- *Corresponding Author:
- Sheeja Koliyote
Department of Pharmaceutics, Principal K. M. Kundnani College of Pharmacy, Cuffe Parade, Colaba, Mumbai, Maharashtra 400005, India
E-mail: sheejakoliyote@gmail.com
| Date of Received | 30 March 2021 |
| Date of Revision | 23 December 2022 |
| Date of Acceptance | 03 March 2023 |
| Indian J Pharm Sci 2023;85(2):299-309 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of this study was to investigate the ability of Tinospora cordifolia mediated synthesis of gold nanoparticle at room temperature and to study the properties of the nanoparticles thus produced. The synthesized nanoparticles were characterized and investigated by ultraviolet-visible spectroscopy spectrophotometry, high-performance thin-layer chromatography screening, Fourier transform infrared, transmission electron microscope, scanning electron microscope, X-ray diffraction, zeta potential, in vitro cytotoxicity test and antioxidant activity. Ultraviolet-visible spectrum of the aqueous medium containing, gold nanoparticles showed a peak at 546 nm. The hexagonal shaped nanoparticles were well dispersed with particle size ranging from 30-60 nm, that were confirmed by transmission electron microscope and scanning electron microscope respectively. Fourier transform infrared showed shift in position and intensity of the peaks. High-performance thin-layer chromatography screening showed that even after forming gold nanoparticles, it retains most of its phytochemical constituents. In vitro stability studies have confirmed that gold nanoparticles are stable in biological fluids at physiological pH and also in salt solutions. X-ray diffraction studies confirmed crystalline nature of the synthesized nanoparticles. Zeta potential value of the synthesized gold nanoparticles is -29 mV at 25° showing good stability of nanoparticles. From in vitro cytotoxicity test it is seen that gold nanoparticles formed are biocompatible. In vitro antioxidant study showed that 2,2-diphenyl- 1-picrylhydrazyl activity increased in a dose dependent manner. The potential of this biosynthesized nanoparticles for the development of value-added products can be used to a good advantage in drug delivery.
Keywords
Gold nanoparticle, Tinospora cordifolia, high-performance thin-layer chromatography, toxicity, antioxidant activity
Synthesis of Gold Nanoparticles (GNP) has fascinated the scientific community due to their unique potential in therapeutics, biomedical diagnostics and numerous technological applications[1]. Several physical and chemical methods have been used for conventional GNP synthesis. Most of these methods use costly and hazardous chemical reagents[2] as reducing and capping agent which remain in the colloidal gold solution rendering the GNP inappropriate for biological applications. Greener substrate such as fungi, yeast, bacteria, virus, algae have also been successfully reported in the synthesis of GNP[3-5]. Green synthesis of GNPs using plant extract is recommended as an eco-friendly alternative to biological and chemical methods that reduces the maintenance of septic environment and eliminates the generation of toxic byproducts[6]. Plant mediated synthesis of GNP is gaining more importance owing to its simplicity, environmental healthy protocol, rapid rate of synthesis of nanoparticles with good control over their sizes and forms, as well as their optical properties and biocompatibility[7]. Recently there have been a lot of reports on synthesis of GNP using different plant parts like Hibiscus rosa-sinensis leaves[8], Eleutherococcus senticosus stem[6], Gnidia glauca flower[4], Dillenia indica fruit[1], Acalypha indica weed[9], Mimosa tenuiflora bark[10], Acorus calamus rhizome extract[11].
Tinospora cordifolia which belongs to Menispermaceae family is a large, deciduous, climbing shrub, found throughout India and also in Sri Lanka, Bangladesh, Nepal and China[12,13]. It is also known as Giloy (in Hindi), Guduchi (in Sanskrit) and Moonseed plant (in English). It is one of the most important herbs of Ayurveda, designated as Rasayana, recommended to enhance general body resistance, rejuvenator activity with wide ranging health benefits, hence included in wide range of Ayurvedic products[14,15]. It contains alkaloids, diterpenoid lactones, glycosides, steroids, saponins, sesquiterpenoid, phenolics, aliphatic compounds and polysaccharides[16]. It has been used to treat fever, jaundice, dengue, gout, skin diseases, diabetes and rheumatoid arthritis[17,18].
The main goal of the present study was the facile green synthesis of GNP using the Hydroalcoholic (HA) stem extract of the medicinally important herb Guduchi. The synthesized GNPs were characterized using Ultraviolet-Visible (UV-Vis) spectroscopy, Transmission Electron Microscope (TEM), Scanning Electron Microscope (SEM), Selected Area Electron Diffraction (SAED), Energy Dispersive X-ray (EDS), Fourier Transform Infrared (FTIR), High-Performance Thin-Layer Chromatography (HPTLC), X-Ray Diffraction (XRD) and zeta potential. The antioxidant efficacy of the synthesized GNP was assessed in vitro against 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and compared with the stem extract. Further, MTT test in which 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) reagent is used, is an in vitro test to assess the efficacy of synthesized GNP and compared with the stem extract for its biocompatibility.
Materials and Methods
Chemicals and reagents:
Hydrogen tetrachloroaurate (III) (HAuCl4 3H2O, 99.98 %), was used as a gold precursor, Bovine serum albu- min and 1,1-Diphenyl-2-picrylhydrazyl was purchased from Sigma-Aldrich (St. Louis, MO, USA). 3-[4,5-di- methylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide, dimethyl sulphoxide were purchased from Himedia, In- dia. All of the reagents were of analytical quality.
Preparation of Tinospora cordifolia stem extract:
Fresh stem of Tinospora cordifolia were collect- ed from Mumbai, India and authenticated by Dr. Rajendra D. Shinde Director of Blatter Herbarium St. Xaviers College, Mumbai (Specimen number P/D/2868/2018). Fresh stems were cut into small pieces, washed with water and dried in tray drier at 50° for a mo. The dried stems were milled to a coarse powder, 1 kg of the dried powder was extracted with HA solvent by maceration at room temperature. The rotavap was used to remove the alcohol from the fil- tered HA extract and the aqueous extract was dried to a semisolid mass which was stored in a refrigerator at 4° for further studies. For the rest of the work Distilled Water (DW) was used.
