- *Corresponding Author:
- A. Swargiary
Department of Zoology, Pharmacology and Bioinformatics Laboratory, Bodoland University, Kokrajhar, Assam 783370, India
E-mail: ananbuzoo101@gmail.com
| Date of Received | 01 October 2021 |
| Date of Revision | 01 October 2021 |
| Date of Acceptance | 15 June 2022 |
| Indian J Pharm Sci 2022;84(3):772-782 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Lindernia crustacea (L.) F. Muell is a small herbaceous plant with several ethnomedicinal values. The present study investigated the phytochemical content and alpha-amylase and alpha-glucosidase inhibitory property of Lindernia crustacea. Methanolic crude extract of plant was obtained following the Soxhlet method. The crude extract was studied for alpha-amylase and alpha-glucosidase inhibitory activity. Phytochemical analysis was carried out using the gas chromatography-mass spectrometry technique. Furthermore, docking study was carried out with the phytocompounds to see the binding affinity with the enzymes. In silico drug-likeness and pharmacological properties were also carried out using Swiss absorption, distribution, metabolism and excretion, and absorption, distribution, metabolism, excretion and toxicity lab tools. The plant extracts showed concentration-dependent inhibition of enzyme activities with half-maximal inhibitory concentration values of 3.11 mg/ml and 548.9 μg/ml for alpha-amylase and alphaglucosidase, respectively. Gas chromatography-mass spectrometry study identified ten phytocompounds with molecular weights ranging from 264.4 to 561 g/mol. Docking study showed 1-(4-Hydroxybenzoyl)- 6,7-dimethoxyisoquinoline as the best binding compound with the enzymes. Phytocompounds identified from Lindernia crustacea were predicted to have substantial drug-likeness and absorption, distribution, metabolism, excretion and toxicity properties. The enzyme inhibition study and binding interactions of phytocompounds suggest promising alpha-amylase and alpha-glucosidase inhibitory activity of Lindernia crustacea. Therefore, the aerial part of Lindernia crustacea may be further investigated to know the exact mode of biological actions.
Keywords
Lindernia crustacea, alpha-amylase, alpha-glucosidase, docking, drug-likeness
Type-2 Diabetes (T2D) is a major health problem of the contemporary world affecting millions of people. According to World Health Organization (WHO), globally, about 422 million people had diabetes in 2014, with about 1.6 million deaths, most of them are from low and middle-income countries[1]. International Diabetes Federation estimated about 578 million adults with diabetes by 2030 and 700 million by 2045[2]. South- East Asia, including India, Sri Lanka and Bangladesh, accounted for more than 70 million cases of diabetes in 2013 and is expected to reach up to 135 million by 2035[3]. There are many reasons for diabetes, including abnormal insulin secretion by pancreatic cells, insulin resistance or both[4,5]. Influenced by both genetic and environmental factors, T2D is a multifactorial disorder. Today, several medications are used to lower the blood glucose level and diabetes management[6,7]. Inhibition of carbohydrate-hydrolyzing enzyme is one of the most important therapeutic approaches in diabetes management. Human alpha (α)-amylase (EC 3.2.1.1) and α-glucosidase (EC 3.2.1.20) are two crucial enzymes that catalyze the release of glucose from polysaccharides[8]. Inhibition of these enzymes, therefore, reduces blood glucose levels in the body[9]. However, the use of antidiabetic drugs is reported to have undesirable effects[7]. Plants and plant products have several medicinal values and have been used as medicines since ancient times[10-12]. Plant-derived medicines are safer, cheaper and sometimes more effective than synthetic drugs. In recent times, several plants have been investigated for their antidiabetic and antihyperglycemic properties[13,14].
Lindernia crustacea (L.) F. Muell (L. crustacea) belonging to the family Linderniaceae, locally known as ‘na bikhi’ (in Bodo language), is a small diffuselybranched herb having several ethnomedicinal values. Traditionally, L. crustacea is used as anthelmintic and antidiabetic agents by the tribal communities of Chirang and Kokrajhar district of Assam[15,16]. Aerial parts of L. crustacea is known to possess significant antiinflammatory, analgesic and antipyretic activities[17]. The leaves of L. crustacea are known to cure excess bile secretion, ringworm and boil[18]. Recent studies by Tsai et al.[19] isolated 33 compounds from the plant of which phytol, aloe-emodin, byzantionoside-B, a mixture of trans-martynoside and cis-martynoside and luteolin-7-O-beta (β)-D-glucopyranoside showed significant inhibitory effects on the herpes virus. Cheng et al.[20] reported two new compounds linderside A and lindersin B, from L. crustacea that were found to have neuritogenesis properties. Recent studies have revealed anticancer and antiproliferative properties of the L. crustacea[21,22]. However, there is very little information about the antidiabetic property of the plant. The present study, therefore, investigates the potential antihyperglycemic property of the aerial part of L. crustacea. In vitro and in silico enzyme inhibitory study was carried out on two key enzymes linked to T2D.
Materials and Methods
Plant material:
The aerial part of L. crustacea was collected from Tinali area of Kokrajhar district of Assam. The plant sample was identified in the Department of Botany, Bodoland University (Identification no. BUBH2018048). After collection, plants were properly washed and completely dried in a hot-air oven below 50°.
Preparation of plant extract:
The dried plant part was ground into powder form using a mechanical grinder. Plant powders were soaked into 80 % methanol for 72 h and filtered using Whatman filter paper no.1. The process was repeated three times and the filtrates obtained were evaporated to dryness using a rotary evaporator. After complete evaporation, dry, solid L. crustacea (LCME) obtained was stored at -20° till further use. Preparation of crude plant extract was carried out as per the method described in our earlier publication[23].
