.03 kV and the scan speed was fixed at 2500 Hz. The solvent cut time was set at 2.0 min, and the scan start time and end time were 2.0 min and 34.0 min, respectively, with a start m/z of 35.0 and an end m/z of 700.0. The software employed to handle mass spectra and chromatograms is LabSolutions GC-MS Solution Version 2.70 software. The NIST 11 library database was used for identifying the chemical components.
Results and Discussion
C. caesia rhizome extracts in ethanol by Soxhlet extraction and UAE were scrutinized for the presence and assessment of phytoconstituents. The outcomes of the extractive value experiments are exhibited in Table 1. The extractive value in ethanol was found higher as equated to n-hexane and ethyl acetate, which indicates the enhanced extraction power of ethanol.
| S No. | Solvent | % Extractive value |
|---|---|---|
| 1 | n-hexane | 3.2694 |
| 2 | ethyl acetate | 7.747 |
| 3 | ethanol | 13.2894 |
Table 1: Extractive Values Obtained for Different Solvents
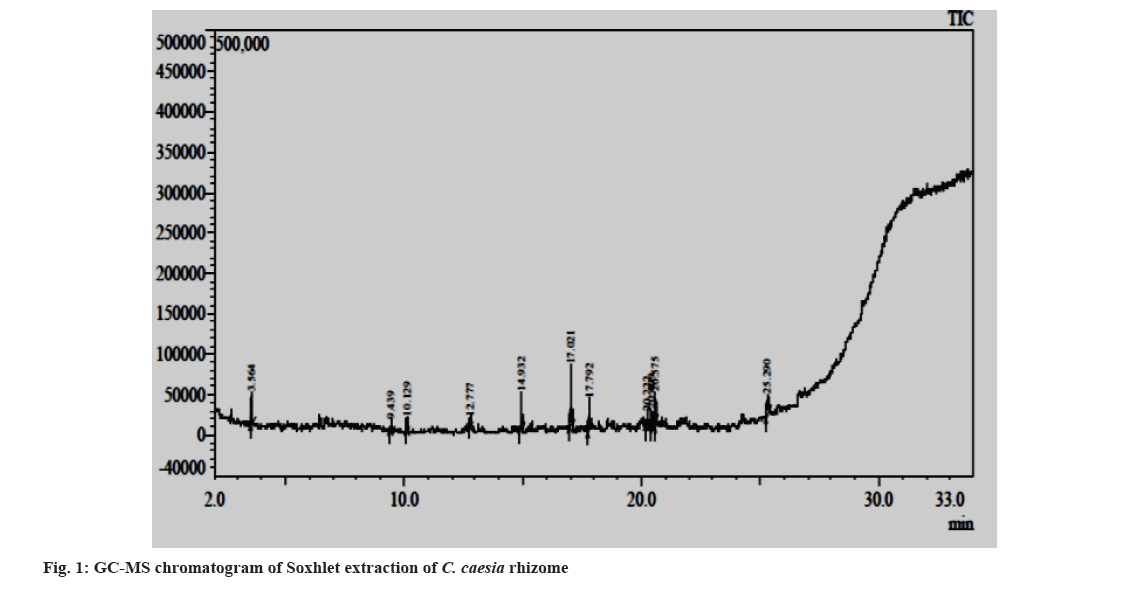
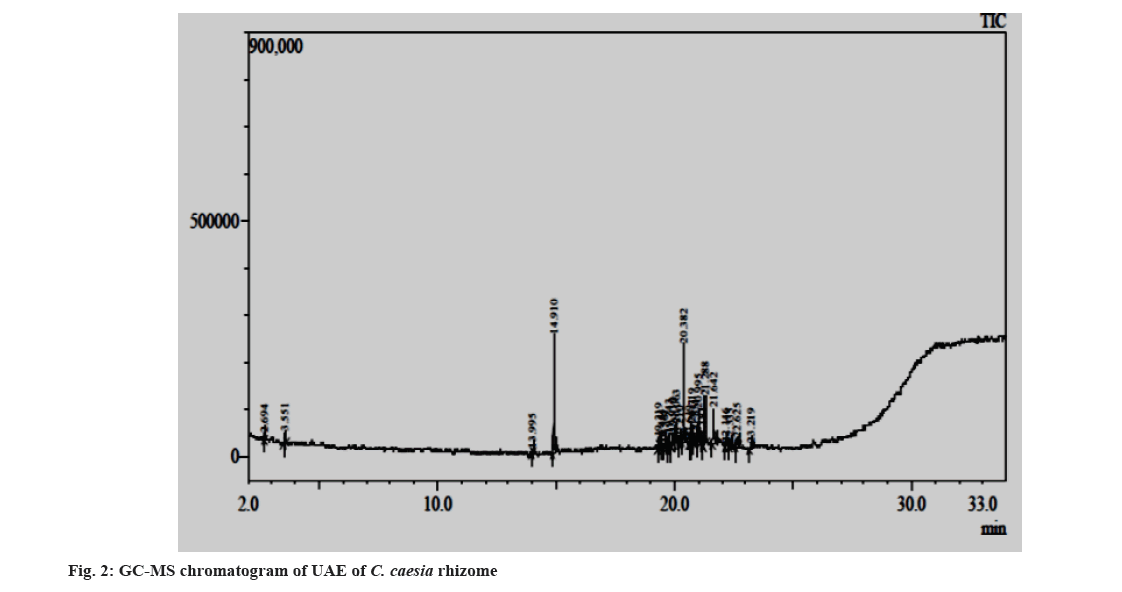
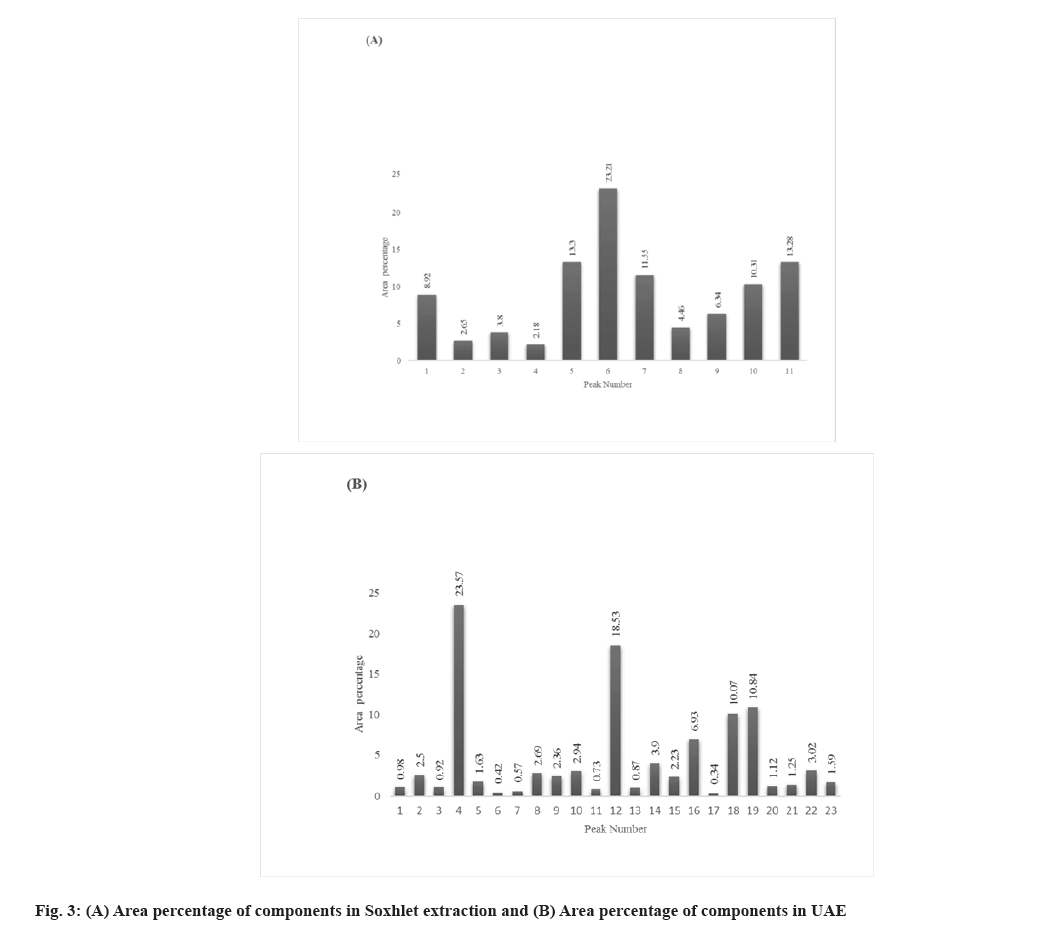
Fig. 1 and fig. 2 represent the GC-MS chromatograms of the ethanol extract by Soxhlet extraction and by UAE. The details of phytoconstituents with their retention time, concentration (Area percentage), Molecular Weight (Mol Wt), and Molecular Formula (Mol Formula), along with the structure obtained from the