- *Corresponding Author:
- Mahmut Yildiztekin
Department of Herbal and Animal Production, Vocational School of Koycegiz, Mugla Sıtkı Kocman University, 48800, Turkey
E-mail: mahmutyildiztekin@mu.edu.tr
| Date of Submission | 24 October 2016 |
| Date of Revision | 13 January 2017 |
| Date of Acceptance | 16 May 2017 |
| Indian J Pharm Sci 2017;79(4):536-543 |
Abstract
This study reports the first ever chemical study on Crocus mathewii, a Turkish endemic and forgotten flowering plant. Silver nanoparticles were green synthesized from one mmol×l-1 silver nitrate extract of C. mathewii. The water-suspended silver nanoparticles showed a peak at 423 nm in UV/Vis spectrophotometer that suggested smaller particle size due to its less surface plasmon resonance. Transmission electron microscopy analysis also proved excellent polydispersity of quasi-spherical particle size distribution with average particles of 7.5 nm (2.5-25 nm). The presence of silver in the nanoparticles was analyzed by X-ray diffraction, while Fourier transform infrared spectroscopy analysis showed the presence of various –OH and –NH containing organics. The results proved that the crude extract of C. mathewii can be used for green synthesis of silver nanoparticles in future for medicinal uses.
Keywords
Silver nanoparticles, Crocus mathewii, endemic plant, TEM, XRD
Crocus belongs to subfamily Crocoideae of Iridaceae, a family of around 80 genera and almost 1500 species [1,2]. Crocus is a genus of 90 flowering species that are grown from the corm [3]; most of the species are cultivated for ornamental purposes. Crocus species are native to woodland, scrub and meadows. They grow from sea level to high altitudes in Europe, North Africa, Middle East, Aegean and China [4]. Crocus is derived from Greek word ‘krokos’ that means saffron (Crocus sativa L.). Crocus species are very important from both a historical and commercial point of view. Harvest and cultivation of C. sativus were first documented in the Mediterranean Crete Island [5].
C. mathewii, also known as Dream Dancer, discovered in 1994 by Kerndorff and Pasche [6]. C. mathewii is endemic to south Turkey, distributed in few regions of Taurus Mountain. It grows during long summer days at dry mountain slopes from 400-1100 m. Its corm and whitish flowers with dark purple zone in the throat can characterize it. C. mathewii was named in the honor of Brian Mathew who was famous as the “Crocus guru” [7]. He proposed properties for the taxonomic classification of Crocus [8].
C. mathewii is an infant to the scientific community. Careful literature survey reflected that researchers have totally ignored this plant. There is no report on the phytochemistry or any other scientific study on C. mathewii. C. sativus, a sister species has been investigated for various bioactivities like antioxidant [9,10], antityrosinase [11], free radical scavenging and ferric reducing [12], antibacterial [13] and inhibitory activity on amyloid-β aggregation [9].
Exploitation of green chemistry for synthesizing biocompatible silver nanoparticles (AgNPs) has gained substantial awareness in recent years for prospective application in biomedicine. Metal nanoparticles are of interest in both research and technology, due to their particular properties not offered in isolated molecules or bulk metals. Because of these properties nanoparticles have many imperative applications in catalysis, sensing and imaging etc. Among the gracious metals (e.g. Ag, Pt, Au and Pd), silver (Ag) is the metal of abundance for prospective applications in the field of biological systems, living organisms and medicine. Due to their elite properties, AgNPs appear to have quite a lot of applications, such as catalysts in chemical reactions [14,15], electrical batteries and in spectrally discriminative coatings for absorption of solar energy [16,17] as optical elements, pharmaceutics and in chemical sensing and biosensing [18-20].
Nanoparticles are gaining importance due to medical applications especially against cancer since 1995 [21]. In a detailed review, Truong emphasized that not only the size and surface of the nanoparticles is important, but also the nanoparticle morphology when dealing with cancer [22]. In a similar study, Liu also stated the importance of nanoparticle shape in drug delivery system [23].
Due to the unique features and applications of nanoparticles, they have gained importance especially in the field of biotechnology, medical imaging and catalysts. AgNPs are among the most widely used nanoparticles that show a broad spectrum of antibacterial activity. Studies have shown that AgNPs prevent replication of the AIDS virus (HIV) and their impact is much greater than that of gold nanoparticles [24]. AgNPs are used in the treatment of wounds. In general, injuries such as cuts, abrasions, burns, warts, fungal diseases, and other skin diseases can be treated with AgNPs [25,26]. Over the past few decades, the use of plants with different applications in medicine and industry has been growing increasingly in the world. In recent years, many environmentally friendly methods have been employed in the synthesis of nanoparticles [27]. The biological methods for AgNPs synthesis using bacteria, fungi, proteins, polypeptides, nucleic acids and plant extracts are simple, nontoxic, affordable and environmentally friendly. These biological methods can be used to generate nanoparticles with acceptable size and morphology [28,29].