Gas Chromatography-Mass Spectrometry (GCMS) analysis:
The phytochemical components of the plant extracts were analyzed by GC-MS system (TQ-8030 Shimadzu Corporation Kyoto, Japan). GC-MS was run on an EB-5MS capillary column (30 m×0.25 mm, internal diameter (i.d.); 0.25 μm) at 57.4 kPa pressure with an initial temperature of 50° and maintained at the same temperature for 2.5 min. Next, the oven temperature was raised to 300° at 15°/min and maintained for 8 min. Injection port temperature was ensured at 300° and helium flow rate as 1 ml/min. The ionization voltage was 70 eV. The plant sample was injected in split mode as 20:1. The mass spectral scan range was set at 0-700 (m/z). Compound identification was performed by comparing the spectra with the databases (NIST-11)[24].
Enzyme inhibition study:
Inhibition of α-amylase activity: The inhibition of α-amylase enzyme activity of plant extract was carried out following Kwon et al.[25]. The crude plant extracts were dissolved in 5 % Dimethyl Sulfoxide (DMSO). Different concentrations of plant extracts and acarbose were mixed with 200 μl of α-amylase enzyme (0.5 mg/ ml). The assay mixture was incubated at 25° for 10 min. Next, 0.5 ml of 1 % starch solution was added and reincubated for another 20 min at 37°. After the incubation, 0.5 ml of 3,5-Dinitrosalicylic acid (DNS) reagent was added to stop the reaction and the assay mixture was boiled for 5 min. The reaction mixture was then diluted after adding 5 ml distilled water and the Absorbance (Abs) was measured at 540 nm in Ultraviolet/Visible (UV/VIS) double beam spectrophotometer.
The percent (%) inhibition of enzyme activity was calculated using the following formula:
Inhibition (%)=Abs control-Abs sample/Abs control×100 (1)
Abs control means absorbance of assay mixture without extract and acarbose. Abs sample means absorbance of assay mixture with extract or acarbose.
Inhibition of α-glucosidase activity: α-glucosidase inhibition assay was carried out following the method of Elya et al.[13]. Plant extract was dissolved in 5 % DMSO and α-glucosidase in 100 mM sodium phosphate buffer, pH 6.9. Different concentrations of plant extracts and acarbose were mixed with 50 μl α-glucosidase (0.5μg/ ml) and incubated for 10 min at 37°. Next, 100 μl of 5 mM p-nitrophenyl-α-D-glucopyranoside is added and incubated for another 20 min at 37°. The reaction was stopped by adding 2 ml of 0.1 M Sodium carbonate (Na2CO3). The color development was measured at 405 nm using a double-beam UV/VIS spectrophotometer.
Inhibition (%) of α-glucosidase activity was calculated using equation (1).
Molecular docking:
Preparation of ligands and enzymes: GC-MS reported compounds of plants were retrieved from the PubChem database. Acarbose was used as a reference inhibitor. The crystal structure of α-amylase (Protein Data Bank (PDB): 2QV4) and α-glucosidase (maltase) (PDB: 2QMJ) was downloaded from the PDB database (http:// www.rcsb.org/pdb). Downloaded protein structures were cleaned by removing the attached ligands, Hetero-Atoms (HETATMs) and water molecules. Polar hydrogens and energy were added to the cleaned protein structures. The amino acids interacting with the co-crystalized ligand of three Dimensional (3D) protein structures were selected as the active site amino acid residues. The active site amino acid residues were Ile51, Trp58, Trp59, Tyr62, Gln63, His101, Gly104, Asn105, Ala106, Val107, Leu162, Thr163, Gly164, Leu165, Arg195, sp197, Ala198, His201, Glu233, Ile235, His299, Asp300 and His305 for α-amylase enzyme. Similarly, the amino acids in the active site of the α-glucosidase enzyme were Asp203, Thr205, Asn207, Tyr299, Asp327, Ile328, Ile364, Trp406, Trp441, Asp443, Met444, Arg526, Trp539, Asp542, Thr544, Phe575, Ala576 and His600.
Docking: After the ligands (phytocompounds and acarbose) and the target enzymes were prepared, docking was performed in AutoDock Vina[26]. The grid parameters for docking were set as x, y, z size coordinate and grid box centre coordinate 13.114, 48.653, 20.864 and 72, 66, 60 for α-amylase and -21.174, -6.030, -10.803 and 68, 54, 68, for α-glucosidase, respectively. The docking algorithm was carried out by keeping the default exhaustiveness at 8. Docking output was visualized in Discovery Studio software.
Analysis of drug-likeness and Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) profile:
The phytochemicals of L. crustacea identified by GC-MS analysis was verified for the drug-likeness properties using SwissADME[27] and PubChem database. The drug-likeness property of compounds was evaluated based on Lipinski’s rule[28]. Similarly, in silico ADMET properties of identified compounds were predicted using ADMETlab[29].
Statistical analysis:
All the results were expressed as means of three experiments±Standard Deviation (SD). Statistical difference between the extract and reference compound was tested by one-way Analysis of Variance (ANOVA) using Statistical Package for Social Sciences (SPSS) software. Other statistical calculations and graphical presentations were prepared in Excel and OriginPro8.5. The significance test was calculated at p≤0.05.
Results and Discussion
L. crustacea is an important herbaceous medicinal plant of Assam. The present study investigated the phytocompounds and α-amylase and α-glucosidase inhibitory property of methanolic extract of aerial part of L. crustacea. The name of the phytocompounds identified by chromatographic analysis and GC-MS parameters are presented in Table 1. A total of ten compounds (C1-C10) were identified from the plant. The GC-MS chromatograms, their retention times and m/z intensities of the identified compounds are presented in fig. 1.
