- *Corresponding Author:
- B. Ahmed
Department of Pharmaceutical Chemistry, School of Pharmaceutical Education and Research, Jamia Hamdard, New Delhi 110062, India
E-mail: bakhan_ph@jamiahamdard.ac.in
| Date of Received | 28 November 2020 |
| Date of Revision | 12 January 2023 |
| Date of Acceptance | 04 April 2023 |
| Indian J Pharm Sci 2023;85(2):491-500 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The exposure of ionizing radiation involve a highly orchestrated series of events that induced amplified by endogenous signaling and culminating in oxidative damage to deoxyribonucleic acid, lipids, proteins and many metabolites. Gallic acid ethyl ester is a major bioactive constituent of Hippophae rhamnoides L. leaves and extract prepared from it exhibited radio protective and pharmacological activity. Radio modifying properties of polyphenol compounds through free radical neutralizing have been reported earlier. The study aims to investigate the in silico and gamma-radio modifying action, oxidative stress estimation, histopathological and Immunohistopathological analysis of gallic acid ethyl ester at a radio protective dose and changes, if any, induced post irradiation. Male C 57 BL/6 mice (28 g-30 g) were administered Gallic acid ethyl ester (250 mg/kg b.w) orally 15 min prior to irradiation. Mice were sacrificed at different time points, plasma and tissues were studied for oxidative stress and pathological analysis. The gamma-radio modifying potential was assessed in terms of mitigating cyclooxygenase-2 expression and oxidative stress parameters levels in liver and kidney post irradiation. Our study suggested the potential use of gallic acid ethyl ester as gamma-radio modifying agent inhibits cyclooxygenase-2 expression and maintains the lipid peroxidation, superoxide, glutathione and alkaline phosphatase activity up to 8 h in comparison to irradiated mice. Moreover, in histopathological analysis, it showed that gallic acid ethyl ester protect and maintain the cellular architect in liver and kidney from the toxic effect of ionizing radiation. These in silico, pre-clinical biomolecules and histopathology result may be useful for study of the bioactive mechanism associated with radiation injury and to develop a potent formulation of gallic acid ethyl ester for clinical application against gamma-radiation.
Keywords
Gallic acid ethyl ester, ionizing radiation, pharmacokinetics, s, oxidative stress
Ionizing radiation composed of various wave lengths including Gamma (γ)-rays and X-rays and released in a form of energy released from atoms. Worldwide the exposure of radiation exposure for different use including medical diagnostic, nuclear medicine procedure and radiotherapies were increasing[1]. Exposure of radiation either from direct and indirect source penetrate living cells or tissue which induce and prompt oxidative stress due to overproduction of several free radicals and disturbed cellular biomolecules. These free radicals are Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) such as Hydroxyl ion (OH-), Hydrogen ion (H+) and Superoxide anions (O2-) which attack nucleic acid, lipid membranes, biomolecules and cause intracellular toxicity. Post radiation exposure either in clinical setup or of some other use of daily basis caused an imbalance between oxidant/antioxidant levels and is partially involved in the pathology of different disorders. Indirect deposition of energy emit by radiation exposure depends upon on production of several free radicals which are highly reactive.
Radiotherapy one of the common approach that partially used as therapeutic approach to inhibit cancer cell proliferations. Exposure of ionizing radiation induce oxidative stress and cause changes in biomolecules at cellular levels and also induces the pathogenesis of various disorder including kidney and liver injury. The alteration of this biomolecules may lead to alter cell division and reduction of stem cell loops, organ dysfunction and lead to cell death. Moreover, from the alteration from radiation sources the different organs part of the body are susceptible to radiation induce damage and it’s also depends upon the population of cells and their productions, differentiation, aging and loss of cells[2]. After irradiation the cell death expression is generally delayed until mitosis. Tissue undergone rapid proliferation has contain specific cells which are capable of renewing indefinite cells and form compartment of proliferating cells after mitotic cell division. Tissue particularly does not have regenerative cells and have low cell proliferative activity. Moreover; the response timing is also depending on the exposure of radiation and their dose[3]. Globally a great deal of time and money spent on the clinical and pre-clinical research with the aim of developing efficacious and safe radio modifying agents that could mitigate the damage induced post radiotherapy. However, until this date none of them were found to be safe and non-toxic for human use. Hippophae rhamnsoides L. (H. rhamnoides) (Sea buckthorn) Elaegnaceae is valuable plant has recently gained attention worldwide due to its nutritional and medicinal properties. Sea buckthorn (H. rhamnoides) is nitrogen fixing deciduous shrub of cold arid region native to Europe and Asia[4]. Sea Buckthorn Leaves aqueous extract (SBL-1) showed radiation protection at a lethal dose of ionizing radiation[5]. Gallic Acid Ethyl Ester (GAE) is one of the major bioactive constituent present in aqueous extract coded SBL-1[6].