In conventional synthesis of nanoparticles, two types of extra components should be added to the metal salts: A) reducing agent to reduce metals to zero valence (M0) so that it can combine with another same metal to develop metal-metal interface and start to develop particles; B) surface covering agents. Plants extracts consists of hundred types of compounds. Some of them reduces the metal while others cover the surface. Plants are in abundance and there is no problem to get a plant extract easily.
Nowadays, the number of studies on the green synthesis of AgNPs has increased. There are number of reviews published on the green synthesis of nanoparticles using plant extracts [30,31]. Most of the green synthesized AgNPs are reported for their antibacterial activities. Banerjee et al. prepared AgNPs from various plants extracts and studied their antibacterial activities and toxicity analysis [32]. Khalil et al. synthesized green AgNPs from olive oil extract and observed AgNPs also possess antibacterial activities [33]. Awwad et al. also reported green synthesis of AgNPs from carob leaves extract having antibacterial activity [34]. Bagherzade et al. reported the biosynthesis of AgNPs via reduction of silver ions using wastage of saffron extract, and they have studied the antibacterial activity of these biosynthesized AgNPs against six bacteria [35]. The general procedure of green AgNPs synthesis is the boiling of plant material in water and adding 1 mM silver nitrate. Most of the organics are not soluble in water. So, we obtained methanol extract, dried it and then processed for AgNPs synthesis.
In the present study, green synthesis of AgNPs from the water extract of C. mathweii was reported. The synthesized AgNPs were also very small and exhibited polydisperse quasi-spherical in size with the average particles size of 7.5 nm.
Materials and Methods
C. mathewii was collected from the region of Fethiye- Babadag, Mugla province of Turkey in the month of October of 2014. The plant material was characterized in the herbarium of Mugla Sitki Kocman University and a specimen (voucher number K.A. 625) was preserved in the herbarium. About 20 g of plant was dried inside a filter paper for 60 d without exposing to light.
Processing of extracts
Aerial parts of the plant material (20 g) were crushed into small pieces with the help of pestle and mortar. These materials were transferred into 250 ml volumetric flasks, which were then filled with 250 ml methanol. Methanol extracts (ME) were obtained after 15 d (3 times). Combined MEs were dried under vacuum in a rotary evaporator, the residue was dissolved in deionized water and boiled for 30 min. The crude aqueous extract (AE) was filtered through a 45 μm filter paper. The clear solution thus obtained was processed for AgNP preparation.
Preparation of AgNPs
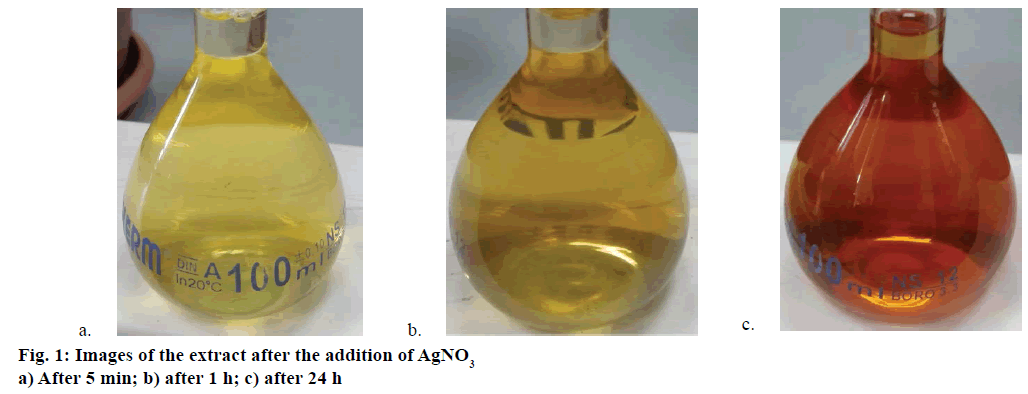
About 17 mg (1 mmol×l-1) of silver nitrate (AgNO3) crystals were added to 100 ml of AE (pH 6.5) of C. mathewii with stirring. The solution was stored at room temperature for 24 h; exposed to daylight but not to direct sunlight. Colour of the mixture changed from light yellow to tea-red (Figure 1). When the solution became cloudy it was filtered through 45 μm filter paper. The obtained clear solution was centrifuged at 14 000 rpm for 30 min at 4°. Aqueous layer was decanted and AgNPs were washed with ethanol and resuspended in deionized water. Obtained AgNPs were stored at 4° in the dark.
