- *Corresponding Author:
- N. K. Rangra
Department of Pharmaceutical Sciences and Technology, Birla Institute of Technology, Mesra, Ranchi-835 215, India
E-mail: nareshrangra@gmail.com
| Date of Submission | 08 August 2016 |
| Date of Revision | 08 March 2017 |
| Date of Acceptance | 05 September 2017 |
| Indian J Pharm Sci 2017;79(6): 885-892 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
A very simplistic, selective, sensitive, and reproducible procedure based on a reversed-phase high-performance liquid chromatography was used and developed for the determination of praziquantel in rat plasma. For the separation of praziquantel from the internal standard (diazepam) on a C18 column, enable (250×4.6 mm, 5 µm particle size), with the retention time of 6.4, 8.5 min, respectively. For praziquantel with UV detector at 225 nm. The mobile phase was a mixture of acetonitrile:water in a ratio of (60:40 v/v), running through the column at the flow rate of 1 ml/min. Sample preparation of 200 µl of plasma was done by a protein precipitation by using perchloric acid. Calibration curve was found to be linear with correlation coefficients (r2) is 0.9989 prepared in plasma at the concentrations of 5 to 1000 ng/ml. The precision of the above method was based on interday, and intraday repeatability, and reproducibility (day-to-day variation) were found to be within the limit of 15%. Limit of quantification was accepted and found to be 5 ng/ml using 200 µl samples. The method appears to be robust and has been applied to a pharmacokinetic study of praziquantel in three groups of rats with a single oral dose of 40 mg/kg body weight.
Keywords
Praziquantel, reversed-phase high-performance liquid chromatography, limit of quantification, rat plasma
Praziquantel (PRQ) is a derivative of pyrazinoiso quinoline with IUPAC name [2-(cyclohexylcarbonyl)- 1,2,3,6,7,11b-hexahydro-4H-pyrazino [2,1- a]isoquinoline-4-one]. Due to its high efficacy and low toxicity, it is widely used for the treatment of many diseases, such as human trematode, cestode infections, schistosomiasis, and many other infections pathogenic conditions to human [1]. From the very beginning different analytical methods were developed by different researchers and reported its level in animals and humans from a biological fluids (plasma, serum), tissue organ extracts. Some of the analytical methods, which include radiometric assay [2], fluorometric assay [3], enzyme-linked immunosorbent assay (ELISA) [4], thinlayer chromatography (TLC), gas chromatography (GC) [5,6], high-pressure liquid chromatography (HPLC) [7,8-14].
Literature demonstrates that a number of researchers have reported the sample preparation procedures and methods but due to its lengthy time-consuming single step or three-step liquid-liquid extraction [15] process, it is not reproducible technically.
To avoid this drawbacks and making this process reproducible to produce clean samples clear chromatograms this method was developed for the estimation of PRQ in rat plasma using diazepam as an internal standard.
This method is simple, sensitive and a selective for determination of PRQ in plasma. In this research work, we reported a very simple and single step sample preparation method based on the protein precipitation by using perchloric acid as a protein precipitating agent. The protein precipitation method was very efficient in giving a clear chromatogram that does not require solvent evaporation by a gentle stream of nitrogen or oxygen. Moreover, this method uses less plasma in this study.
Materials and Methods
PRQ was obtained from Micro Labs Ltd., Goa, India. Diazepam was received from Hetero Drugs Ltd. Hyderabad India as a gift samples. Acetonitrile and methanol were purchased from S. D. Fine-Chem Limited, Mumbai, India. Water was used to HPLC grade. Perchloric acid of an analytical grade was purchased from Merck Specialties Private Limited, Worli, Mumbai, India.
Preparation of standard stock solutions
A stock solution of 1 mg/ml PRQ and diazepam were prepared in methanol. The standard solution of PRQ and diazepam were prepared by mixing and diluting it with an exact amount of an individual stock solution of methanol. The final concentration of the standard solution were prepared in the concentration range of 10 000, 7500, 5000, 2500, 1000, 750, 500 and 50 ng/ml. And the fixed concentration of the internal slandered (IS) was taken as 5000 ng/ml. Different concentration of 10 000, 7500, 5000 50 ng/ml were also developed for its precision and accuracy by the same method with a fixed concentration of the IS (5000 ng/ml). The stock solutions were refrigerated when not in use replaced on a weekly basis. The fresh standard solution was prepared for each day for analysis and validation.