GC-MS analysis of ethanol extracts prepared by Soxhlet extraction and UAE, are listed in Table 2 and Table 3, respectively. The study discovered sesquiterpenoids, steroids, phenolic chemicals, and saturated fatty acids in the extracts. A graphical representation of the phytoconstituents (Peak No.) from the above tables with their respective concentrations (Area percentage) for both extracts is presented in fig. 3 for simple understanding.
| Peak no. | Name | Retention time (min) | Area % | Molecular Wt | Molecular formula | Structure |
|---|---|---|---|---|---|---|
| 1 | Eucalyptol | 3.564 | 8.92 | 154 | C10H18O | 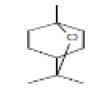 |
| 2 | Isobornyl acetate | 9.439 | 2.65 | 196 | C12H20O2 | 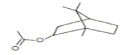 |
| 3 | Cyclohexasiloxane, dodecamethyl- | 10.129 | 3.8 | 444 | C12H36O6Si6 | 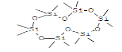 |
| 4 | Cycloheptasiloxane, tetradecamethyl- | 12.777 | 2.18 | 518 | C14H42O7Si7 | 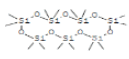 |
| 5 | 2CEHT | 14.932 | 13.3 | 178 | C11H14O2 | 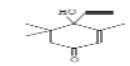 |
| 6 | (-)-Aristolene | 17.021 | 23.21 | 204 | C15H24 | 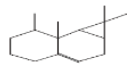 |
| 7 | CPPC | 17.792 | 11.55 | 276 | C20H36 |  |
| 8 | 9,19-Cycloergost-24(28)-en-3-ol, 4,14-dimethyl-, acetate, (3 beta,4 alpha, 5 alpha)- | 20.222 | 4.46 | 468 | C32H52O2 | 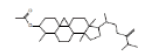 |