| Code | Name of the compounds | RT | Area (%) | Height (%) | m/z |
|---|---|---|---|---|---|
| C1 | 1-(4-Hydroxybenzoyl)-6,7-dimethoxyisoquinoline | 8.868 | 6.44 | 14.97 | 638 |
| C2 | Molybdenum, [(1,2,3,4,5-.eta.)-1-(1,1-dimethylethyl)-2,4-cyclopentadien-1-yl]bis(.eta.3-2-propenyl) | 9.295 | 6.35 | 7.06 | 264 |
| C3 | 1-Propylpentachlorotriphosphazene | 12.020 | 9.22 | 6.22 | 453 |
| C4 | Terephthalic acid, 2,2-dichloroethyl undecyl ester | 14.820 | 6.96 | 8.78 | 427 |
| C5 | N-Ethyl-N'-isopropyl-6-phenoxy-[1,3,5]triazine-2,4-diamine | 14.925 | 2.91 | 5.79 | 50 |
| C6 | Manganese, pentacarbonyl(2,3,3,4,4,5,5,6,6-nonafluoro-1-cyclohexen-1-yl)- | 15.007 | 6.35 | 9.42 | 94 |
| C7 | Thiophene, 3-methyl-5-octadecyl-2-pentadecyl | 16.220 | 15.15 | 7.97 | 560 |
| C8 | Bis(t-butyldimethylsilyl) selenite | 16.615 | 16.16 | 6.52 | 208 |
| C9 | Phenol, pentabromo | 19.501 | 10.89 | 16.03 | 592 |
| C10 | 6-Methoxy-9H-purine TBDMS | 20.359 | 8.16 | 9.63 | 208 |
Table 1: Name of The Phytocompounds Identified By GC-MS From Aerial Part of L. crustacea
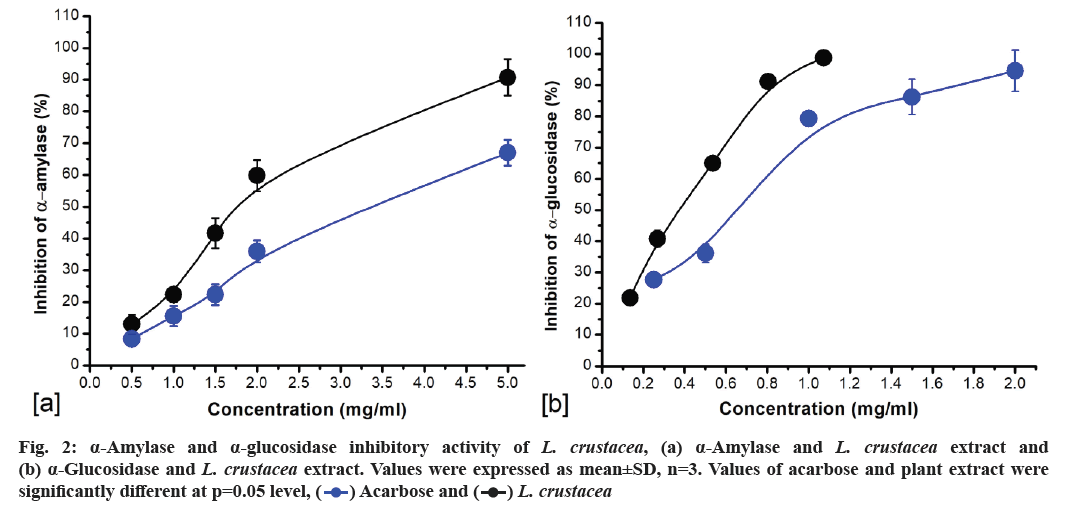
The α-amylase and α-glucosidase inhibitory properties of methanolic crude extract of L. crustacea are presented in fig. 2. Plant extract showed concentration-dependent inhibition of both the enzymes. At concentration 0.5- 5.0 mg/ml, the % inhibition of α-amylase was found to be 8.32 %±1.87 % to 67 %±4.11 % (R2=0.9776). Similarly, the % inhibition for reference drug, acarbose ranged from 12.99 %±2.97 % to 90.73 %±5.19 % at 0.5-5.0 mg/ml concentration (R2=0.9102) (fig. 2a). The half-maximal inhibitory concentration (IC50) of the L. crustacea extract and acarbose against α-amylase was 3.11 mg/ml and 1.78 mg/ml, respectively. Plant extract and acarbose showed stronger inhibitory activity against α-glucosidase compared to α-amylase. At concentration range of 0.25-2 mg/ml, the percent inhibition of α-glucosidase enzyme was found to be 27.66 %±1.54 % to 94.64 %±6.57 % (R2=0.9471). Like α-amylase, acarbose also showed stronger inhibition against α-glucosidase with 21.36±2.15 to 96.21±1.89 at concentrations 0.125 to 1.0 mg/ml (R2=0.9621) (fig. 2b). The IC50 values for L. crustacea and acarbose for α-glucosidase enzyme inhibition was found to be 548.9 μg/ml and 304.14 μg/ml, respectively.
The binding energies of α-amylase and α-glucosidase enzymes with compound C1 identified from L. crustacea are presented in Table 2. The binding energy ranged from -5.2 to -8.1 kcal/mol and -5.0 to -7.9 kcal/mol for α-amylase and α-glucosidase enzymes, respectively. Compound C1 showed the best binding affinity with both enzymes. On the contrary, compound C2 showed the weakest binding affinity with -5.2 kcal/ mol for both α-amylase and α-glucosidase enzymes, respectively. Acarbose showed an almost similar binding affinity with 7.9±0.22 kcal/mol and 7.3±0.29 kcal/mol for α-amylase and α-glucosidase enzymes.