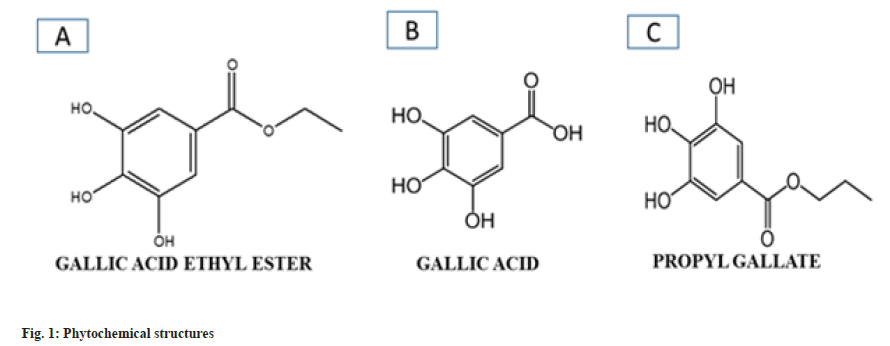
GAE, Syringic acid (C9H10O5) has molecular weight 198.17 g/mol and is a colorless or slightly yellow crystalline compound possessing antiinflammatory[7], antibacterial[8] and anticancer properties[9]. It is a derivative of the shikimic acid pathway. The secondary metabolites of plants are a very good source for the discovery and development of lead molecule. GAE has been reported in extracts of some other plants also such as Terminalia chebula, which have shown neuroprotective effects[10].
In our previous in silico and in vitro studied with GAE and different analogs showed potent anti-oxidant and anti-inflammatory by notable binding affinity with the Superoxide Dismutase (SOD) and Cyclooxygenase-2 (COX-2) receptors in comparison to different analogs. In vitro GAE confirmed promising antioxidant potential by 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) and SOD anion inhibition assay[11]. In another study GAE a major bioactive constituent of H. rhamnoides leaves showed radio modifying action against 5Gy of γ-radiation exposure. The GAE showed the radio modifying potential in terms of mitigating Nuclear Factor Kappa B (NF-κB) activity and serum glutamic-pyruvic transaminase, serum glutamicoxaloacetic transaminase, urea and creatinine levels in liver and kidney post irradiation [12].
Materials and Methods
Chemical reagents:
Reduced Glutathione (GSH), Nicotinamide Adenine Dinucleotide Phosphate (NADPH), 1,2-Dithio-Bisnitrobenzoic Acid (DTNB), Nitroblue Tetrazolium (NBT), glutathione oxidized and Folin’s reagent were purchased from Sisco laboratories Ltd., Mumbai, India. Sodium carbonate and trichloroacetic acid was purchased from Himedia, India. Thiobarbituric acid, bovine serum albumin and Ethylenediaminetetraacetic Acid (EDTA) were purchased from Sigma Aldrich., St. Louis, MO, USA. Ethanol, Methanol and Xylene and Hematoxylin stain were purchased from Sigma Aldrich.
Isolation, extraction and characterization:
The aerial part of H. rhamnoides leaves were powdered finely and extracted with 95 % alcohol, filtered and dried in rotavapor. The extract was dissolved and filtered. The residue of filtrate was neutralized with an aqueous solution of Hydrochloric acid (HCl) n/10=(1 n). The precipitate obtained was filtered and washed with cold water to remove traces of HCl. The solid residue obtained was recrystallized from the minimum quantity of boiling water, on cooling the crystal will appear and crystal was collected, dried and their melting point was recorded.
The isolated compound of GAE was further characterized by using Fourier-transform infrared spectroscopy as described in previous method[12] and data was recorded using a Nicolet 8700 spectrometer equipped with a Deuterated Triglycine Sulfate- Thermoelectrically Cooled (DTGS-TEC) detector and the data were analyzed on OMNIC software. The scan was collected at a resolution of 4cm-1 and a data spacing of 1.928 cm-1 in order to achieve sufficient signal to noise ration.
Gallic Acid (GA) derivative preparation:
GA was dissolved in dried absolute ethyl alcohol followed by passing dried fumes of hydrochloric acid through a glass tube, for about 2 h-3 h and the content were poured in ice cold water. The ester were collected as per method described in previous studies[11].
In silico molecular docking studies:
Molecular docking was applied on reported chemical entities by using Schrodinger, (LLC, Cambridge, USA). The NF-kB and Glutathione Peroxidase (GPx) receptors were retrieved from the Protein Data Bank (PDB) (http://www.rscrb.org/pdb). Molecular docking of ligands deal with ligand interaction, H-bonding and hydrophobic effects with receptors.
Selection of target:
Selection and identification of appropriate target is an important part in drug designing. Three Dimensional (3D) structure of GPx were obtained from PDB with id’s 2P31 respectively. The complexes bound to the receptor molecule, such as nonessential water molecule, including heteroatoms were removed from the target receptor molecule.
Tool used:
Chem draw was used for designing the ligands. The receptors (PDB ID: 2P31) were accessed from the database of Schrodinger tools. These receptors refined, optimized and energy minimized using protein preparation wizards. The binding sites were identified using sitemap (version 3.4) for the receptors without co-crystallized ligands[12,13].