Synthesis of GNP:
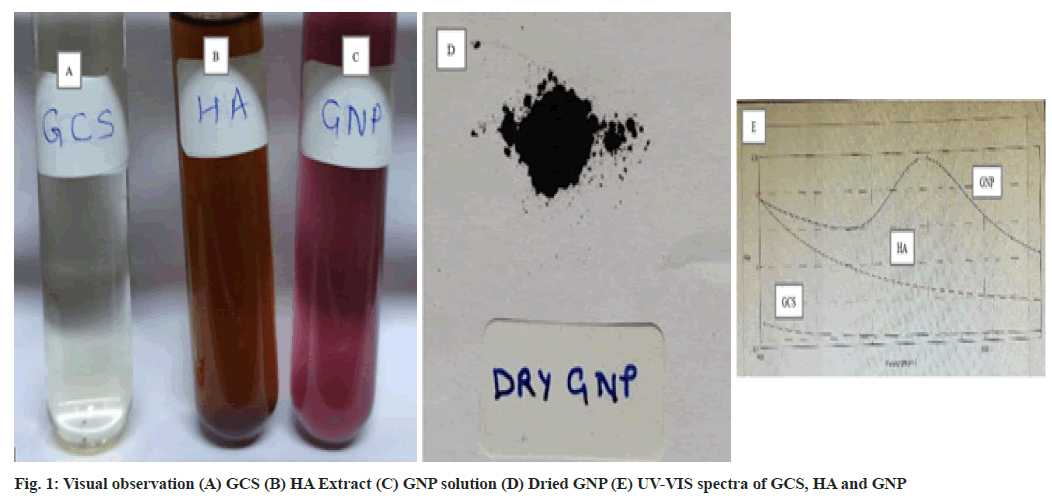
1 mM Gold Chloride Solution (GCS) (fig. 1A) and 1.25 % of HA extract (fig. 1B) was prepared in DW. Filtered extract and GCS was mixed in 1:9 ratio and kept on a magnetic stirrer at 400 rpm for 3 h at room temperature for synthesis of GNP. The change in the colour of the solution from brown colour to violet colour indicated the reduction of Au3+ ions to Au0. The formation of violet colored solution confirmed the formation of GNP (fig. 1C). The solution was centrifuged at 10 000 g for 15 min[9,19]. Supernatant was discarded and pellets were washed with DW. Centrifugation and washing step was repeated thrice for better separation of any biological entities in the GNP[8]. Finally the GNP pellets (fig. 1D) were dried at 37° and stored for further studies[2].
Qualitative chemical evaluation and HPTLC fingerprint analysis:
The extract thus obtained and the synthesized GNP were qualitatively evaluated for the presence of var- ious phytochemical constituents like alkaloid, gly- coside, saponin, reducing sugars, steroids, tannins and flavonoids. Further HPTLC fingerprint analy- sis was also carried out on the HA extract of Tino- spora cordifolia and the GNP with solvent system Toluene:Chloroform:Ethanol (4:4:1 v/v) and 10 % methanolic sulphuric acid as detecting agent using CAMAG HPTLC system consisting of linomat V spotting and scanner 3. The chromatogram obtained was studied under 254 nm and 366 nm[20,21].
Characterization of GNP:
The formation of GNP was confirmed by measuring the UV-VIS spectrum, the most confirmatory tool for the detection of surface plasmon resonance property is by doing UV-VIS spectral analysis using JASCO V-630 Spectrophotometer in the range of 450-650 nm. The dried GNP sample was used for further characterization[22]. The bio reduction compounds responsible for the reaction were determined using FTIR. FTIR spectrum of Tinospora cordifolia extract and GNP were recorded using BRUKER ALPHA FTIR spectrophotometer. Number of significant bands was observed in the region of 500-4250 cm-1. To characterize the size and shape of the synthesized GNP, TEM images were obtained using Philips CM 200 operated at an accelerating voltage of 200 kv. The specimen was suspended in distilled water ultrasonically. 2-3 drops of the suspension was deposited onto carbon coated copper grid. After removal of excess solution with filter paper it was finally dried under InfraRed (IR) lamp. To characterize the size and surface morphology of the synthesized GNP, SEM analysis was done using JEOL JSM-7600F FEG-SEM operating at accelerating voltage of 30 kv. Compositional analysis on the sample was carried out by EDS attached with SEM. The sample was prepared by placing a drop of very fine suspension of nanoparticles in water over a copper tape which was pasted on the stub made of brass. This was then dried under IR lamp. The stub was platinum coated to ensure high conductivity. To determine the structure of synthesized GNP, powder XRD spectroscopy was done. The data was recorded using XPERT-PRO diffractometer instrument operating at a voltage of 45 kv and current of 40 mA with Cu Kα radiation (λ=1.5406 Å) in the region of 2θ from 10-80°. To study the stability of synthesized GNP Zeta-potential measurement was done with a Zetasizer Nano ZS (Malvern Instrument) in a disposable cell at 25°. The assessment was carried out at a pH of 7.26±0.13 to mimic physiological pH.
Effect of pH on biosynthesis of GNP:
In this study 10 ml 1 mM GCS was adjusted to pH 2,4,6,8 and 10 to which 1 ml of 1.25 % HA extract was added and kept overnight at room temperature without stirring[23].
In vitro stability studies of synthesized GNP using Tinospora cordifolia:
Tinospora cordifolia gold nanoparticle was tested in the presence of 10 % NaCl, 0.9 % NaCl, 0.5 % Bovine Serum Albumin (BSA), and at various pH of 2, 4, 6, 8, 10 respectively[24]. Typically, 2 ml of GNP solution was added to 2 ml of 10 % NaCl, 0.9 % NaCl, 0.5 % BSA and for the effect of pH, 4 ml of the GNP solution was adjusted to varying pH in the range of 2, 4, 6, 8 and10 and incubated for 30 min and 24 h. The stability and identity of the GNP were measured by visual observation and recording UV- VIS spectra after 30 min and 24 h.
DPPH radical scavenging activity:
Free radical scavenging activity of the synthesized GNP was examined by the colorimetric assay using its ability to trap the DPPH free radicals[25]. Different concentrations (100-350 ppm) of HA/GNP were treated with 1 ml of 0.1 mM methanolic DPPH solution. The mixture was forcefully vortexed and allowed to reach equilibrium in dark at room temperature for 30 min. The absorbance of the mixture was measured spectrophotometrically at 517 nm. Ascorbic acid was used as standard compound. The percentage radical scavenging activity was calculated using the blank sample absorbance and the HA/GNP samples analysis. For each sample the experiment was done in triplicates.