| Compounds | α-Amylase | α-Glucosidase | ||||
|---|---|---|---|---|---|---|
| AC | -7.8 | -8.2 | -7.7 | -7.2 | -7.7 | -7 |
| C1 | -8 | -8 | -8.1 | -7.9 | -7.9 | -8 |
| C2 | -5.2 | -5.2 | -5.2 | -5.2 | -5.2 | -5 |
| C3 | -5.6 | -5.6 | -5.6 | -5.2 | -5.2 | -5 |
| C4 | -6.1 | -7 | -6.1 | -5.8 | -5.5 | -6 |
| C5 | -6.6 | -6.5 | -6.7 | -6.4 | -7.5 | -7 |
| C6 | -7.1 | -7.1 | -7.1 | -7.2 | -7.7 | -7 |
| C7 | -5.8 | -5.7 | -6.1 | -5.2 | -6.1 | -5 |
| C8 | -5.9 | -6.1 | -6 | -5.7 | -5.6 | -6 |
| C9 | -5.3 | -5.3 | -5.3 | -5.3 | -5.3 | -5 |
| C10 | -7.3 | -7.3 | -7.3 | -6.1 | -6.1 | -7 |
Note: Number of replicates, n=3; AC: Acarbose; C1-C10: Phytocompounds
Table 2: Docking Score (Binding Energies In kcal/mol) of L. crustacea Phytocompounds With α-Amylase and α-Glucosidase Enzymes
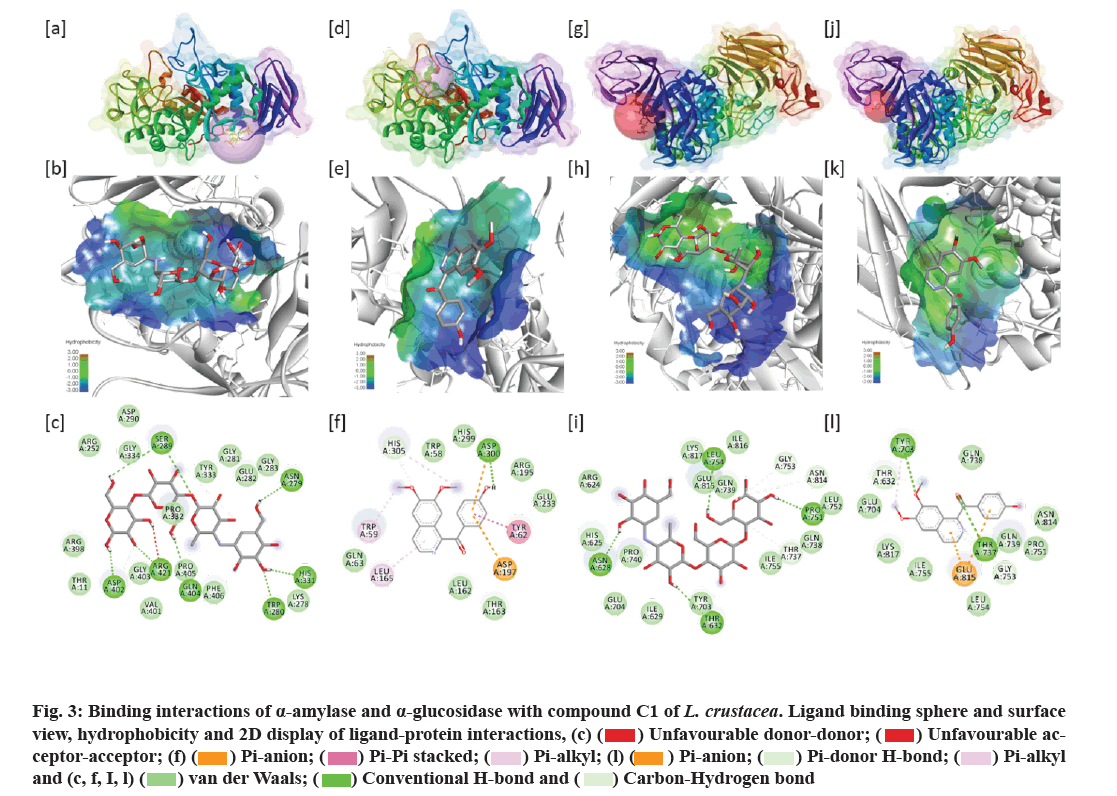
The binding interactions between C1 and α-amylase and α-glucosidase enzymes are shown in fig. 3. The ligandbinding sphere view shows compound C1 has a slightly different binding site compared to acarbose (fig. 3a and fig. 3d). While, in the case of the α-glucosidase enzyme, both the ligands, C1 and acarbose, showed similar binding sites (fig. 3g and fig. 3j). Hydrophobicity study showed that the active pocket of both the α-amylase and α-glucosidase enzymes is occupied mainly by hydrophilic amino acid residues (fig. 3b, fig. 3e, fig. 3h and fig. 3k). Of the 22 and 13 amino acid residues of α-amylase active pocket interacting with Acarbose and C1, 20 and 8 residues are hydrophilic. In contrast, with α-glucosidase enzyme, 15 and 11 amino acid residues out of 20 and 13 are hydrophilic. 2D display of ligandbinding site showed that a total of 22 and 13 amino acid residues of α-amylase enzyme were involved in the formation of different interactions with C1. Acarbose showed seven H-bonds with α-amylase followed by C-H bond and van der Waals interactions, including two unfavorable bonding (fig. 3c). The different types of interactions and amino acid residues involved in the interactions are presented in Table 3. Compound C1 made six different interactions, including one H-bond with Asp300 residue of α-amylase enzyme (Table 3). Similarly, α-glucosidase made three different interactions, including four H-bonds with acarbose (fig. 3i). Compound C1, on the contrary, made six different interactions involving 13 residues that include two H-bonds with Tyr703 and Thr737 residues of α-glucosidase (Table 3).