| 9 | TTUE | 20.409 | 6.34 | 218 | C15H22O | 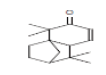 |
| 10 | DTUD | 20.575 | 10.31 | 210 | C13H22O2 | 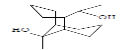 |
| 11 | Dihydroartemisinin, 5-deshydroxy-6-deshydro | 25.29 | 13.28 | 266 | C15H22O4 | 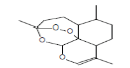 |
Table 2: Phytoconstituents in Soxhlet Extraction of Curcuma Caesia Rhizome
The extracts obtained through the process of Soxhlet extraction consisted of eleven compounds. Sesquiterpenoids such as (-)-aristolene, dihydroartemisinin, 5-deshydroxy-6-deshydro, 10,11-Dimethyl-Tricyclo [4.3.1.1(2,5)] Undecane- 10,11-diol (DTUD), along with 2-Cyclohexen-1-one, 4-Ethynyl-4-Hydroxy-3,5,5-trimethyl- (2CEHT), and cyclohexene, 4-Pentyl-1-(4-Propylcyclohexyl)- (CPPC), were the major components detected in the GC-MS analysis. 23 compounds are identified in the ethanolextractpreparedbythe UAE.Asesquiterpenoid, 2,2,7,7-Tetramethyltricyclo[6.2.1.0(1,6)] Undec- 4-en-3-one (TTUE), along with boldenone, Tetracyclo[ 5. 4. 3 .0 (7 ,11 )] Tetradeca- 2, 5, 10- Trione,1,4,6,14-Tetramethyl-4-vinyl-(TTTTV), Ethenone,1-(1-Hydroxy-2,6,6-Trimethyl-2,4- Cyclohexadien-1-yl)- (EHTC), and 2-(1-(Beta-d- Glucopyranosyloxy)-1-methylethyl)-2,3-Dihydro- 7-oxo-7H-Furo (3,2-g) Chromene, (R) (2BGDFC) were the leading phytoconstituents spotted in the GC-MS examination. Out of 23 components, twelve had an area percentage (concentration) of more than 2 %.