UV/Vis analysis of AgNPs
The AgNPs so obtained were suspended in deionized water and sonicated for 5 min under room temperature to assure complete dispersion. UV/Vis spectrum of the suspension was obtained on spectrophotometer (Biochrom Libra S70) using deionized water as reference. Infrared analysis was performed using Fourier-transform infrared spectroscopy (FTIR) Thermo-Scientific by preparation of AgNPs KBr disk.
Transmission electron microscopy (TEM)
TEM images were obtained using TEM (Jeol JEM 2100F HRTEM). AgNPs were suspended in ethanol, and sonicated for 10 min to completely digest them. Few drops from the sample mixture was dropped on a copper grid and dried for 10 min. The grid was placed in a specimen holder (Single Tilt Holder) at 200 kV. Other settings were as follows, magnification: 50-30 0000×; FEG (Electron gun): ultra-high vacuum; Beam diameter TEM mode: 2-5 nm, line resolution 0.1 nm; point resolution: 0.19 nm; camera length: 80-2000 nm; condenser lens aperture. Images with scale 5-200 nm were selected for discussion.
X-ray diffraction (XRD)
XRD analysis was performed using Rigaku Smart Lab XRD in the range of 20-60 (2θ) with the following parameters: Cu Ka=1.54178 Å; Anton Paar HTK 16N/HTK 2000N model, XG: Cu/40 kV/30 mA; Goniometer: SmartLab; Scan speed: 0.2 deg/min; measurement axis: Theta/2-theta; incident slit: 2/3 deg; vertical divergence slit: 2/3 deg; receiving slit #1: 2/3 deg; receiving slit #2: 0.300 mm.
Results and Discussion
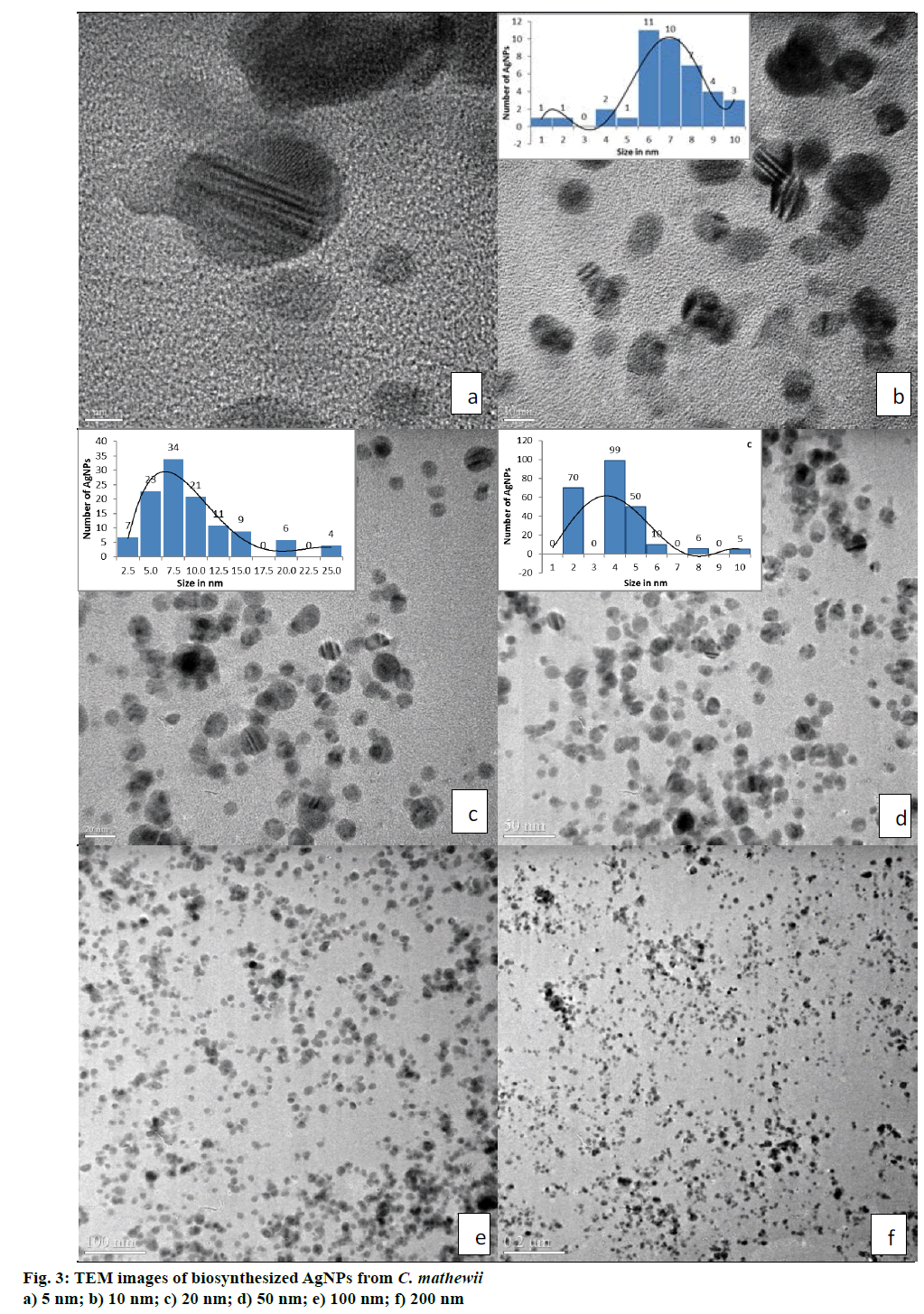