Ligand preparation:
Three compounds GA, GAE and Phloroglucinol (PG) were used for docking studies (fig. 1A- fig. 1C). Structure of compounds obtained from the PubMed and PubChem literature showed and inhibitory action toward inflammation[14]. The ligands were sketched in Chem Draw and the mol/sdf files were imported to LigPrep followed by ligand preparation and energy minimization by using LigPre v2.9 module under the force field OSPL3. The possible ionization states of all the ligands were generated using the ionization module in pH range.
Experimental animals and irradiation:
Pre-clinical studies were performed in black C57BL/6 male mice weighing 28 g-30 g and maintained at the Institutional Animal Experimental Facility. Animals were fed according to standard chow diet having free access to food and water ad libitum. The environmental condition was maintained at room temperature (22°±3°), relative humidity (47 %-60 %) and 12 h dark/light cycle controlled facility. In this study, mice were randomly assigned to four different groups (n=21) i.e. Untreated Control (UC), GAEtreated (GAE), GAE+Radiation (GAE+R), Radiation group (R).
Mice fasted overnight with free access to water before being dosed of GAE at 250 mg/kg body weight. The experiment was carried out in accordance to regulation and guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and study was approved by Institutional Animal Experimental Committee (IAEC), Institute of Nuclear Medicine and Allied Science, Delhi, India. The GAE was administered orally 10 min prior irradiation and the whole-body radiation (10 Gy) was carried out using Bhabahtron II, Telecobalt machine, Panacea medical technology, Bangalore. The mice exposed at a dose rate of 0.961 Gy/min at field 35×35cm2 and the distance maintained from the source was 80 cm.
Tissue extraction and sampling:
Blood, liver and kidney of mice were collected after GAE administration (3 mice per time). Blood was obtained from retro orbital plexus of mice and collected in heparinized Eppendorf tubes, followed by centrifugation at 2500 g for 15 min. The resulting plasma layer was separated and stored in tubes at -20° for further biochemical analysis. Tissue samples (liver and kidney) were weighed and put into the normal saline solution to remove the blood by blotting on filter paper and were minced and homogenized with physiological saline solution (1:2 w/v) thoroughly in an ice bath. The homogenates were stored at -80° until the analysis.
Biochemical parameters:
The oxidative stress parameter were analyzed by using different parameters; prior to analysis of antioxidant defense system protein quantification was done by using Bradford assay, Lipid Peroxidation (LPx) were measured in terms of Malondialdehyde (MDA) formation per unit protein and it was measured by method[15]. Total-Thiol (T-SH) content (mM/mg). SOD activity was expressed in terms of percentage inhibition of NBT reduction and chromagen intensity[16] and Glutathione Reductase (GR)[17]. GPx activity was performed in 50 mM phosphate buffer (pH 7.4) containing 1 mM EDTA, 0.12 mM NADPH, 0.85 mM GSH.
Histopathological studies:
Histopathology changes in liver and kidney were studied by the staining of tissue sections with haematoxylin and eosin. Liver and kidney were fixed in 10 % formalin. Tissues were then processed for dehydration, clearing and impregnated into wax blocks and further sectioning the tissue at 5 μ and stained the slides from Haematoxylin and Eosin by using standard protocols.
Immunohistochemistry of COX-2:
Immunofluoorescent staining of COX-2 was performed by using formalin fixed paraffin embedded tissue by using standard procedure and protocol. The liver and kidney section 5 μm were immunohistochemical stained. Primary polyclonal antibody mouse to prostaglandin endoperoxidase synthase 2 (1:2000) was used. After washing, the slides were counterstained with ethidium bromide (EtBr) and incubated for 1 h at room temperature. Prostaglandin Endoperoxidase Synthase (PGE2) activity was visualized under a fluorescent microscope Axioscope A1 make Carl Zeiss. The images was visualized and captured by the use of Axiovision software.
Results and Discussion
As per previous reported method by Pandey et al.[11] GAE exhibited peaks at 3250 cms due to presence of hydroxyl group, 1260 cm-1 due to C-O (Phenolic group) on the aromatic ring. The peak at 2950 cm-1 due to the CH2 group was also found indicating the ethyl group presence on it. Another peak at 1700 cm-1 was present due to carboxyl group. The IR data confirmed the presence and purity of GAE.