In vitro cytocompatibility assay:
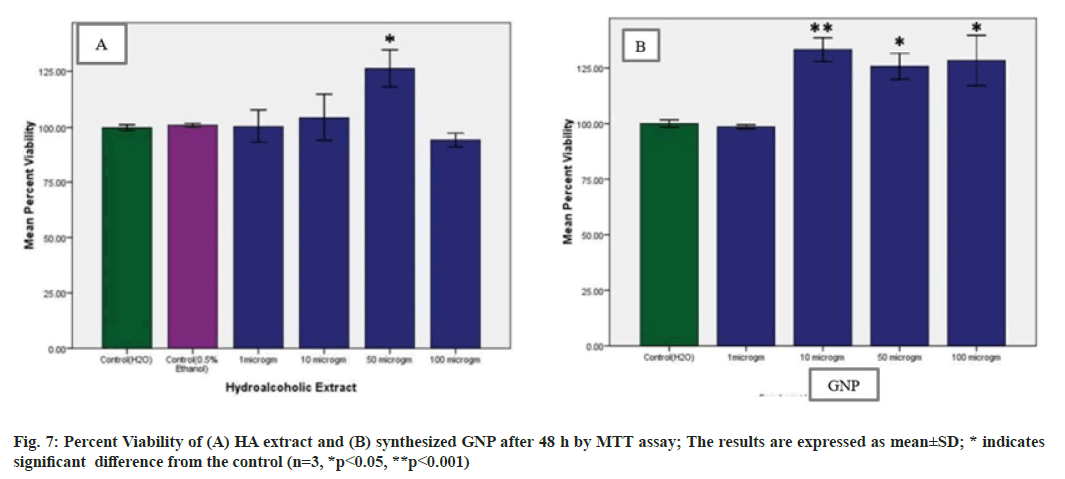
To assess the cytocompatibility of the synthesized GNP, the MTT assay was carried out. MTT assay is a colorimetric assay used in assessing viability and cell proliferation[26]. It can also be used to determine cytotoxicity of agents since the agent would stimu- late or inhibit cell viability. The Peripheral Blood Mononuclear Cells (PBMCs) were isolated from blood by using Ficoll density gradient method. After isolation, PBMCs at the density of 10 000 cells/well were seeded in 96 well plates and incubated at 37°, 5 % CO2 for 24 h. After 24 h the cultured cells were treated with different concentrations namely 1, 10, 50, 100 µg/ml of extract and GNP for 48 h. Sterile water and 0.5 % alcohol served as control. After the incubation the proliferation/cytotoxicity rate was de- termined by MTT assay.
20 µl of MTT (5 mg/ml) was added to each well and incubated at 37° for 4 h. Subsequently MTT solution was removed and blue formazan crystals formed were dissolved in 100 µl of Dimethylsulfoxide (DMSO). The absorbance was read at 570 nm using a micro plate reader. Percent Viability was calculat- ed by using formula, Percent Viability=OD(Sample)/ OD(Control)×100
Statistical analysis:
Statistical analysis was performed using SPSS software version 16. All values were expressed as mean percent viability±standard deviation (n=3). One way ANOVA followed by Tukey’s multiple comparison test was used to examine differences between means, *p<0.05 and **p< 0.001 was considered statistically significant.
Results and Discussion
Green synthesized GNP was confirmed by analyzing the excitation due to applied magnetic field of surface plasmon resonance using UV-VIS spectrophotometer at 546 nm. The change in the colour from light yellow colour of GCS to brown colour of extract to violet colour confirmed the formation of GNP (fig. 1C)[27]. The UV-VIS absorption spectra were recorded for GCS, extract and GNP (fig. 1E).
The time taken for reduction of gold ions and hence appearance of violet colour was found to be temperature dependent[28]. Time taken for change in the colour visually is presented (Table 1). The colour change took place in 3 h at room temperature to within 3 s at 100°. Formation of GNP at 50° shows a distorted spectra which indicates the formation of polydispersed bigger particles which sediments and does not remain suspended. While GNP formed at room temperature gave a smooth spectrum.
| Variables | Time |
|---|---|
| Without stirring at RT | 8 h |
| Shake flask at RT | 3 h |
| Magnetic stirrer at RT | 3 h |
| Refrigerator | 24 h |
| Sonication | 1.5 h |
| Heat at 50° | 15 min |
| Heat at 100° | 3 s |
| Microwave oven | 15 s |
Table 1: Impact of Different Variables in Biosynthesis of GNP
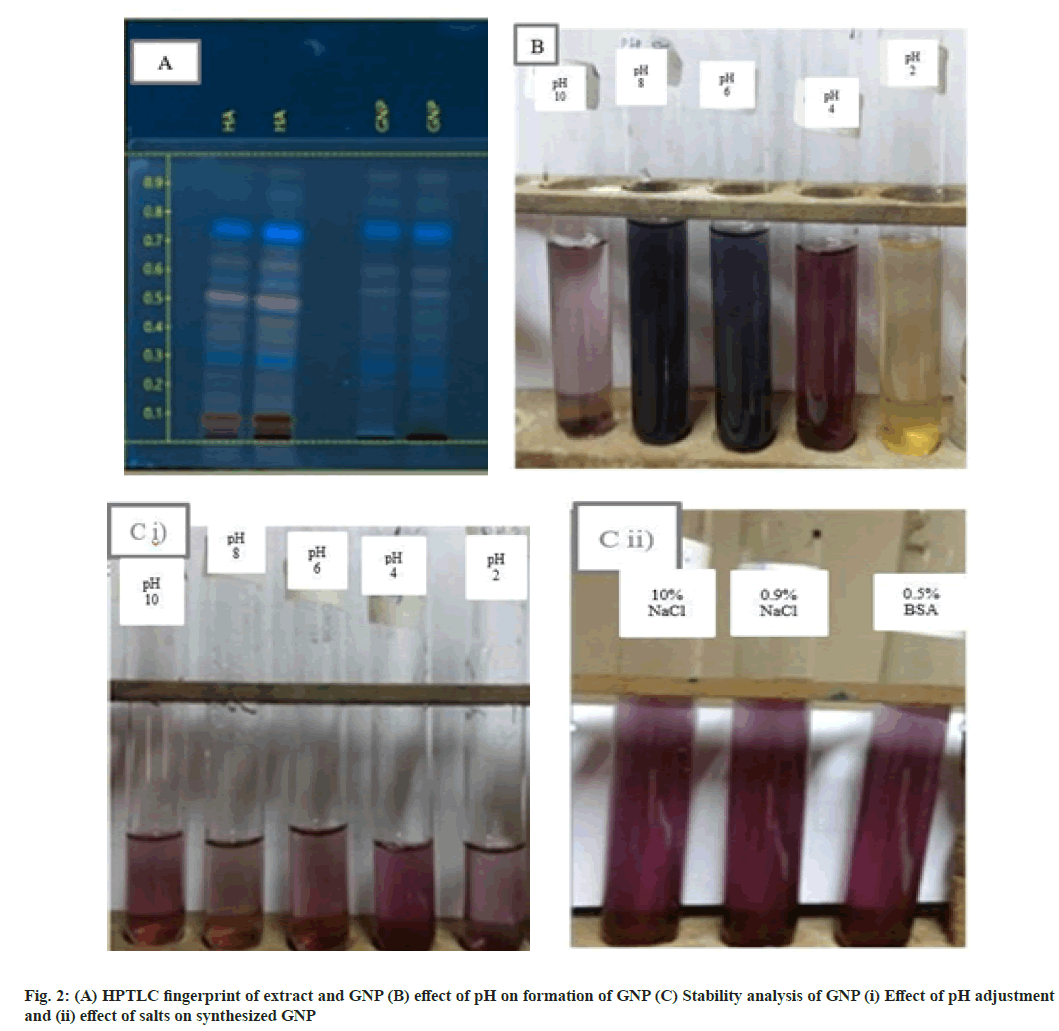