Chromatography
Reversed-phase high-performance liquid chromatography (RP-HPLC, Shimadzu, Kyoto, Japan) system composed of a pump LC-20AT Prominence solvent delivery module, having manual rheodyne injector with a 20-μl fixed loop having a SPD-20A Prominence UV/Vis detector set at 225 nm. Analyte and IS were separated by using an Enable C18 column (column length: 250×4.6 mm i.d.; 5 μm; particle size) at an ambient temperature. The data acquisition was made with Spinchrom Chromatographic Station® CFR Version 2.4.195 (Spinchrom Pvt. Ltd., Chennai, India). The mobile phase consists of acetonitrile:water in a ratio of 60:40 for plasma sample at a flow rate of 1.0 ml/min.
Sample collection
The study protocol was approved by the Institutional Animal Ethics Committee of Sigma Institute of Pharmacy, Vadodara. Healthy albino Wister rats of weight 150-250 g was used to carry out the procedure. The animals were allowed to acclimatize to approved environmental conditions. About 2 ml of blood sample was collected from the vein into a heparinized-coated micro-centrifuge tube from retro-orbital plexus of albino rats. It was centrifuged at 15 000 rpm for 15 min, and the plasma was collected. Plasma was stored at –20°. The blood sample was collected from the rats on a regular time-interval. To avoid unbiased in analysis technique, an experiment was continued till separation completed.
Calibration curves
A blood sample of the different group of animals was collected and plasma was separated. Absence drug contents collected plasma was used as a blank. The calibration curve was prepared by spiking 200 μl of plasma with 20 μl of each PRQ and diazepam as an internal standard solution was vortexed for a few minutes. The calibration curves for plasma were in the range of 5-1000 ng/ml at fixed concentration of the IS (500 ng/ml). After plasma was spiked and it was subjected to further sample preparation before analysis.
Sample preparation
The plasma samples were treated with the protein precipitating agent for the analysis of PRQ. To each 200 μl spiked plasma with PRQ (different concentration) and diazepam (fixed concentration) were taken in 1.5 ml micro-centrifuge tubes. Simultaneously a blank plasma (200 μl) and diazepam were also taken in a 1.5 ml micro-centrifuge tube. Different protein precipitating agents were used such as ammonia sulphate, 15% trichloro acetic acid, 10% sodium tungstate in water, 5-sulphosalicylic acid, zinc sulphate in methanol, different ratio of organic solvents (acetone, acetonitrile, methanol) and the different percentages of perchloric acid in water. Lastly, it was found that 8.25% of perchloric acid gave a clear chromatogram without any interference with PRQ and diazepam. Perchloric acid was used as a protein precipitating agent to precipitate proteins to carried out this study. To each 200 μl of the plasma spiked sample was mixed with 45 μl of 8.25% perchloric acid for 30 s. The samples were centrifuged at 12 000 rpm for 15 min. This gave a clear supernatant liquid at the top in comparison to above-mentioned protein precipitating agents. The 20 μl of clear supernatant liquid was transferred in Hamilton syringe injected through the Rheodyne injector to HPLC column for analysis.
Validation of the developed method
The proposed method was validated as per the Center for Drug Evaluation and Research (CDER) guidelines. The developed method was validated by estimating the different parameters such as linearity, precision, accuracy, recovery, quantification and limit stability. Coefficients of variation and relative errors should be less than 15%. This limit was acceptable for the quantification method. These values were established at 20%, as recommended in the literature [16-18].
Linearity
The linearity was examined in the concentration range of 1000, 750, 500, 250, 100, 75, 50 and 5 ng/ ml for PRQ at a fixed concentration of IS 500 ng/ ml. The calibration curve plotted and evaluated by its coefficient of determination (r2).