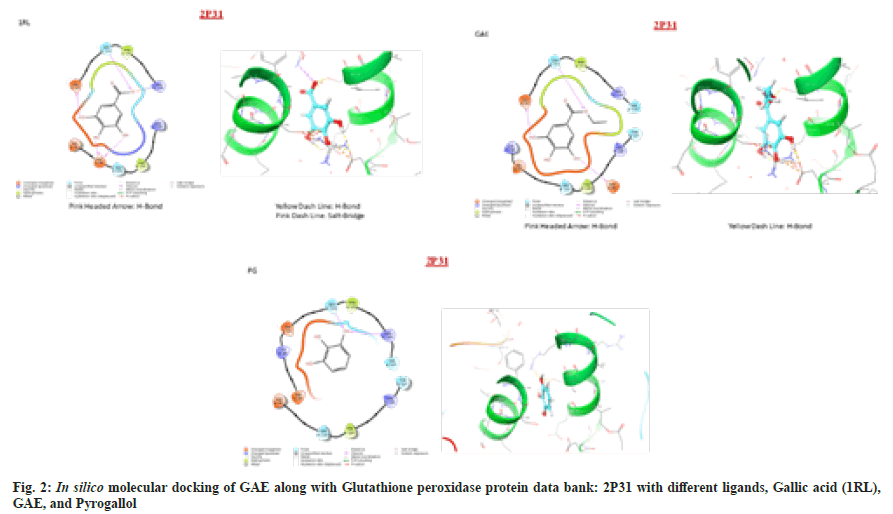
Intermolecular flexible docking simulation was performed to explore the binding site of different ligands with different receptors. Energy was calculated from the information obtained while docking the confirmation of different receptor complexes. To determine the best compound affinity and develop a lead molecule, different compounds were compared to reference ligands and screening were based on Gibbs free energy values, affinity, confirmation of the structure and hydrogen bonding interaction between compounds and target proteins.
The interpretation of ligands with different receptors showed polar and hydrophobic interaction, some ligands are forming H-bonds and some form salt bridges. GA is considered as Reference Ligand (1RL) and it was found to bind with residue Glu99, Ser102 and Arg106 of chain A through polar interaction, while Phe103 of the same protein chain interacted via Hydrophobic interaction. On the other hand, chain B residue Phe103 was interacted via hydrophobic while Asp95, Glu99, Ser102 and Arg106 of the same protein chain interacted with polar and negatively charged interactions. Four of the four hydroxyl groups were forming an H-bond with chain A residue Glu99 with negative charged, while the second hydroxyl group was interacting with the chain A Ser102 residue with polar interaction and through H-bond formation. The third and fourth-hydroxyl groups of ligand are negatively polar interacted with Asp95 of chain B residue through H-bonds formation. The molecule represented a symmetrical orientation to both the chains of the dimeric proteins. It was observed that the molecule interacts at the symmetrical interfaces of the protein. The interfacial positioning of the ligand offered maximum stability to the receptorligands complex and thus suggested a suitable site of interaction with optimum activity.
PG was found strongly interacted via two H-bonds with Ser102 and Arg106 of chain a residue through polar and positive charged interaction. One of three hydroxyl groups formed an H-bond with Chain a residue. While on the other hands Phe103 of chain a residue and Phe103 of chain B residue interacted with hydrophobic interaction. In spite of undergoing an orientation change in the structure, the ligand accommodated itself at the protein interfaces, indicating its activity almost similar to the 1RL. Therefore, the molecule remains same inside the active site, reflecting similar behavior toward the protein (2D and 3D ligand interaction diagram of the ligands with 2P31 proteins).
GAE was found to interact via two H-bond and bind with residue, Asp95, Glu99 of chain a through negative charged interaction, whereas Ser102 of chain a residue interacted with OH group through polar and hydrophobic interaction. On other hand, the chain B residue Lys99, Glu99, Ser102, Phe103, Arg106, Thr107 interacted with negative charge. Two of three hydroxyl group was formed and H-bond with chain a residue Glu99 and Asp95. The chain A and chain B residue formed the hydrophobic cavity with polar and negative charged interaction around the ligand, while the chain A residue Glu99, Ser102, Phe103, Arg106, Thr107 were interacting through hydrophobic polar and positive charge interaction, while Asp99 interacted with negative charge interaction. Moreover, the chain B residue Lys98, Glu99, Ser102, Phe103, Arg106, and Thr107 were interacting through negative charged interaction. In spite of undergone an orientation change in the structure, the ligand accommodated itself at the protein interfaces, indicating it’s almost similar activity to the 1RL. The observation was further assured from the G-score (-5.8) which were higher (fig. 2). Therefore, the molecule remains the same inside the active.