Phytochemical screening reveals the presence of alkaloids, glycosides, saponins, steroids, reducing sugars, tannins and flavonoids (Table 2)[29]. But after forming GNP the phytoconstituents quantity was found to be present in lesser amount indicating that these phytoconstituents are responsible in reducing the GCS and they also help as capping agent as the particle size remains in nanosize even after 2-3 mo. HPTLC fingerprint showed decrease in the intensity of bands at Rf 0.1 and Rf 0.5 indicating the involvement of reacting phytochemicals for the formation of GNP (fig. 2A). Even after the formation of GNP it can be observed that most of the phytochemical constituents of the extract are retained in the GNPs.
| Phytochemical constituents | HA extract | GNP |
|---|---|---|
| Alkaloids | ++ | + |
| Glycosides | ++ | + |
| Tannins and Flavanoids | ++ | + |
| Steroids | + | - |
| Reducing sugars | ++ | + |
| Saponins | ++ | + |
Note: (++): Appreciable amount and (+): Moderately present
Table 2: Preliminary Phytochemical Analysis of Synthesised GNP and Stem Extract of Tinospora cordifolia
pH is a critical factor in the formation of GNP. pH of the extract is 4.6. It was found that GNP was not formed at pH 2 and very little was formed at pH 10 while at pH 4, 6 and 8 GNPs were formed, but there is a difference in colour of GNP solution (fig. 2B). At pH 6 and pH 8 GNPs are blue colored and their scan are uneven indicating formation of polydispersed GNP. Whereas at pH 4 the GNP scan is smooth indicating formation of monodispersed GNP, which is also the pH of the extract[30]. Therefore it can be concluded that, it is best to make GNP without adjusting the pH by mixing 20 ml of 1 mM GCS with 2 ml of 1.25 % HA.
The stability of GNP synthesized using Tinospora cordifolia was evaluated by monitoring visually and the UV-Vis spectra in 10 % NaCl, 0.9 % NaCl, 0.5 % BSA and various pH of 2, 4, 6, 8, 10 respectively[31]. It can be observed that there is no change in the colour of the synthesized GNP when treated with various pH of 2, 4, 6, 8, 10 and 10 % NaCl, 0.9 % NaCl, 0.5 % BSA at even after 24 h (fig. 2C (i) and (ii)). The absorbance spectra at these various conditions were found to be same as GNP solution. Our results from the in vitro stability studies have confirmed that the synthesized GNP were stable at different salt concentrations and pH conditions. This exceptional stability of the green synthesized GNP can be attributed to protection of GNPs by the capping agents present in Tinospora cordifolia stem extract[32].
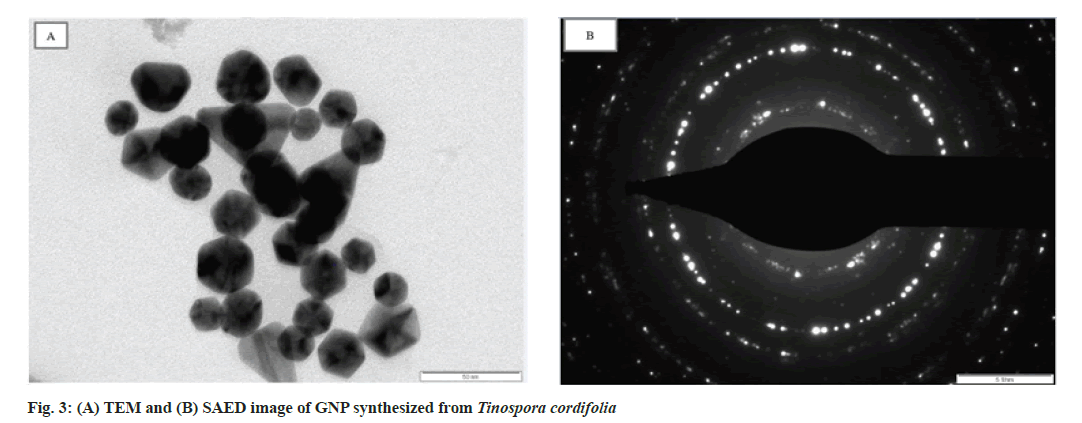
The size and shape of the GNP synthesized was investigated using TEM (fig. 3A). The GNP is well dispersed with Tinospora cordifolia matrix surrounding it indicating that Tinospora cordifolia matrix acts as a capping agent to separate the GNPs from aggregation. It has been observed that the newly formed nanoparticles are polydispersed having various shapes like spherical, triangular, pentagonal, hexagonal with maximum size of 50 nm. Except for the triangular ones the rest look similar[33,34]. This shows that it is an efficient biological method for producing gold particles in nano range. In the Selected Area Electron Diffraction (SAED) pattern (fig. 3B), the ring like pattern with bright circular spots corresponding to Braggs planes, confirmed the crystalline structure of GNP[35].