Fig. 3: Binding interactions of α-amylase and α-glucosidase with compound C1 of L. crustacea. Ligand binding sphere and surface view, hydrophobicity and 2D display of ligand-protein interactions, (c) ( ) Unfavourable donor-donor; (
) Unfavourable donor-donor; ( ) Unfavourable acceptor- acceptor; (f) (
) Unfavourable acceptor- acceptor; (f) ( ) Pi-anion; (
) Pi-anion; ( ) Pi-Pi stacked; (
) Pi-Pi stacked; ( ) Pi-alkyl; (l) (
) Pi-alkyl; (l) ( ) Pi-anion; (
) Pi-anion; ( ) Pi-donor H-bond; (
) Pi-donor H-bond; ( ) Pi-alkyl and (c, f, I, l) (
) Pi-alkyl and (c, f, I, l) ( ) van der Waals; (
) van der Waals; ( ) Conventional H-bond and (
) Conventional H-bond and ( ) Carbon-Hydrogen bond
) Carbon-Hydrogen bond
| Compound C1 | Acarbose | ||||
|---|---|---|---|---|---|
| Nature of bonds | Residues involved |
Bond length (?) |
Residues involved |
Bond length (?) |
|
| α-Amylase | H-bond | Asp300 | 5.09 | Ser289* Asp402 Arg421 Gln404 Asn279 His331 Trp280 |
3.77 3.70 6.13 6.27 5.00 5.11 5.85 |
| C-H bond | His305 | 6.09 | Asp402 Ser289 |
3.72 4.47 |
|
| Pi-anion | Asp197 Asp300 |
7.17 7.14 |
|||
| Pi-Pi-stalked | Tyr62 | 5.12 | |||
| Pi-alkyl | His305 Trp59 Leu165 |
4.90 4.68 6.72 |
|||
| van der Waals | Trp58, His299, Arg195, Glu233, Gln63, Leu162, Thr162 | Arg252, Asp290, Gly334, Tyr333, Gly281, Glu282, Gly283, Pro332, Arg398, Thr11, Gly403, Val401, Pro405, Phe406, Lys278 | |||
| α-Glucosidase | H-bond | Tyr703 Thr737 |
6.18 3.94 |
Asn628 Thr632 Leu754 Pro751 |
4.09 4.66 4.90 3.84 |
| C-H bond | Gly753 Thr632 |
3.73 4.81 |
Gly753 Asn814 Thr737 |
3.74 5.28 4.03 |
|
| Pi-donor H-bond | Thr737 | 4.93 | |||
| Pi-anion | Glu815** | 5.98 5.77 |
|||
| Pi-alkyl | Tyr703 | 5.19 | |||
| van der Waals | Glu704, Lys817, Ile755, Leu754, Gln739, Pro751, Asn814, Gln738 | Ile629, Glu704, Pro740, His625, Arg624, Lys817, Glu815, Gln739, Ile816, Leu752, Glu738, Ile755, Tyr703 |
|||
Note: *Ser289 made two H-bonds; **Glu815 made two Pi-anion bonds and C-H: Carbon-Hydrogen bond
Table 3: Different Bonding Interactions of α-Amylase and α-Glucosidase With Compound C1 and Acarbose
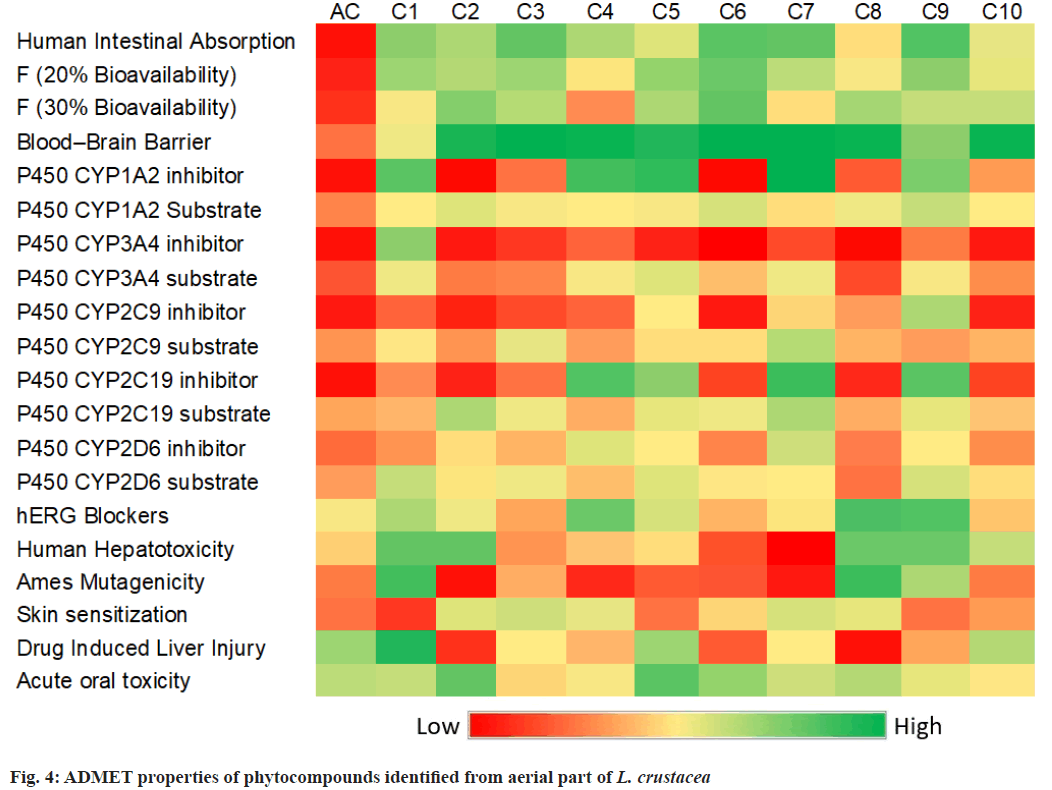
The phytocompounds of L. crustacea identified by GC-MS analysis was studied for the drug-likeness properties using online databases. Table 4 shows the drug-likeness properties of compounds as per Lipinski’s rule of five. According to Lipinski’s rule of five, a drug is orally active if the molecular weight is less than 500 g/mol, LogP<5, Hydrogen Bond Donor (HBD)<5 and the number of Hydrogen Bond Acceptor (HBA) is <5. All the identified phytocompounds showed considerable drug-likeness properties. L. crustacea phytocompounds, C6 and C7 showed violation of the rule in two properties. The Topological Polar Surface Area (TPSA) is less than 100 ?2 for all the identified compounds. Fig. 4 shows the ADMET properties of phytocompounds and acarbose. All the phytocompounds C1-C10 were predicted to have moderate to high absorption by the human intestine, while acarbose showed low absorption. Similarly, the identified compounds showed moderate to high bloodbrain barrier permeation. Because of high absorption and permeability, the phytocompounds can penetrate through the cell membrane and reach the target site.