The UV/Vis spectrum of water suspended AgNPs showed a peak at 423 nm (Figure 2) that suggested the presence of AgNPs due to surface plasmon resonance [36]. Some studies suggests that smaller particles range 5-10 nm absorb UV/Vis light near 420 nm [37]. Desai et al. [38] and He et al. [39] suggested that smaller nanoparticles possess less single surface plasmon resonance that results in blue shift. So, λmax at 423 nm suggests that the synthesized AgNPs have smaller size i.e. <20 nm. TEM images also supported this argument, where, most of the particles were of 7.5 nm (Figure 3). As the particle size growing bigger, they reflect red shift.
TEM analysis of AgNPs obtained from C. mathewii water extract, showed excellent results (Figure 3a, b, c, d, e and f). All of the images in Figure 3 provided particle sizes and their distribution very clearly. AgNPs were mostly polydispersed quasi-spherical in shape with very slight size differences. Figure 3b, c and d suggested that approximately most of the nanoparticles were of the sizes between 4 to 11 nm with the mean size of 5.7 nm. As a whole, our natural product-derived AgNPs were under the size of 15 nm. Few particles were even smaller i.e. 5 nm as clearly visible in Figure 3a. AgNPs derived from plant extracts are generally unstable or larger in size with usual particle size distribution. TEM analyses of AgNPs, synthesized from the extract of C. mathewii were found to be stable when checked after 45 d. This can open doors to the green synthesis of stable AgNPs for the future use in medical science.
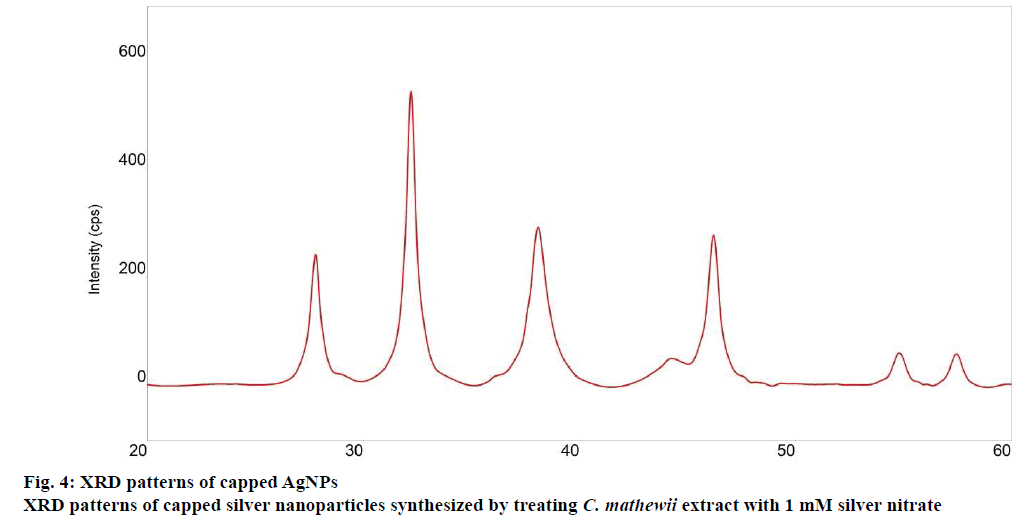
The XRD analysis confirmed that AgNPs formed from the extract of C. mathewii. XRD analysis showed seven peaks. Silver crystals showed peaks at 38.0, 44.1 and 46.2 with peak intensities of 187, 28 and 190, respectively in the XRD (Figure 4). Peaks at 27.8, 32.2, 54.8 and 57.4 with peak intensities of 169, 393, 47 and 38, respectively belong to organic compounds that were incorporated on the surface of silver atoms [40].
