- *Corresponding Author:
- M. Gandhimathi
Department of Pharmaceutical Analysis, Sri Ramakrishna College of Pharmacy, 395, Sarojini Naidu Road, Coimbatore-641 044, India
E-mail: gands72@yahoo.co.in
| Date of Submission | 28 October 2005 |
| Date of Revision | 25 May 2006 |
| Date of Acceptance | 10 February 2007 |
| Indian J Pharm Sci, 2007, 69 (1): 123-125 |
Abstract
A reverse phase high performance liquid chromatographic method to determine aspirin and clopidogrel in combined dosage form is proposed. The chromatographic resolution of aspirin and clopidogrel was obtained in a mobile phase consisting of 0.1% v/v triethylamine (pH 4.0):acetonitrile in the ratio 25:75% (v/v) in an isocratic elution. A detection wavelength of 225 nm and flow rate of 1 ml/min was used in the study. Nimesulide (20 µg/ml) was used as an internal standard. The system suitability procedures as precision, accuracy, LOD, LOQ, number of theoretical plates and tailing factor were studied.
Aspirin [1] is a non-steroidal antiinflammatory drug that exhibits antiinflammatory, analgesic and antipyretic activities. Clopidogrel1 is a platelet aggregation inhibitor and act as an anticoagulant. Tablets containing 75 mg of each of aspirin and clopidogrel are available in market. Reported methods for the determination of aspirin and clopidogrel are spectrofluorimetry [2], RPHPLC [3-7], HPTLC [8] and GC [9]. There is no method reported for the simultaneous estimation of aspirin and clopidogrel in tablets. This paper aims to report a simple, accurate and precise simultaneous RPHPLC method for the determination of aspirin and clopidogrel in tablets.
The HPLC system was Shimadzu, including pump LC-10AT VP, photodiode array detector and Phenomenax, Gemini C18 column (150×4.60 mm, 5 μ) was used as the stationary phase. The pH meter used was Elico Pvt. Ltd., India. Aspirin and clopidogrel were received as gift samples from Micro Pharma, Chennai, India. Triethylamine AR grade, orthophosphoric acid AR grade, acetonitrile for HPLC and water for HPLC were purchased from S. D. Fine Chemicals Ltd, India and Qualigens Fine Chemicals, India.
The chromatographic conditions were optimized to achieve reliable and reproducible resolution (Table 1). A stock solution of aspirin and clopidogrel was prepared by dissolving 10 mg of aspirin and 10 mg of clopidogrel in methanol. A solution of nimesulide (1 mg/ml) was prepared and used as internal standard. A working standard solution containing 1, 2, 3, 4, 5 μg/ml each of aspirin and clopidogrel were prepared from above stock solution. Each of this solution contained 20 μg/ml of nimesulide as internal standard.
| Parameters | Optimized condition |
|---|---|
| Chromatograph | Shimadzu, pump LC-10AT, photodiode array detector |
| Mobile phase | 0.1% (v/v) triethylamine in water: acetonitrile in the ratio 25:75% v/v. (pH adjusted to 4 using dilute ortho phosphoric acid) |
| Column | Phenomenex, Gemini C 18 column (150 x 4.60 mm, 5 μ) |
| Flow rate | 1 ml/min |
| Detection | UV, 225 nm |
| Injection volume | 20 μl |
| Temperature | Room temperature |
| Retention time of aspirin | 2.0 min |
| Retention time of clopidogre | 2.7 min |
| Retention time of nimesulide (Internal Standard) | 5.9 min |
| Run time | 7 min |
Table 1: Optimized Chromatographic Conditions
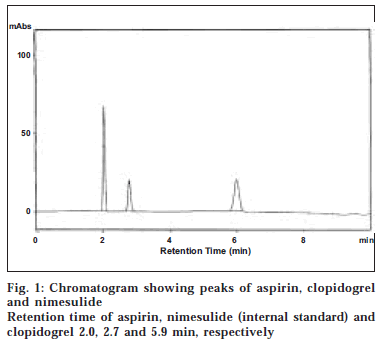
Twenty tablets each containing 75 mg of aspirin and 75 mg of clopidogrel (Clopid-AS, Genx Pharma) were weighed and powdered. A quantity of powder equivalent to 10 mg each of aspirin and clopidogrel was transferred in to a 50 ml standard flask and dissolved in methanol. The volume was made up to 50 ml with methanol. One millilitre of this solution was pipetted in to a 50 ml volumetric flask containing 1 ml of nimesulide (1000 μg/ ml). The solution was filtered using 0.2 μ membrane filter and the filtrate is referred to as formulation solution. Twenty microliters of the standard and formulation solutions were injected onto the column and the chromatogram was recorded (fig. 1). The retention times of aspirin, nimesulide and clopidogrel were found to be 2.0, 2.7, 5.9 min, respectively.