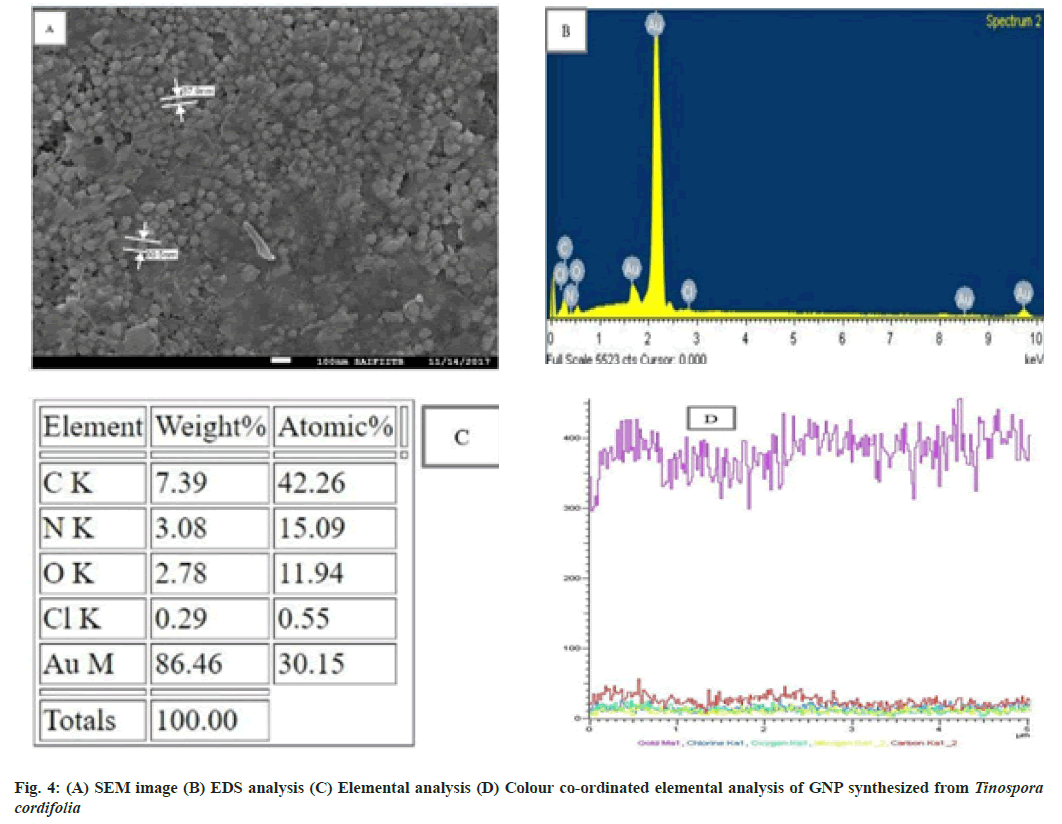
A SEM image further ascertains that the GNP are predominantly spherical in morphology with their size ranging from 30-60 nm in diameter (fig. 4A)[36]. The Energy Dispersive X ray (EDX) spectroscopy is done on SEM instrument itself. It illustrates the chemical nature of synthesized GNP using Tinospora cordifolia stem extract. EDX revealed the peak for gold, carbon, nitrogen and chlorine (fig. 4B). The quantitative analysis using EDX showed high gold content of 86.46 %[37] . Spectrum also shows the presence of carbon, nitrogen, oxygen and chlorine at 7.39 %, 3.08 %, 2.78 % and 0.29 % respectively (fig. 4C). The colour coordinated elemental analysis reveals the highest percentage of gold depicted by violet colour (fig. 4D).
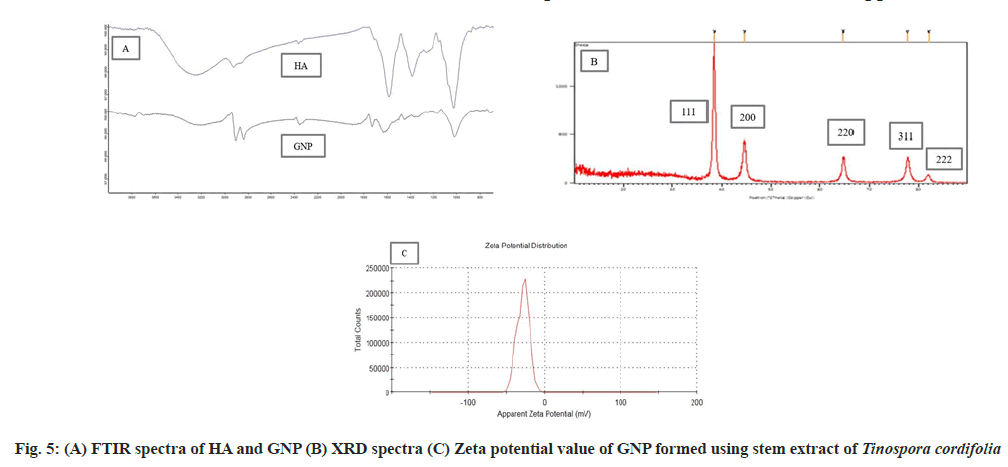
FTIR analysis was carried out in order to investigate the possible mechanism of the gold nanoparticle synthesis by Tinospora cordifolia stem extract. The main goal was to identify absorption peaks that exhibited prominent shift. The Tinospora cordifolia stem extract exhibited a number of absorption peaks, reflecting its complex nature. The stem extract shows characteristic vibrational peaks at 3255, 2351, 1581, 1384 and 1022 cm-1 which correspond to functional groups -OH stretching, C=C group,-NH amide, -CH stretch, C-O stretching vibrations (fig. 5A) HA. A sharp decrease in the band intensity at 3255 cm-1 in synthesized gold nanoparticle was found, as compared to stem extract may be due to binding of -OH groups with AuCl4 ion, which may be responsible for the reduction of metal ion to metal nanoparticles[26,38]. After the formation of GNP, the appearance of strong peak at 1725 cm-1 is attributed to the binding of carbonyl group with nanoparticles (fig. 5A) GNP. More over the FTIR of GNP shows additional signal at 2837 cm-1 signifying the formation of new bonds between metallic nanoparticles and functional groups of biomolecules present in Tinospora cordifolia. The presence of most of the peaks of stem extract in the FTIR of GNP suggests the presence of phytochemicals on the surface of GNP. The capping behavior and stability of the synthesized GNP could be due to the presence of alkaloid, glycoside, saponins, phenols and steroid phytochemicals present in the stem extract.