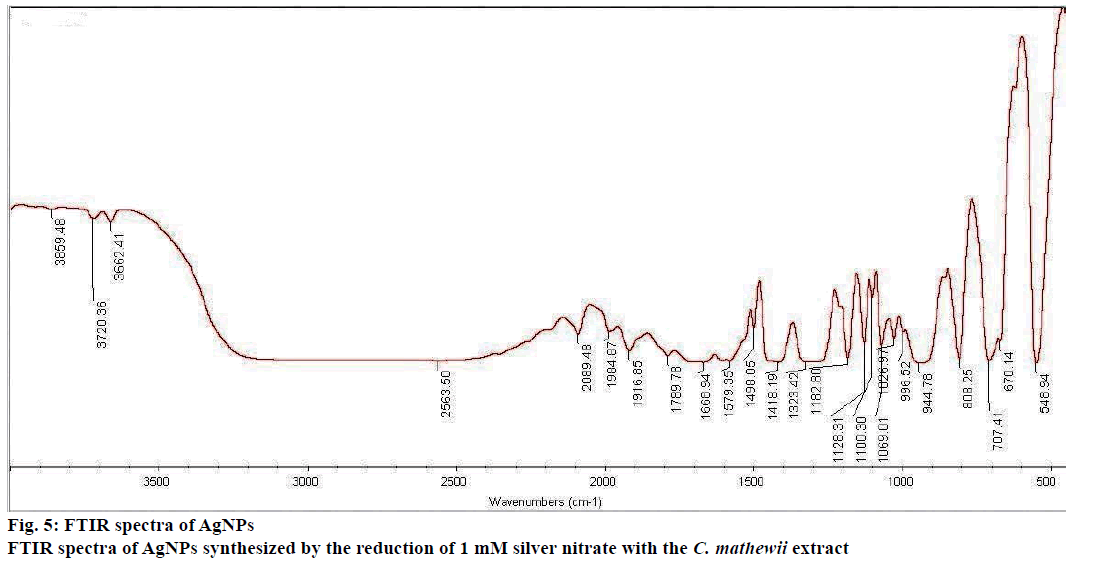
FTIR analysis showed the presence of organic compounds incorporated on the silver atoms surface with various functionalities (Figure 5). A broad peak was observed at 3300 cm-1 that suggests the presence of –OH group that was supported by two sharp peaks at 3720 and 3662 cm-1. The presence of C-O bond was reflected by a peak at 1128 cm-1 in the finger print region. The broad peak at 3300 cm-1 showed the stretching of C-H bond that means the presence of amines or amides. The idea of the presence of primary amines was supported by N-H bending at 1580 cm-1 and N-H waging at 707 and 808 cm-1. Carboxylic acids were present in the nanoparticles that can be observed by O-H peak at 2500 cm-1.
Green synthesis of nanoparticles has become more and more popular among the chemists, biologists and even technologists. AgNPs have already been reported with medicinal importance. C. mathewii is a plant endemic to Turkey that was explored for the first time for antioxidant, anticholinesterase and tyrosinase inhibitory activities in addition to its fatty acid profile [41]. As many of the plant derived AgNPs have different medicinal properties, C. mathewii’s role in the green synthesis of AgNPs could be useful in medicine in future.