A calibration graph was plotted using peak area ratio of the aspirin and clopidogrel to that of internal standard vs. concentration of the standard solutions. The calibration graph was found to be linear in the range of 1 to 5 μg/ ml for both aspirin and clopidogrel. The slope, intercept and correlation values are shown in Table 2.
| Parameters | Aspirin | Clopidogrel |
|---|---|---|
| Concentration range (μg/ml) | 1-5 | 1-5 |
| Slope | 0.4285 | 0.3620 |
| Intercept | 0.2462 | 0.0741 |
| Correlation coefficient | 0.9915 | 0.9865 |
| LOD (μg/ml) | 0.02 | 0.03 |
| LOQ (μg/ml) | 0.9 | 1.0 |
| Theoretical plates | 43220 | 83200 |
| Resolution | - | >2 |
| Tailing Factor | 1.05 | 1.09 |
| Assay Precision (% RSD) | 1.22 | 0.95 |
Table 2: Results of linearity and system suitability.
The system suitability procedures as precision, accuracy, LOD, LOQ, number of theoretical plates and tailing factor were determined (Table 2). Precision at the method was studied by repeatedly injecting the mixture of aspirin and clopidogrel. To study the reliability and accuracy of the method recovery experiments were carried out at 100% level. The %RSD of the recovery studies is 1.03 and 1.17 for aspirin and clopidogrel, respectively. No significant peak was observed from the tablet excipients.
The validation study shows that the developed method is accurate and robust. Further this method eliminates complicated extraction of individual drugs for quantification. Both the drugs were estimated within 6 min. Hence the present method is cost effective and faster analytical method. The application of this method to tablets showed a high percentage of recovery and precision (Table 3). The developed method is simple, sensitive and quick and can be applied to simultaneous determination of aspirin and clopidogrel in tablets.
| Drug | Labeled amount mg/tablet | Amount found mg/tablet | Percentage recovery |
|---|---|---|---|
| Aspirin | 75 | 76.95 ± 0.15 | 101.62 |
| Clopidogrel | 75 | 77.41 ± 0.52 | 100.34 |
Table 3: Results of aspirin and clodetermination in tablets
Acknowledgements
The authors thank Micro Pharma, Ltd., Chennai, India for providing gift samples of aspirin and clopidogrel. The authors also sincerely thank M/s. SNR Sons, Charitable Trust for providing facilities to carry out the research work.
References
- Budavari, S., Eds., In; The Merck Index, 13th Edn., Merck & Co., Inc., Whitehouse Station, NJ, 2001, 856.
- Umapathi, P., Parimoo, S.K., Thomas, V. and Agarwal, J., J. Pharm. Biomed. Anal ., 5, 1997, 1703.
- Shanawany, A., El Sadek, M., Khievier, A.A and Rucker, G., Indian J. Pharm. Sci ., 53, 1991, 209.
- Ivanovic, D., Khecht, H.C., Nivand, C.E. and Guernet, N., Anal. Lett.25, 1992, 1693.
- Omite, C.I., Tebbett, I.R, and Danesh, D., J. Chromatogr ., 22, 1986, 187.
- Sane, R.T., Ghade, J.K., Jani, A.B., Vaidya, A.J. and Kotwal, S.K., Indian Drugs , 29, 1992, 240.
- Gandhimathi, M., Ravi, T.K. and Thomas R., J. Pharm. Biomed. Anal ., 32, 2003, 1145.
- Sharma, J.B. and Chantel, R., J. Planar Chromatog. , 5, 1992, 197.
- Kamble, N.S. and Venkatachalam, A., Indian J. Pharm. Sci ., 67, 2005, 128.