| Compounds | PubChem ID | Molecular formula | Molecular weight (g/mol) | LogP (<5) | HBD (<5) | HBA (<10) | TPS (A2) |
|---|---|---|---|---|---|---|---|
| AC | 41774 | C25H43NO18 | 645.6 | -8.5 | 14 | 19 | 321 |
| C1 | 539259 | C18H15NO4 | 309.32 | 3.4 | 1 | 5 | 68.6 |
| C2 | 250101276 | C15H23Mo | 299.30 | 7.07 | 0 | 0 | 0.00 |
| C3 | 629960 | C3H7Cl5N3P3 | 355.3 | 5.4 | 0 | 3 | 37.1 |
| C4 | 91739665 | C21H30Cl2O4 | 417.4 | 8.5 | 0 | 4 | 52.6 |
| C5 | 23000 | C14H19N5O | 273.33 | 4.2 | 2 | 6 | 72 |
| C6 | 617896 | C11F9MnO5 | 438.04 | 6.28 | 0 | 15 | 5.0 |
| C7 | 635976 | C38H72S | 561 | 18.97 | 0 | 0 | 28.24 |
| C8 | 91714768 | C12H30O3SeSi2 | 357.5 | 5.65 | 0 | 3 | 35.5 |
| C9 | 11852 | C6Br5OH | 488.59 | 5.0 | 1 | 1 | 20.2 |
| C10 | 91734011 | C12H20N4OSi | 264.4 | 3.24 | 0 | 4 | 52.83 |
Note: HBD: Hydrogen Bond Donor; HBA: Hydrogen Bond Acceptor and TPSA: Topological Polar Surface Area
Table 4: Lipinski’s Data of Drug-Likeness Properties of Phytocompounds of L. crustacea
Cytochromes (CYPs) P450 superfamily of enzymes is involved in drug metabolism. Phytocompounds may increase or decrease the activity CYP-enzyme complex by activating or inhibiting the activity. Fig. 4 shows that the identified compounds from L. crustacea exhibit low interference with the CYP-enzyme complex. Phytocompounds C1, C4, C5, C7 and C9 showed slight inhibitory activity to CYP-complex. The half-life of a drug is the time taken for the plasma concentration of a drug to reduce to half of its original value. All the reported phytocompounds of L. crustacea were predicted to have a low half-life time (<3 h). Similarly, the compounds were predicted to be having a low clearance rate (<5 mg/ml/kg). The best docking compound, C1, on the other hand, showed slight hepatotoxic and carcinogenic properties. Most of the phytocompounds showed a minimal toxicity profile.
Plants have been used as a source of medicines since ancient times and the discovery of phyto-based drugs is an emerging avenue in global health management. T2D is a metabolic disorder affecting millions of people worldwide[30]. α-Amylase and α-glucosidase are two crucial metabolic enzymes linked to diabetes. Several plants and plant-derived phytocompounds are reported to possess α-amylase and α-glucosidase inhibitory properties[31,32]. The present study revealed considerable inhibitory properties of the crude extract of L. crustacea against α-amylase and α-glucosidase enzymes in in vitro experiments. The reference drug acarbose showed higher inhibitory properties compared to plant extract. IC50 values of α-amylase and α-glucosidase enzymes were 3.11 mg/ml and 1.78 mg/ml, respectively. Plant extract showed more potent inhibitory activity against α-glucosidase enzyme (IC50, 548.9 μg/ml) compared to α-amylase. Lindernia ciliata, another species belonging to genus Lindernia was reported to exhibit weaker α-amylase and α-glucosidase inhibitory activities with IC50 values of 6.11 mg/ml and 6.10 mg/ ml, respectively[33]. Several studies have shown the inhibitory property of plant extracts against α-amylase and α-glucosidase enzymes. Thengyai et al.[34] investigated 37 plants and five plants (Vitex glabrata, Senna siamea, Phyllanthus amarus, Terminalia catappa and Salacia chinensis) showed potential α-amylase and α-glucosidase inhibitory activity with IC50 values 8.91±2.92 μg/ml to 20.89±1.87 μg/ml and 11.22±1.70 μg/ml to 25.11±1.44 μg/ml, respectively. Musa balbisiana is an important plant and the rhizome extract was reported to possess strong α-amylase and α-glucosidase inhibitory activity[35]. The aerial part decoction and methanolic extracts of Salvia aegyptiaca and Salvia verbenaca showed potential α?amylase and α?glucosidase activities with IC50 values 86 and 101 μg/ ml and 97 and 150 μg/ml, respectively[36].
Molecular docking is a crucial in silico technique used to study the interactions between proteins and ligands at the molecular level and can be used to design new drug molecules against disease-causing biological molecules[37]. In the present study, molecular docking was carried out to explore the possible binding interactions between phytocompounds of L. crustacea and α?amylase and α?glucosidase enzymes. Compound C1 showed almost similar binding affinity to that of reference inhibitor acarbose with both the enzymes. Many studies have reported the inhibitory activity of phytocompounds against α?amylase and α?glucosidase enzymes. Similarly, Iheagwam et al.[38] showed the binding affinity of ethyl-α-D-glucopyranoside with α? amylase and α?glucosidase enzymes and the binding energies were found to be -6.0 and -5.1 kcal/mol. The present study revealed that the in silico analysis showed almost similar results with in vitro studies in which L. crustacea extract showed weaker inhibitory activity than acarbose. In a similar study, Nadeem et al.[39] investigated crude extract of Cuscuta reflexa and isolated 13 isolated phytocompounds showing a similar pattern of binding affinity between crude extract and isolated phytocompounds against α?amylase and α? glucosidase enzymes. In a recent study, Konappa et al.[40] identified 25 bioactive compounds from Amomum nilgiricum and the docking study revealed that serverogenin acetate was the best binding compound with α?amylase and α?glucosidase. The present study shows the influence of phytochemicals on the inhibition of enzyme activity. Similarly, Shanak et al.[41] reported inhibitory action of Ocimum basilicum methanol extract and their phytocompounds on α-glucosidase and α-amylase. The biochemical assays and in silico study suggested the possible α?amylase and α?glucosidase enzyme inhibitory property of L. crustacea.