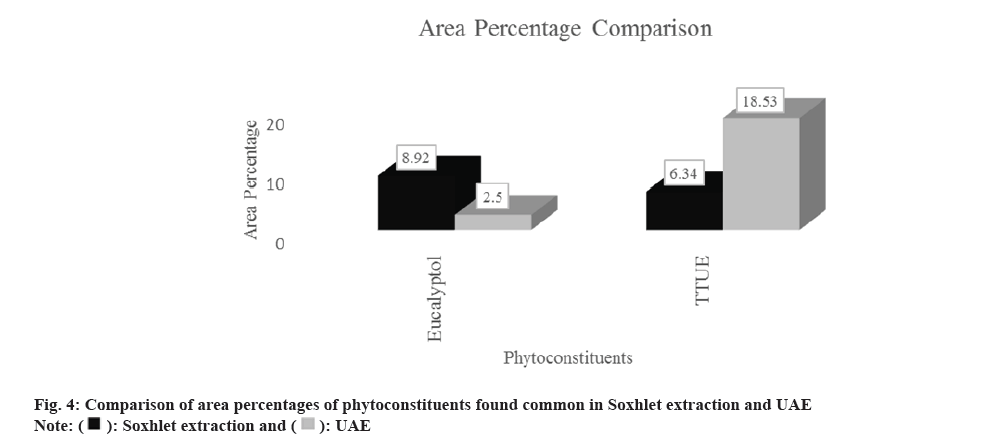
Eucalyptol and TTUE were commonly found in both extracts. A graphical comparison of the percentage area concentrations of both of these components is presented in fig. 4. Literature is available on the anti- inflammatory activity of Eucalyptol[15]. Eucalyptol is also reported as a major component in the essential oil of C. caesia rhizome from Northeast India[4]. The essential oil (hexane extract) of roots or rhizomes of Cyperus articulatus L is found to contain TTUE which is a is a sesquiterpene ketone[16]. It is also reported to be found in essential oil extracted by hydro distillation of Clerodendrum bungei Steud.[17] and is one of the main components of the essential oil from the aerial part of Aristolochia mollissima Hance[18]. The information on the application of UAE with GC-MS for different species from the Curcuma genus is listed below in Table 4. It is clear from Table 3 that UAE is being used for the extraction of essential oils from rhizomes and to perform comparative analysis with other extraction techniques to gain an understanding of the phytoconstituents.