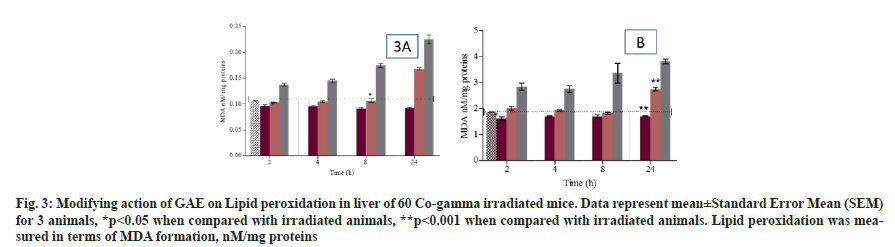
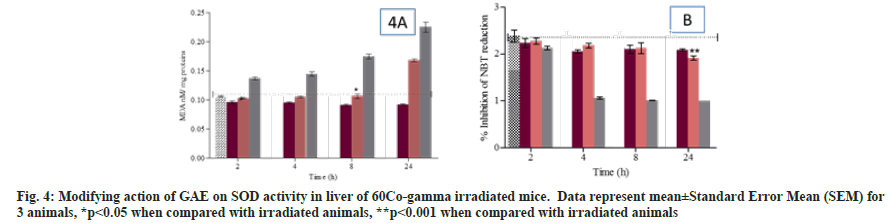
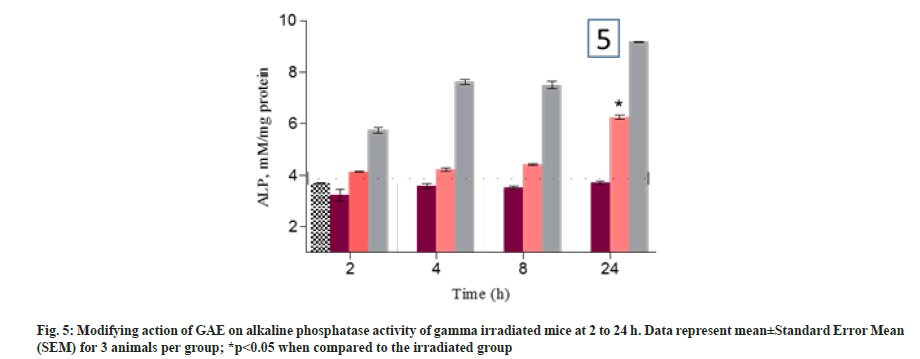
Lipid peroxidation was measured in term of MDA formation. The animal treated with GAE prior to irradiation, showed the significant reduction in lipid peroxidation up to 8 h (p>0.05) and increase in 24 h when compared to untreated control animals. Animals treated with GAE prior to irradiation, significantly increase (p<0.005) lipid peroxidation in kidney and it was observed at 24 h when compared to untreated control group (fig. 3A and fig. 3B). Superoxide dismutase activity was measured in treated and untreated animal at different time points in liver and kidney. The activity differed significantly (p<0.005) in liver of GAE treated animals in comparison to irradiated animals. In kidney the SOD activity was significantly (p<0.01) reduced at all different time points from 4 h to 24 h in irradiated animals when compared to untreated control groups. In GAE treated animals the SOD activity differed significantly (p<0.005) up to 24 h in comparison to irradiated groups (fig. 4A and fig. 4B). Alkaline phosphatase level was measured in plasma of mice to check the severity and damage of liver post irradiation. Alkaline Phosphatase (ALP) level was measured at all different time points when compared to untreated control animals. The administration of GAE prior to irradiation significantly reduced the ALP levels (p<0.01) up to 8 h when compared to irradiated groups (fig. 5), while in 24 h the ALP level was high.
Fig. 3: Modifying action of GAE on Lipid peroxidation in liver of 60 Co-gamma irradiated mice. Data represent mean±Standard Error Mean (SEM) for 3 animals, *p<0.05 when compared with irradiated animals, **p<0.001 when compared with irradiated animals. Lipid peroxidation was measured in terms of MDA formation, nM/mg proteins
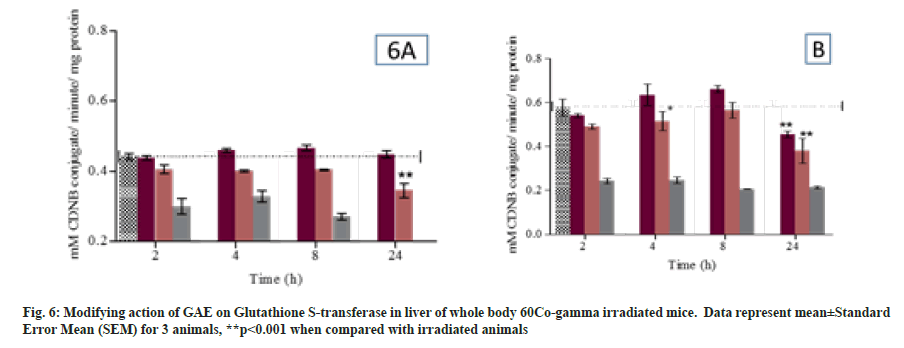
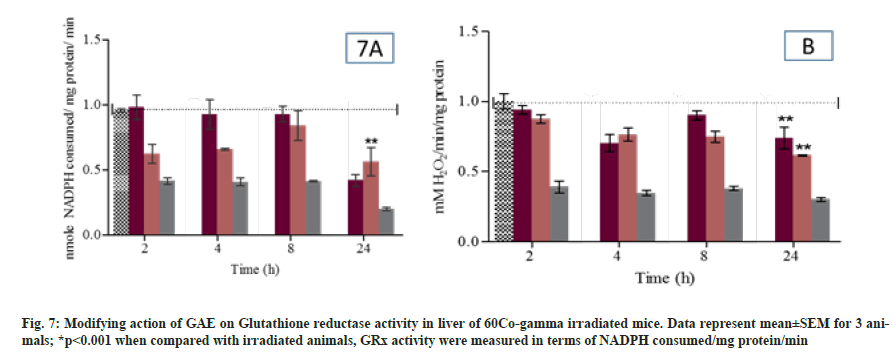
Glutathione S Transferase activity was measured in liver and kidney of different group mice at different time points. In GAE treated animals the GST activity was comparable to untreated group animals. Whereas, GAE treated animal prior to irradiation significantly maintain GST activity (p<0.05) in liver or increase when compared to irradiated animals. Moreover, in kidney the GST activity significantly (p<0.05) maintain up to 8 h when compared to irradiated treated group animals (fig. 6A and D). GR activity was measured at all different time points. In GAE treated animals the GPx activity was comparable to untreated group animals. GAE administration prior to irradiation significantly (p<0.05) reduced the GPx activity at 2 h, while the reductase activity was comparable to untreated control group at 4 h and 8 h whereas at 24 h the activity reduced again. The GPx activity in kidney was comparable to untreated group animals at all study time points. In animal treated with GAE prior irradiation showed the significant (p<0.05) increase in GPx activity at 2 h to 24 h when compared to irradiated group. The T-SH content was significantly (p<0.01) reduced from 2 h to 24 h in irradiated animals liver when compared to untreated control animals (fig. 7A). Level were significantly increased (p<0.01) in GAE treated animals at all different time when compared to irradiated group animals. The significant reduction in T-SH content was observed in irradiated animal’s kidney at 8 h and 24 h when compared to untreated control. Moreover, the T-SH level was significantly increased (p<0.01) at different time points in GAE treated animals when compared to irradiated animals as shown in fig. 7B.