In silico drug-likeness and toxicology study is essential for predictive lead compound identification in the present day drug discovery pipeline. According to Lipinski’s rule, an oral drug is predicted to be the most effective if it follows all four rules[42]. On the contrary, a molecule shall not be orally active if it violates two or more of the four rules[42]. The present study found that C6 and C7 slightly violated the rule in two properties while the rest of the identified compounds showed considerable drug-likeness properties. In addition to Lipinski’s rule, a potential drug candidate must fulfill desirable pharmacokinetic properties. ADMET study predicts the drug-likeness property of a compound. Undesirable pharmacokinetics properties and high toxicity are the leading causes of failure at the clinical trial stage. Therefore, the ADMET study offers a valuable guideline for further optimization and drug discovery[43]. Most of the phytochemicals identified from L. crustacea showed promising ADMET properties. The high absorption property, high permeability and less en route metabolism suggest the potential antihyperglycemic property of L. crustacea and the identified phytocompounds.
The present study showed promising α-amylase and α-glucosidase inhibitory activity of L. crustacea. The GC-MS identified compounds also revealed a strong binding affinity to both the enzymes. Drug-likeness and pharmacokinetic study also revealed considerable medicinal properties of L. crustacea phytocompounds. Therefore, the strong α-amylase and α-glucosidase inhibitory properties of the plant suggest that the aerial part of L. crustacea could be a potential source of antihyperglycemic agents.
Acknowledgements:
Authors would like to thank Science and Engineering Research Board-Department of Science and Technology (SERB-DST), Government of India, for providing financial assistance to carry out this research work in the form of the research project to AS (File No., EEQ/2017/000071). We also thank Institute of Advanced Study in Science and Technology (IASST), Boragaon, Assam for providing GC-MS facility.
Conflict of interests:
The authors declared no conflict of interest.
References
- WHO. Diabetes. World Health Organization; 2020.
- IDF Diabetes Atlas. 9th ed. International Diabetes Federation; 2019.
- Ramachandran A, Snehalatha C, Ma RC. Diabetes in South-East Asia: An update. Diabetes Res Clin Pract 2014;103(2):231-7.
[Crossref] [Google Scholar] [PubMed]
- Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 2001;104(4):517-29.
[Crossref] [Google Scholar] [PubMed]
- Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J Diabetes 2010;1(3):68-75.
[Crossref] [Google Scholar] [PubMed]
- Krentz AJ, Patel MB, Bailey CJ. New drugs for type 2 diabetes mellitus. Drugs 2008;68(15):2131-62.
[Crossref] [Google Scholar] [PubMed]
- Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front Endocrinol 2017;8:6.
[Crossref] [Google Scholar] [PubMed]
- Raptis SA, Dimitriadis GD. Oral hypoglycemic agents: Insulin secretagogues, α-glucosidase inhibitors and insulin sensitizers. Exp Clin Endocrinol Diabetes 2001;109(2):265-87.
[Crossref] [Google Scholar] [PubMed]
- Azad SB, Ansari P, Azam S, Hossain SM, Shahid MI, Hasan M, et al. Anti-hyperglycaemic activity of Moringa oleifera is partly mediated by carbohydrase inhibition and glucose-fibre binding. Biosci Rep 2017;37(3):1-11.
[Crossref] [Google Scholar] [PubMed]
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 2001;109(1):69-75.
- Swargiary A, Boro H, Brahma BK, Rahman S. Ethno-botanical study of anti-diabetic medicinal plants used by the local people of Kokrajhar district of Bodoland Territorial Council, India. J Med Plants Stud 2013;1(5):51-8.
- Sachan AK, Rao CV, Sachan NK. In vitro studies on the inhibition of α-amylase and α-glucosidase by hydro-ethanolic extract of Pluchea lanceolata, Alhagi pseudalhagi, Caesalpinia bonduc. Pharmacognosy Res 2019;11(3):310-4.
- Elya B, Basah K, Mun'im A, Yuliastuti W, Bangun A, Septiana EK. Screening of α-glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae and Rubiaceae. J Biomed Biotechnol 2012;2012:1-6.
[Crossref] [Google Scholar] [PubMed]
- Unuofin JO, Lebelo SL. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxid Med Cell Longev 2020;2020:1-36.
[Crossref] [Google Scholar] [PubMed]
- Swargiary A, Roy MK, Daimari M. Survey and documentation of ethnobotanicals used in the traditional medicines system of tribal communities of Chirang district of Assam against helminthiasis. Biomed Pharmacol J 2019;12(4):1923-35.
- Daimari M, Roy MK, Swargiary A, Baruah S, Basumatary S. An ethnobotanical survey of antidiabetic medicinal plants used by the Bodo tribe of Kokrajhar district, Assam. Indian J Tradit Know 2019;18(3):421-9.
- Smriti Rekha CD, Ahmed AB, Saha D, Chanda I. Scientific evidence of Lindernia crustacea (L) F. Muell, an indigenous plant: Folklore medicine used traditionally. Int Res J Pharm 2019;10(1):176-83.
- Panda A, Misra MK. Ethnomedicinal survey of some wetland plants of South Orissa and their conservation. Indian J Tradit Know 2011;10(2):296-303.
- Tsai YC, Hohmann J, El-Shazly M, Chang LK, Dankó B, Kúsz N, et al. Bioactive constituents of Lindernia crustacea and its anti-EBV effect via RTA expression inhibition in the viral lytic cycle. J Ethnopharmacol 2020;250:112493.
- Cheng L, Ye Y, Xiang L, Osada H, Qi J. Lindersin B from Lindernia crustacea induces neuritogenesis by activation of tyrosine kinase A/phosphatidylinositol 3 kinase/extracellular signal-regulated kinase signaling pathway. Phytomedicine 2017;24:31-8.