| Peak No. | Name | Retention time (min) | Area % | Molecular wt | Molecular formula | Structure |
|---|---|---|---|---|---|---|
| 1 | Tetraethyl silicate | 2.694 | 0.98 | 208 | C8H20O4Si | 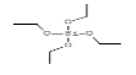 |
| 2 | Eucalyptol | 3.551 | 2.5 | 154 | C10H18O |  |
| 3 | Cyclohexane, 1,2-dimethyl-3,5-bis(1-methylethenyl)- | 13.995 | 0.92 | 192 | C14H24 | 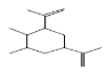 |
| 4 | Boldenone | 14.91 | 23.57 | 286 | C19H26O2 | 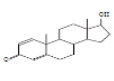 |
| 5 | Cyclohexane, 1,1-bis(5-methyl-2-furyl)- | 19.319 | 1.63 | 244 | C16H20O2 | 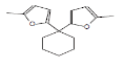 |
| 6 | 4-t-Butyl-.alpha.,.alpha.'-bis[dimethylamino]-2,6-xylenol | 19.454 | 0.42 | 264 | C16H28N2O | 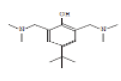 |
| 7 | 2,6-Pyrazinedicarbonitrile, 3-amino-5-(diethylamino)- | 19.561 | 0.57 | 216 | C10H12N6 |  |
| 8 | 1,6-Dimethyl-9-(1-methylethylidene)-5,12-dioxatricyclo[9.1.0.0(4,6)]dodecan-8-one | 19.743 | 2.69 | 250 | C15H22O3 |  |
| 9 | n-Hexadecanoic acid | 19.919 | 2.36 | 256 | C16H32O2 |  |
| 10 | 2,11-Dioxatetracyclo[4.3.1.1(3,10).0(6,9)]undec-4-ene, 3,7,7,10-tetramethyl | 20.063 | 2.94 | 206 | C13H18O2 | 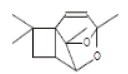 |
| 11 | 3.alpha.,7.beta.-Dihydroxy-5.beta.,6.beta.-epoxycholestane | 20.21 | 0.73 | 418 | C27H46O3 | 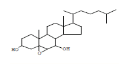 |
| 12 | TTUE | 20.382 | 18.53 | 218 | C15H22O | 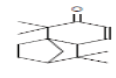 |
| 13 | 3,4-Dihydrocoumarin, 4,4,5,7,8-pentamethyl-6-cyano- | 20.619 | 0.87 | 243 | C15H17NO2 | 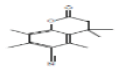 |
| 14 | 1,2-Dimethyl-5-nitroadamantane | 20.719 | 3.9 | 209 | C12H19NO2 | 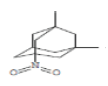 |
| 15 | Phenol, 3-ethyl-, acetate | 20.851 | 2.23 | 164 | C10H12O2 | 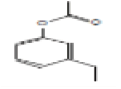 |
| 16 | 2BGDFC | 20.995 | 6.93 | 408 | C20H24O9 | 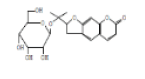 |
| 17 | 17-Oxo-4-nor-3,5-seco-5-androsten-3-oic acid, methyl ester | 21.181 | 0.34 | 304 | C19H28O3 | 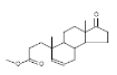 |
| 18 | EHTC | 21.288 | 10.07 | 180 | C11H16O2 | 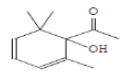 |
| 19 | TTTTV | 21.642 | 10.84 | 316 | C20H28O3 | 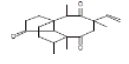 |
| 20 | Octadecanoic acid | 22.146 | 1.12 | 284 | C18H36O2 | 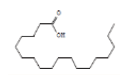 |
| 21 | 4-(3,3-Dimethyl-but-1-ynyl)-4-hydroxy-2,6,6-trimethylcyclohex-2-enone | 22.335 | 1.25 | 234 | C15H22O2 | 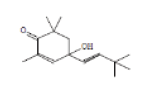 |
| 22 | 2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexa-1,3,5-trienyl]cyclohex-1-en-1-carboxaldehyde | 22.625 | 3.02 | 324 | C23H32O | 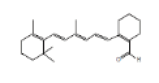 |
| 23 | [1,3]Dioxane-4,6-dione, 2-tert-butyl-2-methyl-5-(4-methylbenzylidene)- | 23.219 | 1.59 | 288 | C17H20O4 | 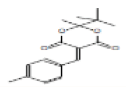 |
Table 3: Phytoconstituents In UAE Of Curcuma Caesia Rhizome
| Species | Extract | Reference |
|---|---|---|
| Curcuma amada Roxb. | Extraction of rhizome essential oil | [19] |
| Curcumae rhizoma and Curcumae radix | Comparative analysis of essential oil | [20] |
| Curcuma wenyujin | Quantitative analysis and discrimination of steamed and non-steamed rhizomes | [21] |
Table 4: Details on UAE Application with GC-MS for Different Species From Curcuma Genus
The information reported in the present study provides details of phytoconstituents present in ethanolic extract of C. caesia rhizomes detected by GC-MS. Both the extraction procedures are applicable for studying the phytoconstituents present in the C. caesia plant rhizome. Biological activities are reported for some of the phytoconstituents present in the ethanolic extract. This study offers a chance for investigators working on C. caesia plants to further probe, develop new procedures for the isolation of phytoconstituents and assess their biological activities.
