- *Corresponding Author:
- Ravi T.K
Department of Pharmaceutical Analysis, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore - 641 044, India
E-mail: tkravi2004@yahoo.co.in
| Date of Received : | 5 May 2006 |
| Date of Revised : | 29 March 2007 |
| Date of Accepted : | 6 October 2007 |
| Indian J. Pharm. Sci., 2007, 69 (5): 684-686 |
Abstract
A simple, fast, precise and accurate high performance thin layer chromatographic method has been developed for the simultaneous estimation of lansoprazole and domperidone in tablet formulations. This method allows the determination of 100-500 ng/spot of lansoprazole and 100-500 ng/spot of domperidone. The mobile phase composition was n-butanol:glacial acetic acid:water (9.3:0.25:0.5, v/v/v). Densitometric analysis of lansoprazole and domperidone was carried out in the absorbance mode at 288 nm. The R f values of lansoprazole and domperidone were found to be 0.78 and 0.21 respectively. The limit of detection for lansoprazole and domperidone were found to be 10 and 30 ng/spot, respectively. The limit of quantification for lansoprazole and domperidone were found to be 40 and 65 ng/spot, respectively. The amounts of drug present in the tablet and recovery studies were also carried out. The method was validated for precision, accuracy and reproducibility.
Keywords
Lansoprazole, domperidone, high performance thin layer chromatography
Lansoprazole [1] (L), 2-({3-methyl-4-(2,2,2- trifluoroethoxy)-2-pyridyl)methyl} sulfinyl benzimidazole, is used as a gastric proton pump inhibitor. Domperidone1 (D), 5-chloro-1- {1-[3-(2-oxobenzimidazolin-1-yl)propyl]-4- piperidyl}benzimidazolin-2-one, is a dopamine antagonist and is used as antiemetic and for the treatment of nausea. A combination of these drugs, L (30 mg) and D (30 mg) is available commercially as Lans-Dx. Methods have been reported for the determination of L and D, individually [2-10]. However, no high performance thin layer chromatographic (HPTLC) method is reported for the simultaneous determination of these drugs. The present work describes a simple, precise and accurate HPTLC method for simultaneous estimation of L and D in combined dosage forms.
Drugs, L and D were obtained as gift samples from Natco Pharmaceuticals Ltd., Hyderabad, India. All chemicals and reagents used were of analytical grade and purchased from S. D. Fine Chemicals, Mumbai. Instrument used for the analysis was a Camag HPTLC system (with TLC scanner 3, Win CATS Software and Linomat 5 as application device). The samples were spotted in the form of bands of width 6 mm with a Hamilton syringe on precoated silica gel aluminium plate 60 F254 (Machery-Nagel, Germany). The mobile phase consisted of n- butanol:glacial acetic acid:water (9.3: 0.25: 0.5, v/v/v). Linear ascending development of chromatogram was carried out in a Camag twin trough glass chamber saturated with the mobile phase. The chamber saturation time for mobile phase was optimized at 25 min. The length of chromatogram run was 85 mm. Subsequent to development, the TLC plates were dried in a current of air. Densitometric scanning was performed using Camag TLC Scanner 3 in the absorbance mode at 288 nm. The source of radiation utilized was deuterium lamp.
Standard stock solution containing 0.1 mg/ml of L and 0.1 mg/ml of D were prepared by dissolving L and D in methanol. With the fixed chromatographic conditions 1, 2, 3, 4 and 5 µl of standard solution were applied on plate. The plate was developed and scanned as mentioned above. Calibration curves for L and D were generated by plotting peak areas of drugs versus concentration of drugs spotted.
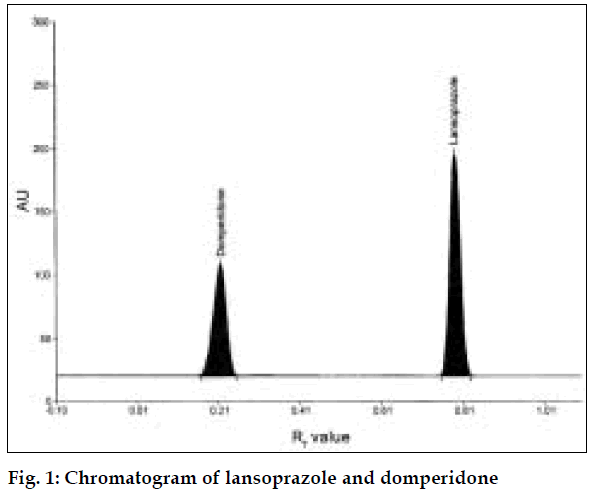
Twenty tablets, each containing quantity equivalent to 10 mg of L and 10 mg of D were weighed; powdered and average weight was calculated. Quantities equivalent to 10 mg of L and 10 mg of D were weighed accurately, transferred to a 100 ml volumetric flask. The drugs were extracted with the addition of little quantities (20 ml) of methanol and volume was made upto 100 ml. This solution was filtered from which suitable aliquots were applied. The plate was developed and scanned as mentioned above (fig. 1). Peak areas were recorded and the amount of L and D present in formulations were estimated using the calibration curve for L and D. Results of analysis of formulation are tabulated in Table 1.
| Drug | Labeled amount (mg/tablet) | Amount found (mg/tablet) | % Label claim | % RSDa |
|---|---|---|---|---|
| Lansoprazole | 30 | 29.14 | 97.13 | 0.1670 |
| Domperidone | 30 | 29.46 | 98.20 | 0.1380 |
aMean and RSD of six observations. Tablets were procured from local market
Table 1: Estimation of Lansoprazole and Domperidone from Formulation.