Linearity can be defined as an analytical method with a definite range and the result obtained is directly proportional to the concentration. That data were obtained from the analytes present in the sample. The calibration curve was plotted from the peak area ratio of PRQ to IS versus concentration of PRQ. In the X-axis concentration of PRQ was taken and on the Y-axis as the peak area ratio of PRQ to IS. The replicate analysis was done by taking all eight different concentration levels (therefore n=8) the linear relationship was obtained by using the method of least square. The coefficient of determination (r2) for PRQ in plasma was found to be 0.999 as shown in Figure 1A.
Accuracy
Accuracy was determined by replication analysis. Five sets of plasma samples were spiked with four different concentration levels of PRQ (5, 500, 750 1000 ng/ml) with a fixed concentration of diazepam (500 ng/ml). Finally, the comparison was made a difference between the plasma spiked value (theoretical value) to that of actually found value.
Precision
The precision of the method was based on with-inday repeatability and day-to-day reproducibility. It was determined by the replicate analysis. Five sets of plasma spiked with four different concentration level of PRQ (5, 500, 750 and 1000 ng/ml) with a fixed concentration of diazepam (500 ng/ml). The day-today variation reproducibility of this above method was validated using the same concentration range of plasma as described above. A single determination of each concentration was made in three different days. Relative standard deviation (RSD) was calculated from the ratio of standard deviation (SD) to the mean expressed in percentage.
Recovery
For an analytical recovery of sample preparation procedure was to estimate the peak height obtained from the sample (plasma). Here sample is PRQ and diazepam was taken as an internal standard. Samples were first prepared in methanol solution, and it was diluted by an equivalent amount of PRQ and IS in plasma. The analysis was carried out in triplicate at the concentration range of 5 to 1000 ng/ml for PRQ at a fixed concentration of 500 ng/ml for IS.
Selectivity
Selectivity is a method to verify by checking for the interferences of various drugs and their metabolites in the same categories.
As PRQ is an antihelminthic drug, therefore we have verified checked with different antihelminthic drugs such as albendazole, albendazole sulphoxide (active metabolite of albendazole), including the commonly used antibiotics such as ampicillin, penicillin after subjecting them to sample preparation because PRQ is given in filariasis geohelminths in combination with albendazole-ivermectin geohelminths.
Limit of quantification (LOQ)
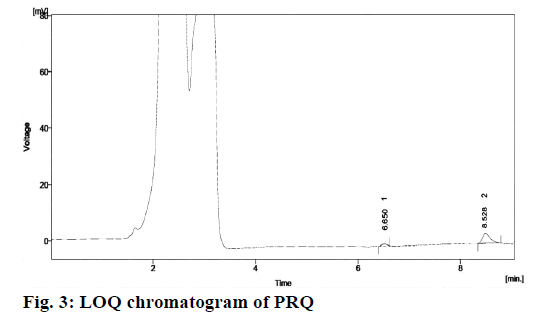
LOQ is an assay procedure to estimate the lowest concentration of PRQ (spiked plasma sample), which will give raise a peak height thrice the baseline noise at a sensitivity of 0.005 AUFS (absorbance unit full scale) in a 200 μl of the sample.
Stability
PRQ stability was estimated from the spiked plasma samples having a concentration of 5, 1000, 5000 ng/ml with a fixed concentration of diazepam (500 ng/ml). It was stored, and analysis carried for three times for each concentration i.e. triplicate analysis. Spiked plasma samples of different concentration were stored at –20° freezer (Sanyo, Japan) for 6 mo. Periodically the three concentrations were measured for 1, 2, 4 and 6 mo, consiquitevely. The technique for freeze-thaw stability was performed for all the three different concentration of the plasma samples were frozen at –20° for 24 h at room temperature (25°). When thawed completed, the spiked plasma samples were transferred back to the original freezer again refrozen for 24 h. The above process was repeated for three cycles.