Powder X-ray diffraction pattern shows that GNP synthesized using HA extract of Tinospora cordifolia has crystalline structure (fig. 5B). A number of Bragg reflections with 2θ values of 38.44°, 44.60°, 64.75°, 77.70° and 82.10° which corresponds to the (111), (200), (220), (311) and (222) sets of lattice planes are detected proving the structure of the GNP to be of face center cubic crystal structure of gold[39]. The data achieved matched with file No. 04-0783 of the Joint Committee on Powder Diffraction Standards (JCPDS) database. The intensity of the peak of (111) at 38.44° diffraction was found to be the strongest indicating extreme reactivity due to high rate of electron transfer.
The XRD results were consistant with the SAED pattern recorded for GNP (fig. 3B). The peak height, Full-Width at Half-Maximum (FWHM) left, d-spacing and relative intensity of the synthesized GNP[40] were also recorded (Table 3).
| Pos. (°2Th.) | Height (cts) | FWHM Left (°2Th.) | ԁ-spacing (Å) | Rel.Inst. (%) |
|---|---|---|---|---|
| 38.4443 | 1395.98 | 0.1771 | 2.34163 | 100 |
| 44.6067 | 386.22 | 0.5314 | 2.0314 | 27.67 |
| 64.7516 | 250.12 | 0.2952 | 1.43974 | 17.92 |
| 77.7065 | 222.75 | 0.5904 | 1.22892 | 15.96 |
| 82.1073 | 68.11 | 0.4133 | 1.17384 | 4.88 |
Table 3: Peak Parameters of Synthesized GNP
The stability of synthesized GNP was performed using zeta potential. Clear disposable zeta cell was used to measure zeta potential of the particles at 25°. A zeta value of ±30 mV is needed for a suspension to be physically stable. A high zeta potential value indicates a high electric charge on the surface of nanoparticles, resulting in strong repellant forces which prevent aggregation amongst the particles[2,30]. Zeta potential value of the synthesized GNP was found to be -29 mV (fig. 5C). It can be concluded that Tinospora cordifolia is a good capping agent for the formed GNP.
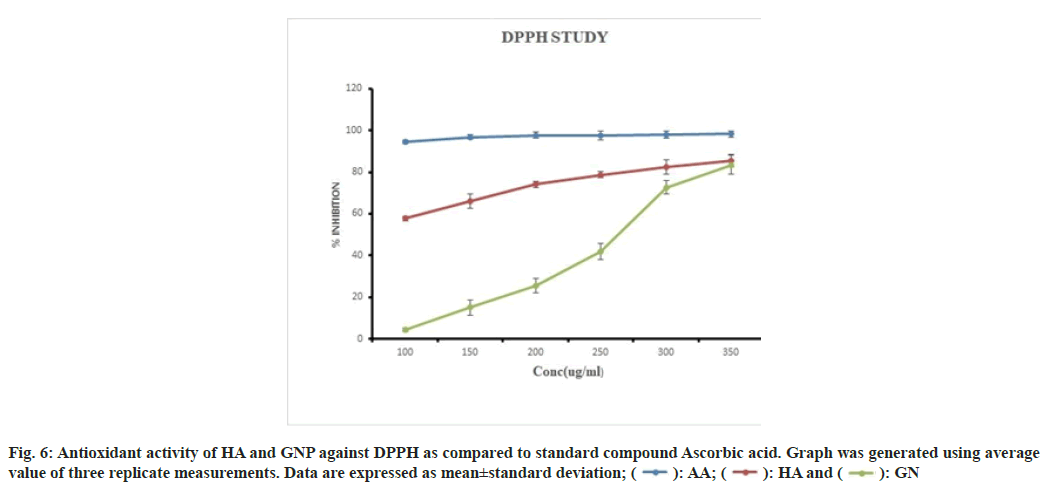
The DPPH assay is widely used to evaluate the properties of compounds for scavenging free radicals such as antioxidants. DPPH is a stable synthetic free radical that is easily reduced by antioxidants, either by accepting or donating electrons, formation of hydrazine molecules changes the colour of DPPH from purple to yellow. The method is based on the spectrophotometric measurement of the DPPH concentration change resulting from the reaction with an anti-oxidant[41,42]. The antioxidant activity of the HA and GNP was estimated by comparing the percentage inhibition of DPPH radicals of standard ascorbic acid. The DPPH scavenging activity of HA increased with increasing concentrations namely 57±1.13 %, 100 µg/ml; 65±3.34 %,150 µg/ml; 74±1.54 %, 200 µg/ ml; 79±1.39 %, 250 µg/ml; 82±3.43 % 300 µg/ml and 85±3.13 l%, 350 µg/ml whereas, GNPs showed the same trend but at a very slow rate of 4±1.02 %, 100 µg/ ml; 15±3.72 %, 150 µg/ml; 25±3.57 %, 200 µg/ml; 42±3.73 %, 250 µg/ml; 72±3.30 %, 300 µg/ml and 83±4.47 %, 350 µg/ ml due to the less solubility of GNP. The DPPH scavenging activity of ascorbic acid used as standard was found to be 98 % at all the concentrations. Both HA and GNP showed dose dependant activity (fig. 6). Many kinds of antioxidants in the extract could perform synergistically. During the synthesis of the GNPs, these bio-compounds are adsorbed onto the surface of the GNPs. Thus antioxidant effects of the GNP might be the result of an active physicochemical interaction of Au atoms with the functional groups of the HA.