The developed method was validated for specificity precision and accuracy. The method was found to be specific, since it resolved the peak of L (Rf value= 0.78) and D (Rf value= 0.21) in presence of excipients in the formulations. The linear regression data showed good linear relationship over a concentration range of 100-500 ng/spot for L (r= 0.9990) and 100-500 ng/spot for D (r= 0. 9983). The regression equation and validation parameters are given in Table 2. Precision studies were carried out and the parameters studied were intra-day precision, inter-day precision, repeatability of measurement and repeatability of sample application. Low % RSD. values indicate that the developed method has good precision (Table 2). Stability studies were carried out for the plate and the developed plate was found to be stable for about 2 h. Accuracy of the method was evaluated by carrying out the recovery studies. Recovery studies were carried out at 50 and 100% levels. Good recovery values indicate that the method is free from interference and excipients present in formulation (Table 2).
| Parameter | Results | |
|---|---|---|
| Lansoprazole | Domperidone | |
| LOD (ng/spot) | 10 | 30 |
| LOQ (ng/spot) | 40 | 65 |
| Linearity range (ng/spot) | 100-500 | 100-500 |
| Regression equation (Y= a + bc) | ||
| *Slope (b) | 16.221 | 10.073 |
| *Intercept (a) | 690.070 | 365.916 |
| *Correlation Coefficient (r) | 0.9990 | 0.9983 |
| Recovery studiesc | ||
| *50% level | 98.47 | 98.21 |
| *100% level | 101.35 | 98.82 |
| Precision (% RSD) | ||
| *Intra-day (n=3) | 0.3489 | 0.2328 |
| *Inter-day (n=3) | 0.6795 | 0.5170 |
| *Repeatability of sample application(n=6) | 0.4640 | 0.5434 |
| *Repeatability of measurement (n=6) | 0.2603 | 0.2122 |
cMean of five replicate samples
Table 2: Method Validation Parameters.
The developed HPTLC technique is precise, specific and accurate. There was no interference from the excipients used in the tablet formulation and hence this method can be used for routine analysis of L and D in combined dosage form. It may also be extended for simultaneous analysis of L and D in plasma and other biological fluids.
Acknowledgements
The authors acknowledge M/s SNR and Sons Charitable Trust, Coimbatore, India for providing the facilities to carry the experiment, Natco Pharmaceuticals Ltd., Hyderabad, for supplying pure sample of L and D and Tamil Nadu Pharmaceutical Sciences Welfare Trust, Chennai for awarding Scholarship for the work.
References
- Parfitt K, editor. Martindale, the complete drug reference. Pharmaceutical Press: London; 1999.
- Gerloff J, Mignot A, Barth H, Heintze K. Pharmacokinetics and absolute bioavailability of lansoprazole. Eur J ClinPharmacol 1996;50:293-7.
- Masatomo M, Tada H, Suzuki T. Simultaneous determination of lansoprazole enantiomers and their metabolites in plasma by liquid chromatography with solid-phase extraction. J Chromatogr B AnalytTechnol Biomed Life Sci 2004;804:389-95.
- Pandya KK, Mody VD, Satia MC, Modi RI, Chakravarthy BK, Gandhi TP. High performance thin-layer chromatographic method for the detection and determination of lansoprazole in human plasma and its use in pharmacokinetic studies. J Chromatogr B Biomed SciAppl 1997;693:199-204.
- Wu MS, Gao L, Cai XH, Wang GJ. Determination of domperidone in human plasma by LC-MS and its pharmacokinetics in healthy Chinese volunteers. ActaPharmacol Sin 2002;23:285-8.
- Zavitsanos AP, MacDonald C, Bassoo E, Gopaul D. Determination of domperidone in human serum and human breast milk by high performance liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed SciAppl 1999;730:9-24.
- Trivedi C, Soni K, Khan IJ, Loya P, Manglani U, Saraf MN. High-performance liquid chromatographic analysis with ultra violet detection for the determination of domperidone in human plasma. Indian Drugs 2005;42:461-4.
- Vinodhini C, Vaidyalingam V, Ajithadas A, Niraimathi V, Shantha A. Simultaneous estimation of cinnarizine and domperidone in solid dosage form by high performance liquid chromatographic method. Indian Drugs 2005;42:516-8.
- Vinodhini C, Vaidyalingam V, Kalidoss AS. Simultaneous estimation of cinnarizine and domperidone by high performance thin layer chromatographic method in tablets. Indian Drugs 2005;42:600-3.
- Kobylinska M, Kobylinska K. High-performance liquid chromatographic analysis for the determination of domperidone in human plasma. J Chromatogr B Biomed SciAppl 2000;744:207-12.