[Crossref] [Google Scholar] [PubMed]
- Barusrux S, Weerapreeyakul N, Phiboonchaiyanan PP, Khamphio M, Tanthanuch W, Thummanu K. Anticancer activity of Lindernia crustacea (L.) F. Muell. var. Crustacean on human HCT116 colon cancer cell viacellular lipid and β-sheet protein accumulation. Walailak J Sci Technol 2020;17(11):1211-20.
- Swargiary A, Verma AK, Singh S, Roy MK, Daimari M. Antioxidant and antiproliferative activity of selected medicinal plants of lower Assam, India: An in vitro and in silico study. Anticancer Agents Med Chem 2021;21(2):267-77.
[Crossref] [Google Scholar] [PubMed]
- Swargiary A, Daimari A, Daimari M, Basumatary N, Narzary E. Phytochemicals, antioxidant and anthelmintic activity of selected traditional wild edible plants of lower Assam. Indian J Pharmacol 2016;48(4):418-23.
[Google Scholar] [PubMed]
- Kalita H, Boruah DC, Deori M, Hazarika A, Sarma R, Kumari S, et al. Antidiabetic and antilipidemic effect of Musa balbisiana root extract: A potent agent for glucose homeostasis in streptozotocin-induced diabetic rat. Front Pharmacol 2016;7:102.
[Crossref] [Google Scholar] [PubMed]
- Kwon Y, Apostolidis E, Shetty K. Inhibitory potential of wine and tea against α?amylase and α?glucosidase for management of hyperglycemia linked to type 2 diabetes. J Food Biochem 2008;32(1):15-31.
- Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 2010;31(2):455-61.
[Crossref] [Google Scholar] [PubMed]
- Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 2017;7(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Lipinski CA. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov Today Technol 2004;1(4):337-41.
[Crossref] [Google Scholar] [PubMed]
- Dong J, Wang NN, Yao ZJ, Zhang L, Cheng Y, Ouyang D, et al. ADMETlab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J Cheminform 2018;10(1):1-11.
- Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. The lancet 2017;389(10085):2239-51.
[Crossref] [Google Scholar] [PubMed]
- Swargiary A, Daimari M. Identification of bioactive compounds by GC-MS and α-amylase and α-glucosidase inhibitory activity of Rauvolfia tetraphylla L. and Oroxylum indicum (L.) Kurz: An in vitro and in silico approach. Clin Phytosci 2020;6(1):1-11.
- Swargiary A, Daimari M. GC-MS analysis of phytocompounds and antihyperglycemic property of Hydrocotyle sibthorpioides Lam. SN Appl Sci 2021;3(1):1-11.
- Momina SS, Rani VS. In vitro studies on α-amylase and α-glucosidase inhibitory activity of some bioactive extracts. J Young Pharm 2020;12(2s):s72.
- Thengyai S, Thiantongin P, Sontimuang C, Ovatlarnporn C, Puttarak P. α-Glucosidase and α-amylase inhibitory activities of medicinal plants in Thai antidiabetic recipes and bioactive compounds from Vitex glabrata R. Br. stem bark. J Herb Med 2020;19(1):100302.
- Swargiary A, Daimari M. Identification of major compounds and α-amylase and α-glucosidase inhibitory activity of rhizome of Musa balbisiana Colla: An in vitro and insilico study. Comb Chem High Throughput Screen 2022;25(1):139-48.
[Crossref] [Google Scholar] [PubMed]
- Mamache W, Amira S, Ben Souici C, Laouer H, Benchikh F. In vitro antioxidant, anticholinesterases, anti?α?amylase and anti?α?glucosidase effects of Algerian Salvia aegyptiaca and Salvia verbenaca. J Food Biochem 2020;44(11):e13472.
[Crossref] [Google Scholar] [PubMed]
- Bhattacharyya MK, Dutta D, Nashre-ul-Islam SM, Frontera A, Sharma P, Verma AK, et al. Energetically Significant antiparallel π-stacking contacts in Co (II), Ni (II) and Cu (II) coordination compounds of pyridine-2, 6-dicarboxylates: Antiproliferative evaluation and theoretical studies. Inorganica Chim Acta 2020;501:119233.
- Iheagwam FN, Israel EN, Kayode KO, de Campos OC, Ogunlana OO, Chinedu SN. GC-MS analysis and inhibitory evaluation of Terminalia catappa leaf extracts on major enzymes linked to diabetes. Evid Based Complement Alternat Med 2019;2019:1-14.
- Nadeem M, Mumtaz MW, Danish M, Rashid U, Mukhtar H, Irfan A, et al. UHPLC-QTOF-MS/MS metabolites profiling and antioxidant/antidiabetic attributes of Cuscuta reflexa grown on Casearia tomentosa: Exploring phytochemicals role viamolecular docking. Int J Food Prop 2020;23(1):918-40.
- Konappa NM, Siddaiah CN, Krishnamurthy S, Singh B, Ramachandrappa NS. Phytochemical screening and antimicrobial activity of leaf extracts of Amomum nilgiricum (Thomas) (Zingiberaceae) from Western Ghats, India. J Biol Act Prod Nat 2017;7(4):311-30.
- Shanak S, Bassalat N, Albzoor R, Kadan S, Zaid H. In vitro and in silico evaluation for the inhibitory action of O. basilicum methanol extract on α-glucosidase and α-amylase. Evid Based Complement Alternat Med 2021;2021:1-9.
[Crossref] [Google Scholar] [PubMed]
- Guan L, Yang H, Cai Y, Sun L, Di P, Li W, et al. ADMET-score-A comprehensive scoring function for evaluation of chemical drug-likeness. Med Chem Comm 2019;10(1):148-57.
[Crossref] [Google Scholar] [PubMed]
- Jia CY, Li JY, Hao GF, Yang GF. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov Today 2020;25(1):248-58.
[Crossref] [Google Scholar] [PubMed]