The MTT dye conversion assay is a colorimetric assay performed to assess the cell metabolic activity. Viable cells mitochondria are able to produce NADPH dependent oxidoreductase enzyme which can reduce MTT to a violet-coloured insoluble crystals of farmazan. But in case of metabolically inactive cells the violet colour change will not occur. When treated with different concentrations namely 1, 10, 50, 100 µg/ml of extract and GNP, no toxicity was noticed. After 48 h it was found that, treatment with HA extract and GNP did not significantly p<0.05 and p<0.001 respectively, decrease proliferation of PBMCs. Almost 80 % of PBMCs retained their viability on treatment with HA extract at 100 µg/ml and with GNP all the concentrations retained their viability (fig. 7). Thus suggesting that the synthesized GNP were non-toxic and biocompatible providing an excellent platform for various biomedical applications[43].
In conclusion Tinospora cordifolia extract has shown excellent capability of green synthesis of GNP from gold salt solution. This biosynthesis is a simple, single step, rapid, reproducible and efficient method by which GNP are produced at room temperature. The spectroscopic characterization using various analytical instruments was useful in confirming the formation, size and shape of GNPs. FTIR and HPTLC fingerprint confirmed the presence of bio-reducing organic compounds responsible for nanoparticle synthesis. XRD study shows that the formed GNP is crystalline in nature having good stability with zeta potential value of -29 mV. The DPPH assay shows dose dependant antioxidant activity. The in vitro stability test and MTT assay proves that the GNP`s are stable to biological fluids and also biocompatible. More investigations on other in vitro activity and in vivo toxicity test remain to be done. The present work may support further studies about using the high medicinal values of the biomolecules present in the GNP synthesized from stem extract of Tinospora cordifolia for the enrichment of herbal preparations to strengthen their activities. Thus, this is a safe, simple, rapid and highly reproducible green synthesized herbal gold nanoparticle which provides an opportunity to use these GNP for application in drug delivery.
Acknowledgements:
We are deeply thankful to Dr. Ashish Phadke and Dr.Vinayak Naik for their valuable suggestions during this work.
Conflict of interest:
References
- Sett A, Gadewar M, Sharma P, Deka M, Bora U. Green synthesis of gold nanoparticles using aqueous extract of Dillenia indica. Adv Nat Sci Nanosci Nanotechnol 2016;7(2):1-8.
- Shabestarian H, Homayouni-Tabrizi M, Soltani M, Namvar F, Azizi S, Mohamad R, et al. Green synthesis of gold nanoparticles using sumac aqueous extract and their antioxidant activity. Mater Res 2016;20:264-70.
- Singh AB, Sharma MM, Batra AM. Synthesis of gold nanoparticles using chick pea leaf extract using green chemistry. J Optoelectron Biomed Mater 2013;5(2):27-32.
- Ghosh S, Patil S, Ahire M, Kitture R, Gurav DD, Jabgunde AM, et al. Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J Nanobiotechnol 2012;10:1-9.
[Crossref] [Google Scholar] [PubMed]
- Elia P, Zach R, Hazan S, Kolusheva S, Porat ZE, Zeiri Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int J Nanomed 2014;9:4007.
- Abbai R, Mathiyalagan R, Markus J, Kim YJ, Wang C, Singh P, et al. Green synthesis of multifunctional silver and gold nanoparticles from the oriental herbal adaptogen: Siberian ginseng. Int J Nanomed 2016;11:3131-43.
[Crossref] [Google Scholar] [PubMed]
- Hariharan A, Begum TN, Ilyas MH, Jahangir HS, Kumpati P, Mathew S, et al. Synthesis of plant mediated gold nanoparticles using Azima tetracantha Lam. leaves extract and evaluation of their antimicrobial activities. Pharmacogn J 2016;8(5):507-12.
- Yasmin A, Ramesh K, Rajeshkumar S. Optimization and stabilization of gold nanoparticles by using herbal plant extract with microwave heating. Nano Converg 2014;1(1):12.
[Crossref] [Google Scholar] [PubMed]
- Boomi P, Ganesan R, Prabu Poorani G, Jegatheeswaran S, Balakumar C, Gurumallesh Prabu H, et al. Phyto-engineered gold nanoparticles (AuNPs) with potential antibacterial, antioxidant, and wound healing activities under in vitro and in vivo conditions. Int J Nanomed 2020:7553-68.
[Crossref] [Google Scholar] [PubMed]
- Rodríguez-León E, Rodríguez-Vázquez BE, Martínez-Higuera A, Rodríguez-Beas C, Larios-Rodríguez E, Navarro RE, et al. Synthesis of gold nanoparticles using Mimosa tenuiflora extract, assessments of cytotoxicity, cellular uptake, and catalysis. Nanoscale Res Lett 2019;14(1):1-6.
- Ganesan RM, Prabu HG. Synthesis of gold nanoparticles using herbal Acorus calamus rhizome extract and coating on cotton fabric for antibacterial and UV blocking applications. Arab J Chem 2019;12(8):2166-74.
- Mittal J, Sharma MM, Batra A. Tinospora cordifolia: A multipurpose medicinal plant-A. J Med Plant 2014;2(2):32-47.
- Khatun H, Kundu S, Kazi MM, Ahmed MG. Guduchi (Tinospora cordifolia (Wild), A traditional Indian herbs and its medicinal importance-An ayurvedic approach with contemporary view. Int J Ayurvedic Herb Med 2016;41(6):2260-67.
[Crossref]
- Choudhary N, Siddiqui MB, Azmat S, Khatoon S. Tinospora cordifolia: Ethnobotany, phytopharmacology and phytochemistry aspects. Int J Pharma Sci Res 2013;4(3):891.
- Sankhala LN, Saini RK, Saini BS. A review on chemical and biological properties of Tinospora cordifolia. Int J Med Arom Plant 2012;2(2):340-4.
- Singh S, Devi P. Pharmacological potential of Tinospora cordifolia (Willd.) Miers ex hook. & Thoms.(Giloy): A review. J Pharmacogn Phytochem 2017;6(6):1644-7.
- Patni K. The amazing pharmacological properties of Tinospora cordifolia: A short review. Int J Ayu Pharm Chem 2015;4(2):105-29.
- Sinha K, Mishra NP, Singh J, Khanuja SP. Tinospora cordifolia (Guduchi), a reservoir plant for therapeutic applications: A review.
- Singh AK. Comparative therapeutic effects of plant-extract synthesized and traditionally synthesized gold nanoparticles on alcohol-induced inflammatory activity in SH-SY5Y cells in vitro. Biomedicines 2017;5(4):70-97.