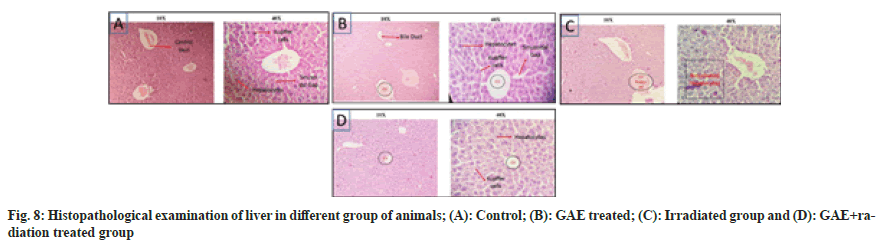
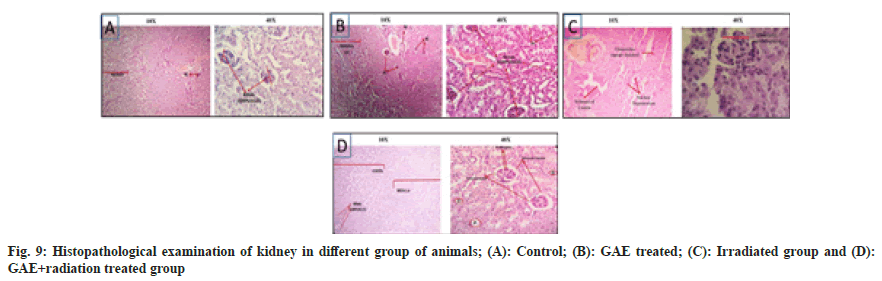
Post irradiation (up to 10 Gy) multiple histopathological changes were observed in liver of mice. The central vein damaged, sinusoidal space is shrinked, kupffer cell were not observed, bile duct was damaged and hepatocytes are clubbed and damaged showed binucleated hepatocytes. Whereas the GAE administration prior to irradiation in animals showed minimal damage in central vein, bile ducts, hepatocytes were binucleated in sinusoidal space were shrinked, no kupffer cells were observed at 24 h (fig. 8) in comparison to untreated control group. Moreover, in kidney the normal appearance of Bowman’s capsule as well distal and proximal convoluted tubules were observed in transverse section of untreated control animals. The kidney section of irradiated animals showed multiple changes such as glomerulus shrinkage, widened of lumen, nuclear degeneration and increased in intertubular spacing observed at 24 h when compared to untreated controls. The animals treated with GAE prior to irradiation showed minimal shrinkage in glomerular, slightly widening of lumen though was observed at 24 h, but when compared to irradiated treated group animals the damage was much smaller in GAE prior to irradiation group. The proximal and distal tubules of GAE treated groups were similar to untreated control groups, whereas the podocytes were also present in well-arranged manner in renal corpuscles; medulla and cortex were also in wellarranged manner (fig. 9).