Most of the literature procedures consist of boiling plant material in water for 25-30 min [34]; while in some cases, the setup took 5 min at 60° to get plant extract. Many other compounds would not be extracted by water from plant materials. In this study, we soaked the C. mathewii in methanol for 15 d to get almost all of the compounds from the cellulose. Methanol extract was dried and redissolved in water. After the addition of AgNO3 (final concentration 1 mmol×l-1) to the crude extract of C. mathewii, the mixture was stored at room so that it could see the indirect daylight. Within 1 h, colour of the solution started to change from light yellow to dark yellow (Figure 1a and b). Another experiment was set in the dark but no colour change was observed after 24 h. During the next 24 h, colour of the solution continuously became darker to tea-red (Figure 1c). That was a positive sign to expect green synthesis of AgNPs. Biosynthesis of AgNPs can be easily detected by naked eye as colour change as reported by many workers [32,42]. The final mixture was filtered through 45-μm filter paper to remove the agglomerates. Transparent dark yellow solution was centrifuged at 14 000 rpm (4°). Centrifuged AgNPs were decanted and washed two times to remove unbound compounds. Pure AgNPs were resuspended in deionized water for further use.
Size of the AgNPs and particle size distribution is different in each study. It has been observed that AgNPs synthesized from plant extracts are formed in various sizes; mostly 10-100 nm average particle size (APS). Shah et al. also reviewed the nanoparticles as nanomedicines [43]. Zhao et al. synthesized graphene oxide (GO)/AgNPs/luminol composites with electrochemiluminescence activity for sensitive detection of DNA methyltransferase activity with the APS of 18 nm [44]. Zhang et al. prepared AgNP-gated, mesoporous silica coated gold nanorods with an APS of 15 nm [45]. Jia et al. developed a trap to kill bacteria by depositing AgNPs onto TiO2 surface [46]. Their APS of AgNPs was 50 nm. Yang et al. prepared bacterial cellulose/polyacrylamide-AgNPs antibacterial nanocomposites of 65 nm APS [47]. El-Kader et al. prepared nanocomposites of poly (n-vinylcarbazole) and AgNPs with the finely distributed nanoparticles with 10 nm APS [48].
C. mathewii is being introduced to the chemists and nanoscientists for the first time through this study. AgNPs synthesized from C. mathewii were obtained with polydispersed quasi-spherical particles with size distribution of 4-11 nm and an average size of 7.5 nm. These smaller sized nanoparticles can be clearly seen in TEM images. UV/Vis spectrum (423 nm) of suspended AgNPs in water also supports this idea. Particles provided that same UV-spectrum after 45 d of storage to support the idea of their stability. C. mathewii can be used for the green synthesis of AgNPs for medicinal use and where polydispersed quasi-spherical particle distribution is desired. C. mathewii is a Turkish endemic plant that should be grown on various parts of the world and should be utilized for various chemical and biological purposes.
The synthesized AgNPs can be used in numerous technologies and incorporated into a wide array of consumer products that take advantage of their desirable optical, conductive, and antibacterial properties. Few examples are diagnostic applications (biosensors, biological tags for quantitative detection), antibacterial applications (in AgNPs incorporated apparel, footwear, paints, wound dressings, appliances, cosmetics, and plastics) in addition to conductive (electrical and thermal) and optical applications, light harvesting, optical spectroscopies including metal-enhanced fluorescence (MEF) and surface-enhanced Raman scattering (SERS) [49].
Acknowledgements
This paper has been granted by the Muğla Sıtkı Koçman University Research Projects Coordination Office (BAP). Project Grant Number: 15/029 and title “Investigations on the antioxidant potential and mineral nutrient uptake of medicinally important and endemic Crocus L. species (Iridaceae) in Muğla Provience”. Authors thank the Central Resource Laboratories of Muğla Sıtkı Koçman University for TEM and XRD analysis and Köyceğiz Vocational High School, Muğla Sıtkı Koçman University for helping in the plant collection from Taurus Mountains and Kenan Akbaş of the herbarium of Mugla Sitki Kocman University for characterizing the plant.
Conflict interests
The authors declare that there is no conflict of interest.
Financial support and sponsorship
Nil.
References
- Goldblatt P. Phylogeny and classification of Iridaceae. Ann Missouri Bot Gard 1990;77:607-27.
- Bremer B, Bremer K, Chase MW, Fay MF, Reveal JL, Soltis DE, et al. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 2009;161:105-21.
- Wani BA, Mohiddin FA. Micropropagation of genus Crocus – a review. Afr J Agric Res 2009;4:1545-48.
- Rudall P. Anatomy and systematics of Iridaceae. Bot J Linn Soc 1994;114:1-21.
- http://www.themodernantiquarian.com/site/10854/knossos.html.
- Kerndorff H. Observations of Crocus (Iridaceae) in Jordan with special reference to Crocus moabiticus. Herberita 1988;44:33-53.