The study evaluates and discusses the phytochemical profiles obtained by GC-MS assessment of the ethanolic rhizome extract of C. caesia synthesised by Soxhlet extraction and UAE techniques. The study furnishes information on the structure, molecular formula, molecular weight, and genre of the phytoconstituents present in the extracts. The current work is a modest stride in fingerprinting the rhizome's chemical phytoconstituents, and this information can serve as a critical step in the characterisation study of C. caesia rhizomes. The ultrasound-assisted ethanol extract study for C. caesia rhizomes is a unique work presented for the first time, and the comparison with an extract processed by a conventional Soxhlet extraction renders a significant understanding for the plant species. This report reinforces the usage of C. caesia as a medicinal plant and emphasises the utilisation and development of the rhizome part of the plant as an herbal drug in the future to meet the unfulfilled medical needs of humankind.
Acknowledgements:
The authors graciously acknowledge the help rendered by Dr. D. Prasath, Principal Scientist, Indian Agricultural Research Institute-Indian Institute of Spices Research, Kozhikode, Kerala, in sharing the rhizomes and would like to express their sincere gratitude for the support.
Conflict of interests:
The authors declared no conflict of interests.
References
- Saggar S, Mir PA, Kumar N, Chawla A, Uppal J, Shilpa, et al. Traditional and herbal medicines: Opportunities and challenges. Pharmacognosy Res 2022;14(2):107-14.
- Ravindran PN, Nirmal Babu K, Sivaraman K. Turmeric: The genus curcuma. CRC Press 2007;504.
- Borah A, Kumar D, Paw M, Begum T, Lal M. A Review On Ethnobotany And Promising Pharmacological Aspects Of An Endangered Medicinal Plant, Curcuma CaesiaRoxb. Turk J Botany 2020;44(3):205-13.
- Paw M, Gogoi R, Sarma N, Pandey SK, Borah A, Begum T, et al. Study of anti-oxidant, anti-inflammatory, genotoxicity, and antimicrobial activities and analysis of different constituents found in rhizome essential oil of Curcuma caesiaRoxb., collected from north east India. Curr Pharm Biotechnol 2019;21(5):403-13.
[Crossref] [Google Scholar] [PubMed]
- Azwanida, NN. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants 2015;4(3):3-8.
- Mukunthan KS, Satyan RS, Patel TN. Pharmacological evaluation of Phytochemicals from south Indian black turmeric (Curcuma caesiaRoxb.) to target cancer apoptosis. J Ethnopharmacol 2017;209:82-90.
[Crossref] [Google Scholar] [PubMed]
- Chaturvedi M, Rani R, Sharma D, Yadav JP. Comparison of Curcuma caesia extracts for bioactive metabolite composition, antioxidant and antimicrobial potential. Nat Prod Res 2019;35(18):3131-5.