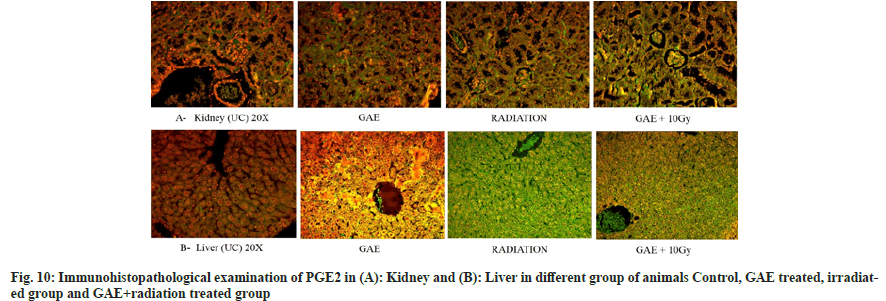
COX-2 is a key mediator pro-inflammatory cytokines play a significant role in inflammation induces post irradiation. The time-dependent study showed major expression by means of immunofluorescence staining. PGE2 positive fluorescence was strongly expressed in the central vein, hepatocytes and sinusoidal gaps of liver sections in irradiated mice and appeared in green color. Similarly, the positive fluorescence expression of COX-2 was expressed in tubules, glomeruli, Bowman capsules and proximal and distal tubules in the kidney section of irradiated mice and appeared in green color. The expression was higher in the irradiated group in comparison to the GAE+irradiated group as shown in fig. 10.
Low linear energy transfer ionizing radiation exposure at lethal dose induces damage to biological macromolecules via overproduction of free radicals, ROS. Due to high reactivity in nature of these radical it damage all biological molecule including lipids, Deoxyribonucleic Acid (DNA) and proteins. Moreover; oxidative damage is directly correlate with the dose of radiation exposure and numerous type of reactive species induce after irradiation. The potential of GAE modify or counter the damage induce post irradiation exposure associated with oxidative stress which is an important biomarker to assess the efficacy against radiation induce injury. The GAE was further investigated for scavenging and neutralizing of free radicals in terms of scavenging of •OH, O2•-. The superoxide (O2•-) radical initiated after the reduction of oxygen at molecular level and mediates via the enzymatic action of the NADPH complex. Moreover, the GAE maintained the enzymatic and nonenzymatic level up to 8 h in GAE+radiation treated groups. Administration of GAE prior to irradiation showed the normal appearance in histopathological examination of kidney was observed up to 8 h (fig. 8). Minimal glomerular shrinkage, slightly widening of lumens was observed at 24 h. Due to escape of O2•- and •OH radicals the un protection was observed at 24 h.
T-SH play an important role in transportation and function of proteins, and it was observed that membrane enzymes help in the reduction of oxidative stress induced after radiation exposure. The modifying effect of thiol’s can act by directly scavenging or neutralizing the free radicals, or indirectly increasing GSH levels. In animals, GAE administration prior to irradiation significantly reduced T-SH from 2 h to 24 h. The experimental result showed the use of GSH in countering the free radicals generation induced after radiation exposure. The significant reduction in GR content was observed from 2 h to 24 h.
Lipid peroxidation is an important biomarker to assess the oxidative stress and damage to cellular membrane induced post irradiation. Moreover; it also observed that MDA formation play an important role in tissue damage mediated via development of oxygen radicals. MDA are the byproducts of lipid peroxidation. In our study result suggested that after whole body radiation exposure the MDA formation was significantly increased at 2 h to 24 h, but prior administration of GAE significantly reduced the lipid peroxidation at 2 h to 24 h. The result indicated that post irradiation animal showed improper detoxification of free radicals which prompt the formation of hydro-peroxide and cause changes in permeability of membrane. Whereas administration of GAE prior to irradiation significantly reduce the MDA formation up to 24 h. A significant decrease in GST activity was observed at 2 h to 24 h showed the probability of increase in lipid peroxidation. Glutathione S Transferase catalyzing enzyme is responsible for the conjugation with glutathione reduced to form the production of byproducts of lipid peroxidation. The administration of GAE prior to irradiation, SOD was increased.
The present study reveal that significant increase enzymatic level such as glutathione antioxidant enzymes system (GSH, GR, GST) along with reduction in lipid peroxidation may have been the reason for potential γ-radio modifying action by GAE in irradiated animals. Although restoration of SOD and by maintain the cellular enzymatic activity showed the modifying action cellular enzymatic and non-enzymatic level, induced post irradiation. The γ-radio modifying action of GAE administration prior to irradiation inhibit the free radical generation by neutralizing or stabilizing the free radicals induce after whole body radiation exposure in animals.
The experimental animals treated with GAE, showed normal cellular architecture of kidney sections. The histopathological study of drug treated kidney showed presence of non-apoptotic cells, hyalinization, and tubular lumen in normal appearance, observation represented GAE as nontoxic in nature. The oxidative stress and inflammation were not found in kidney and revealed that GAE was non-toxic to the kidneys of experimental mice.
The result of in silico molecular docking study suggested that the bioactive constituent present in H. rhamnoides have a noticeable effect on the scavenging of free radicals. The result suggested that comparative in silico analysis of GAE isolated from H. rhamnoides leaves showed the best binding affinity with GPx receptor at highest G-score (-5.8) with hydrophobic and polar interaction of amino acid residue.