- Grey-Wilson C, Mathew B. Bulbs, the bulbous plants of Europe and their allies. London, England: Collins; 1981.
- Petersen G, Seberg O, Thorsøe S, Jørgensen T, Mathew B. A phylogeny of the genus Crocus (Iridaceae) based on sequence data from five plastid regions. Taxon 2008;57:487-99.
- Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, et al. Inhibitory activity on amyloid-β aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem 2006;54:8762-8.
- Ramaan A, Soliman G, Mahmoud SS, Nofal SM, Abdel-Rahman RF. Evaluation of the safety and antioxidant activities of Crocus sativus and Propolis ethanolic extracts. J Saudi Chem Soc 2015;4;16:13-21.
- Sariri R, Sabbaghzadeh R, Poumohamad F. In vitro antioxidant and anti-tyrosinase activity of methanol extracts from Crocus Sativus flowers. Pharmacology Online 2011;3:1-11.
- Karimi E, Oskoueian E, Hendra R, Jaafar HZE. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 2010;15:6244-56.
- Parray JA, Kamili AN, Hamid R, Reshi ZA, Qadri RA. Antibacterial and antioxidant activity of methanol extracts of Crocus sativus L. Front Life Sci 2015;8:40-6.
- Jain D, Dsaima HK, Kachhwaha S, Kothari SL. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti-microbial activities. Dig J Nanomater Biostruct 2009;4:723-7.
- Jiang ZJ, Liu CY, Sun LW. Catalytic properties of silver nanoparticles supported on silica spheres. J Phys Chem B 2005;109:1730-5.
- Joerger TK, Joerger R, Olsson E, Granqvist CG. Bacteria as workers in the living factory: metal-accumulating bacteria and their potential for materials science. Trends Biotechnol 2001;19:15-20.
- Moulin E, Sukmanowski J, Schulte M, Royer FX, Stiebig H. Thin-film silicon solar cells with integrated silver nanoparticles. Thin Solid Films 2008;516:6813-7.
- Nam JM, Park SJ, Mirkin CA. Bio-barcodes based on oligonucleotide modified nanoparticles. J Am Chem Soc 2002;124:3820-1.
- Aymonier C, Schlotterbeck U, Antonietti L, Zacharias P, Thomann R, Tiller JC, et al. Hybrids of silver nanoparticles with amphiphilic hyperbranched macromolecules exhibiting antimicrobial properties. Chem Commun 2002;24:3018-9.
- Songping W, Shuyuan M. Preparation of ultrafine silver powder using ascorbic acid as reducing agent and its application in MLCI Mater. Chem Phys 2005;89:423-7.
- Hu CMJ, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther Deliv 2015;12:323-34.
- Truong NP, Whittaker MR, Mak CW, Davis TP. The importance of nanoparticle shape in cancer drug delivery. Expert Opin Drug Deliv 2015;12:129-42.
- Liu Y, Tan J, Thomas A, Ou-Yang D, Muzykantov VR. The shape of things to come: importance of design in nanotechnology for drug delivery. Ther Deliv 2015;3:181-94.
- Sun RW, Chen R, Chung NP, Ho CM, Lin CL, Che CM. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem Commun (Camb) 2005;40:5059-61.
- Vishnudas D, Mitra B, Sant SB, Annamalai A. Green-synthesis and characterization of silver nanoparticles by aqueous leaf extracts of Cardiospermum helicacabum leaves. Drug Invent Today 2012;4:340-4.
- Kim KJ, Sung WS, Moon SK, Choi JS, Kim JG, Lee DG. Antifungal effect of silver nanoparticles on dermatophytes. J Microbiol Biotechnol 2008;18:1482-4.
- Balayssac S, Trefi S, Gilard V, Malet-Martino M, Martino R, Delsuc MA. 2D and 3D DOSY 1 H NMR, a useful tool for analysis of complex mixtures: application to herbal drugs or dietary supplements for erectile dysfunction. J Pharm Biomed Anal 2009;50:602-12.
- Thomas R, Nair AP, Kr S, Mathew J, Ek R. Antibacterial activity and synergistic effect of biosynthesized AgNPs with antibiotics against multidrug-resistant biofilm-forming coagulase-negative staphylococci isolated from clinical samples. Appl Biochem Biotechnol 2014;173:449-60.
- Kumar PPNV, Pammi SVN, Kollu P, Satyanarayana KVV, Shameem U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their antibacterial activity. Indian Crops Prod 2014;52:562-6.
- Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem 2015 ;13:2638.
- Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, et al. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae 2015;6:35-44.
- Banerjee P, Satapathy M, Mukhopahayay A, Das P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour Bioprocess 2014;1:3.
- Khalil MMH, Ismail EH, El-Baghdady KZ, Mohamed D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab J Chem 2014;7:1131-9.
- Awwad AM, Salem NM, Abdeen AO. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int J Ind Chem 2013;4:29.
- Bagherzade G, Tavakoli MM, Namaei MH. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac J Trop Biomed 2017;7:227-33.
- Maduraiveeran G, Ramaraj R. Silver nanoparticles embedded in functionalized silicate sol-gel network film as optical sensor for the detection of biomolecules. J Anal Chem 2015;68:241-8.
- Whelan AM, Brennan ME, Blau WJ, Kellya JM. Enhanced third-order optical nonlinearity of silver nanoparticles with a tunable surface plasmon resonance. J Nanosci Nanotechnol 2015;4:66-8.
- Desai R, Mankad V, Gupta SK, Jha PK. Size distribution of silver nanoparticles: UV-visible spectroscopic assessment. Nanosci Nanotechnol Lett 2015;4:30-4.
- He R, Qian X, Yin J, Zhu Z. Preparation of polychrome silver nanoparticles in different solvents. J Mater Chem 2015;12:3783-6.
- Rajeshkumar S, Malarkodi C. In vitro antibacterial activity and mechanism of silver nanoparticles against foodborne pathogens. Bioinorg Chem Appl 2014;2014:1-10.
- Yildiztekin F, Nadeem S, Erol E, Yildiztekin M, Tuna AL, Ozturk M. Antioxidant, anticholinesterase and tyrosinase inhibition activities, and fatty acids of Crocus mathewii-A forgotten endemic angiosperm of Turkey. Pharm Biol 2016;54:1557-63.
- Dong C, Zhang X, Cai H, Cao C. Facile and one-step synthesis of monodisperse silver nanoparticles using gum acacia in aqueous solution. J Mol Liq 2015;196:135-41.
- Shah S, Liu Y, Hu W, Gao J. Modeling particle shape-dependent dynamics in nanomedicine. J Nanosci Nanotechnol 2015;11:919-28.
- Zhao HF, Liang RP, Wang JW, Qiu JD. One-pot synthesis of GO/AgNPs/luminol composites with electrochemiluminescence activity for sensitive detection of DNA methyltransferase activity. Biosens Bioelectron 2015;63:458-64.
- Zhang Z, Liu C, Bai J, Wu C, Xiao Y, Li Y, et al. Silver nanoparticle gated, mesoporous silica coated gold nanorods (AuNR@MS@AgNPs): low premature release and multifunctional cancer theranostic platform. ACS Appl Mater Interfaces 2015;7:6211-9.
- Jia Z, Xiu P, Li M, Xu X, Shi Y, Cheng Y, et al. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 2015;75:203-22.
- Chen Y, Zhang H, Tian X, Zhao C, Cai L, Liu Y, et al. Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides ELLIS and Crocus sativus L.: A relationship investigation between antioxidant activity and crocin contents. Food Chem 2015;109:484-92.
- Abd El-Kader FH, Hakeem NA, Elashmawi IS, Menazea AA. Synthesis and characterization of PVK/AgNPs nanocomposites prepared by laser ablation. Spectrochim Acta A Mol Biomol Spectrosc 2015;138:331-9.
- Li WR, Xie XB, Shi QS, Zeng HY, Ou-Yang YS, Chen YB. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol. 2010;85:1115-22.
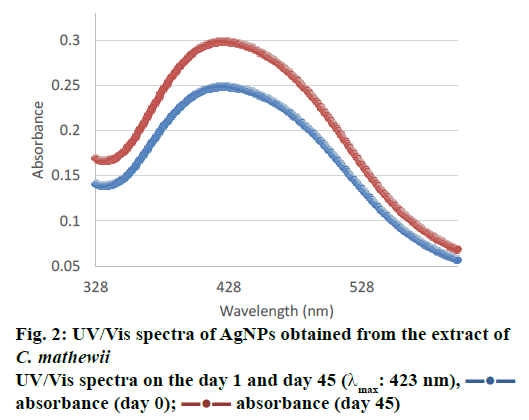
 absorbance (day 0);
absorbance (day 0);  absorbance (day 45)
absorbance (day 45)