[Crossref] [Google Scholar] [PubMed]
- Sivakumar V, Rajan MD. Marker based standardization of Tinospora cordifolia stem extract by HPTLC. IJPR 2012;4(2):93-95.
- Usmani K. HPTLC fingerprint profile of stems of Tinospora cordifolia (wild) miers and roots of Hemidesmus indicus (l.) R.Br with their polyherbal mixture. Int J Pharm Technol 2020;12(2):32111-28.
- Chauhan RP, Gupta C, Prakash D. Methodological advancements in green nanotechnology and their applications in biological synthesis of herbal nanoparticles. Int J Bioassays 2012;1(7):6-10.
- Boruah JS, Devi C, Hazarika U, Reddy PV, Chowdhury D, Barthakur M, et al. Green synthesis of gold nanoparticles using an antiepileptic plant extract: In vitro biological and photo-catalytic activities. RSC Adv 2021;11(45):28029-41.
- Lee YJ, Song K, Cha SH, Cho S, Kim YS, Park Y. Sesquiterpenoids from Tussilago farfara flower bud extract for the eco-friendly synthesis of silver and gold nanoparticles possessing antibacterial and anticancer activities. Nanomaterials 2019;9(6):819.
[Crossref] [Google Scholar] [PubMed]
- Boomi P, Ganesan R, Prabu Poorani G, Jegatheeswaran S, Balakumar C, Gurumallesh Prabu H, et al. Phyto-engineered gold nanoparticles (AuNPs) with potential antibacterial, antioxidant, and wound healing activities under in vitro and in vivo conditions. Int J Nanomed 2020:7553-68.
[Crossref] [Google Scholar] [PubMed]
- Chahardoli A, Karimi N, Sadeghi F, Fattahi A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif Cells Nanomed Biotechnol 2018;46(3):579-88.
[Crossref] [Google Scholar] [PubMed]
- Kumar B, Smita K, Vizuete KS, Cumbal L. Aqueous phase Lavender leaf mediated green synthesis of gold nanoparticles and evaluation of its antioxidant activity. Biol Med 2016;8(3):1-4.
- Ghahremanzadeh R, Yazdi Samadi F, Yousefi M. Green synthesis of gold nanoparticles using three medicinal plant extracts as efficient reducing agents. Iran J Chem Chem Eng 2019;38(1):1-10.
- Bindhani BK, Panigrahi AK. Green synthesis and characterization of gold nanoparticles using leaf extracts of Withania somnifera (Linn.)(Ashwagandha). Int J Mater Sci Appl 2014;3(6):279-84.
- Tripathi A, Kumari S, Kumar A. Toxicity evaluation of pH dependent stable Achyranthes aspera herbal gold nanoparticles. Appl Nanosci 2016;6:61-9.
- J Arunachalam L. Green synthetic route for the size-controlled synthesis of biocompatible gold nanoparticles using aqueous extract of garlic (Allium Sativum). Adv Mater Lett 2013;4(7):548-55.
- Islam NU, Khan I, Rauf A, Muhammad N, Shahid M, Shah MR. Antinociceptive, muscle relaxant and sedative activities of gold nanoparticles generated by methanolic extract of Euphorbia milii. BMC Complement Alternat Med 2015;15(1):160-71.
- Rodríguez-León E, Rodríguez-Vázquez BE, Martínez-Higuera A, Rodríguez-Beas C, Larios-Rodríguez E, Navarro RE, et al. Synthesis of gold nanoparticles using Mimosa tenuiflora extract, assessments of cytotoxicity, cellular uptake, and catalysis. Nanoscale Res Lett 2019;14(1):1-6.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, El-Agamy Farh M, Yang DC. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif Cells Nanomed Biotechnol 2016;44(3):811-6.
[Crossref] [Google Scholar] [PubMed]
- Markus J, Wang D, Kim YJ, Ahn S, Mathiyalagan R, Wang C, et al. Biosynthesis, characterization, and bioactivities evaluation of silver and gold nanoparticles mediated by the roots of Chinese herbal Angelica pubescens Maxim. Nanoscale Res Lett 2017;12:1-2.
[Crossref] [Google Scholar] [PubMed]
- Sun YW, Wang LH, Meng DL, Che X. A green and facile preparation approach, licochalcone A capped on hollow gold nanoparticles, for improving the solubility and dissolution of anticancer natural product. Oncotarget 2017;8(62):105673-81.
[Crossref] [Google Scholar] [PubMed]
- Mishra AN, Bhadauria S, Gaur MS, Pasricha R, Kushwah BS. Synthesis of gold nanoparticles by leaves of zero-calorie sweetener herb (Stevia rebaudiana) and their nanoscopic characterization by spectroscopy and microscopy. Int J Green Nanotechnol Physics Chem 2010;1(2):P118-24.
- Khalil MM, Mahmoud II, Hamed MO. Green synthesis of gold nanoparticles using Laurus nobilis L. leaf extract and its antimicrobial activity. Int J Green Herbal Chem 2015;265.
- Dubey SP, Lahtinen M, Sillanpää M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem 2010;45:1065-71.
- Mukundan D, Mohankumar R, Vasanthakumari R. Comparative study of synthesized silver and gold nanoparticles using leaves extract of Bauhinia tomentosa Linn and their anticancer efficacy. Bull Mater Sci 2017;40:335-44.
- Guler E, Barlas FB, Yavuz M, Demir B, Gumus ZP, Baspinar Y, et al. Bio-active nanoemulsions enriched with gold nanoparticle, marigold extracts and lipoic acid: In vitro investigations. Colloids Surf B Biointerfaces 2014;121:299-306.
[Crossref] [Google Scholar] [PubMed]
- Benedec D, Oniga I, Cuibus F, Sevastre B, Stiufiuc G, Duma M, et al. Origanum vulgare mediated green synthesis of biocompatible gold nanoparticles simultaneously possessing plasmonic, antioxidant and antimicrobial properties. Int J Nanomed 2018;13:1041-58.
[Crossref] [Google Scholar] [PubMed]
- Dey A, Yogamoorthy A, Sundarapandian SM. Green synthesis of gold nanoparticles and evaluation of its cytotoxic property against colon cancer cell line. Res J Life Sci Bioinformat Pharm Chem Sci 2018;4(6):1-7.
 GN
GN