The results of the above study suggested that the bioactive compounds present in the H. rhamnoides have a noticeable effect on the scavenging of free radicals post irradiation and maintain the cellular biomolecules and their integrity. The result suggested that GAE isolated from H. rhamnoides leaves showed the best binding affinity in silico with GPx receptor at highest G-score (-5.8) with hydrophobic and polar interaction of amino acid residue and its strongly interacted with four H-bonds two of three hydroxyl was formed H-bonds with chain-A residue of receptor in comparison to 1RL. Development of GAE as γ-radio modifying agents to protect the living organism by inhibiting or neutralizing the hazardous effects of γ-ionizing radiation is an important and challenging field. Such agents isolated from plants have utility not only in situation such as nuclear warfare or nuclear accidents but could also be investigated for their utility as adjuvant to radiotherapy to ameliorate the Hepatorenal toxicity induced post irradiation. In conclusion the finding suggested that due to its polyphenolic in nature GAE is promising agent inhibiting the free radical generation post irradiation and protects vital organ liver and kidney and maintains their biological macromolecules because of its rapid hydroxylation, which occur during the stabilizing of free radicals generation post irradiation. The presence of hydroxyl group on GAE donates their protons to a reactive oxygen species to quench and neutralize the free radicals post γ-radiation exposure. The hydroxylation at a particular position on GAE also enhances the plasma protein binding as well the ester present on it enhances the binding affinity to bind with plasma proteins. Moreover; the in silico study showed strong binding affinity of GAE with GPx in comparison to other ligands which represent the maintain of hydroxyl ion donation which may help in neutralizing the toxic radicals induced post radiotherapy or radiation exposure.
Conflict of interest:
The authors declared no conflict of interest.
References
- Kim O, Kim MS, Jang HJ, Lee H, Kang Y, Pang Y, et al. Radiation safety education and compliance with safety procedures-the Korea nurses’ health study. J Clin Nurs 2018;27(13-14):2650-60.
[Crossref] [Google Scholar] [PubMed]
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 7th ed. 2006;166:816-7.
- Singh VK, Romaine PL, Seed TM. Medical countermeasures for radiation exposure and related injuries: characterization of medicines, FDA-approval status and inclusion into the strategic national stockpile. Health Phys 2015;108(6):607.
[Crossref] [Google Scholar] [PubMed]
- Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries report of an NCI workshop, December 3–4, 2003. Radiat Res 2004;162(6):711-28.
[Crossref] [Google Scholar] [PubMed]
- Bala M, Prasad J, Singh S, Tiwari S, Sawhney RC. Whole-body radioprotective effects of SBL-1: A preparation from leaves of Hippophae rhamnoides. J Herbs Spices Med Plants 2009;15(2):203-15.
- Bala M, Saini M. Validated HPTLC methods for quantification of marker compounds in aqueous extract of Hippophae rhamnoides leaves. Int J Pharm Sci Rev Res 2013;23(2):58-63.
- Murase T, Kume N, Hase T, Shibuya Y, Nishizawa Y, Tokimitsu I, et al. Gallates inhibit cytokine-induced nuclear translocation of NF-κB and expression of leukocyte adhesion molecules in vascular endothelial cells. Arterioscler Thromb Vasc Biol 1999;19(6):1412-20.
[Crossref] [Google Scholar] [PubMed]
- Cueva C, Mingo S, Muñoz‐González I, Bustos I, Requena T, Del Campo R, et al. Antibacterial activity of wine phenolic compounds and oenological extracts against potential respiratory pathogens. Lett Appl Microbiol 2012;54(6):557-63.
[Crossref] [Google Scholar] [PubMed]
- Kim WH, Song HO, Choi HJ, Bang HI, Choi DY, Park H. Ethyl gallate induces apoptosis of HL-60 cells by promoting the expression of caspases-8,-9,-3, apoptosis-inducing factor and endonuclease G. Int J Mol Sci 2012;13(9):11912-22.
[Crossref] [Google Scholar] [PubMed]
- Chang CL, Lin CS. Phytochemical composition, antioxidant activity, and neuroprotective effect of Terminalia chebula Retzius extracts. Evid Based Complement Alternat Med 2012;2012:125247.
[Crossref] [Google Scholar] [PubMed]
- Pandey PK, Ahmed B, Khan HA, Bala M, Prasad J. In silico molecular docking and comparative in vitro analysis of ethyl 3, 4, 5-trihydroxybenzoate and its derivative isolated from Hippophae rhamnoides leaves as free radical scavenger and anti-inflammatory compound. Pharmacogn Magaz 2019;15(2):S313-9.
- Pandey PK, Ahmed B, Prasad J, Bala M, Khan HA. Radiomodifying action, Pharmacokinetic and Biodistribution of Ethyl 3, 4, 5-trihydroxybenzoate-Implication in development of radiomitigator. Sci Rep 2019;9(1):1-0.
- Schrödinger LL. Prime version 2.2, Lig prep version 2.7, Glide version 6.0. New York; 2014.
- Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 2004;47(7):1739-49.
[Crossref] [Google Scholar] [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
[Crossref] [Google Scholar] [PubMed]
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 984;21(2):130-2.
[Google Scholar] [PubMed]
- Worthington DJ, Rosemeyer MA. Glutathione reductase from human erythrocytes: Catalytic properties and aggregation. Eur J Biochem 1976;67(1):231-8.
[Crossref] [Google Scholar] [PubMed]