[Crossref] [Google Scholar] [PubMed]
- Atom RS, Shaikh SA, Laitonjam WS, Ninghthoujam RS, Kunwar A. Phytochemical profiling of petroleum ether and chloroform extracts of Curcuma caesia rhizome by GC-MS and comparing their bioactivities. J Spices Aromat Crop 2021;30(2):183195.
- Pakkirisamy M, Kalakandan SK, Ravichandran K. Phytochemical screening, GC-MS, FT-IR analysis of methanolic extract of Curcuma caesia Roxb (black turmeric). Pharmacogn J 2017;9(6):952-6.
- Pandey AK, Chowdhury AR. Volatile constituents of the rhizome oil of Curcuma caesia Roxb. from central India. Flavour Fragr J 2003;18(5):463-5.
- Mukunthan KS, Anil Kumar NV, Balaji S, Trupti NP. Analysis of essential oil constituents in rhizome of Curcuma caesia Roxb. from south India. J Essent Oil-Bear Plants 2014;17(4):647-51.
- Borah A, Paw M, Gogoi R, Loying R, Sarma N, Munda S, et al. Chemical composition, antioxidant, anti-inflammatory, anti-microbial and in-vitro cytotoxic efficacy of essential oil of Curcuma caesia Roxb. leaves: An endangered medicinal plant of north east India. Ind Crops Prod 2019;129:448-54.
- Fatt LC, Rahman NWA, Aziz MAA, Isa KM. Identification of the chemical constituents of Curcuma caesia (Black Turmeric) hydrosol extracted by hydro-distillation method. IOP Conf Ser Earth Environ Sci 2021;765(1):012025.
- Warrier PP, Badole M. GC-MS based Phytochemical Profiling of n-Hexane extracts of Curcuma angustifolia Roxb. rhizomes. J Plant Sci Res 2022;38(1):333-41. [Crossref]
[Google Scholar] [PubMed]
- Caceres AI, Liu B, Jabba SV, Achanta S, Morris JB, Jordt SE. Transient receptor potential cation channel subfamily m member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br J Pharmacol 2017;174(9):867-79.
[Crossref] [Google Scholar] [PubMed]
- Metuge JA, Nyongbela KD, Mbah JA, Samje M, Fotso G, Babiaka SB, et al. Anti-onchocerca activity and phytochemical analysis of an essential oil from Cyperus articulatus L. BMC Complement Altern Med 2014;14:1-10.
[Crossref] [Google Scholar] [PubMed]
- Lu XX, Hu NN, Du YS, Almaz B, Zhang X, Du SS. Chemical compositions and repellent activity of clerodendrum bungei Steud. essential oil against three stored product insects. Daru 2021;29(2):469-75.
[Crossref] [Google Scholar] [PubMed]
- Yu JQ, Liao ZX, Cai XQ, Lei JC, Zou GL. Composition, antimicrobial activity and cytotoxicity of essential oils from Aristolochia mollissima. Environ Toxicol Pharmacol 2007;23(2):162-7.
[Crossref] [Google Scholar] [PubMed]
- Narayanankutty A, Sasidharan A, Job JT, Rajagopal R, Alfarhan A, Kim YO, et al. Mango ginger (Curcuma amada Roxb.) rhizome essential oils as source of environmental friendly biocides: Comparison of the chemical composition, antibacterial, insecticidal and larvicidal properties of essential oils extracted by different methods. Environ Res 2021;202:111718.
[Crossref] [Google Scholar] [PubMed]
- Lu DY, Cao Y, Li L, Zhu ZY, Dong X, Zhang H, et al. Comparative analysis of essential oils found in rhizomes curcumae and radix curcumae by gas chromatography-mass spectrometry. J Pharm Anal 2011;1(3):203-7.
- An YW, Hu G, Yin GP, Zhu JJ, Zhang QW, Wang ZM, et al. Quantitative analysis and discrimination of steamed and non-steamed rhizomes of curcuma wenyujin by GC-MS and HPLC. J Chromatogr Sci 2014;52(9):961-70.
[Crossref] [Google Scholar] [PubMed]