Quality control (QC)
QC samples for PRQ were made up in plasma by diluting the stock solution. It was separated previously during the preparation of the calibration curve, at the concentrations range of 5, 500, 1000, 5000 ng/ml along with a fixed concentration of diazepam (500 ng/ml). Samples were stored frozen at a temperature of –20° before the use of each analytical run. Depending upon the results of the QC samples provided gives an indication towards the accepting or rejecting the run. At least two of the four QC samples should be within ±20% of their respective nominal value. Other than that two of the four QC samples may be outside the ±20% of their respective nominal value, but it should not be at the same concentration.
Robustness
Robustness of PRQ by the proposed method as carried out by the slight variation in flow rate, pH, and mobile phase ratio. The percentage recovery and RSD were noted for PRQ.
Application of the method to biological samples
This method was then applied for the pharmacokinetic study of PRQ in twelve healthy albino rats (weighing 150-250 g) followed by a single oral dose of 40 mg/kg body weight. Blood samples (1.5 ml) from a vein were collected into heparinized-coated micro centrifuge plastic tubes (2 ml) from retro orbital plexus of albino rats at different time intervals: 0, 0.5, 1, 1.5, 2, 4, 6, 18, 23, 27, 30 and 48 h of dosing.
Blood was collected from the retro-orbital plexus immediately and it was centrifuged at 15 000 rpm for 15 min. The plasma was collected carefully. The supernatant plasma layer was separated and stored at –20° until analysed. The plasma samples were analysed for PRQ concentration in rat plasma at different time intervals. Sample preparation was carried out by using 8.25% of perchloric acid to precipitate plasma protein. Graph was plotted between time versus observed plasma concentration, which gave area under curve (AUC) calculated by using the linear trapezoidal rule. The maximum observed PRQ concentration (Cmax), the time at which Cmax was observed (Tmax) were reported directly from graph.
Results and Discussion
A high-performance liquid chromatography (HPLC) was used to investigate and optimize the separation of PRQ from IS. The retention time of PRQ was 6.4 min using a stationary phase (Enable C18 reversed-phase column). The UV detection was set at 225 nm. And the mobile phase using acetonitrile and distilled water in the ratio of 60:40 (v/v) was chosen as an appropriate elution solvent as it resulted in effective separation of PRQ and diazepam (IS). The retention time was 6.4 and 8.5 min, respectively. All the chromatograms showed a good baseline with effective separation. Chromatogram of PRQ (1000 ng/ml) and diazepam (500 ng/ml, IS) was shown in (Figure 1B).
Sample preparation process used in this above study involved protein precipitation of the sample, i.e., perchloric acid (8.25%) of 45 μl was used to precipitate the proteins from plasma. This process of precipitating the protein was found to be the most optimal conditions because less time-consuming at the same time it is economical for sample preparation, which resulted in a clean chromatogram.
The spiked plasma samples were analysed and calibrated for a concentration range of 5-1000 ng/ ml for PRQ with a fixed concentration of diazepam (500 ng/ml). The calibration curve gave a linear relationship between correlation coefficients (r2) of 0.9989 with Eqn., Y=0.003x+0.633 where the slope was 0.003 and intercept was 0.633 for PRQ.
The linearity was tested in the concentration range of 1000, 750, 500, 250, 100, 75, 50 and 5 ng/ml for PRQ with a fixed concentration of diazepam (500 ng/ml). The calibration curve was plotted and evaluated by its correlation coefficient (r2). The coefficient of correlation was found to be 0.998. Results suggested a strong linear relationship between the variables, which is summarized in Table 1.
| Parameters | Value |
|---|---|
| Absorption maxima, λmax (nm) | 217 nm |
| Linearity range (ngml-1) | 5-1000 |
| Coefficient of determination (r2) | 0.999 |
| Correlation coefficient (r) | 0.9994 |
| Regression equation (Ya) | Y=0.003x+0.633 |
| Slope (b) | 0.003 |
| Intercept (a) | 0.633 |
| LOD (ngml-1) | 1.515 |
| LOQ (ngml-1) | 5 |
LOD: Limit of detection; LOQ: limit of quantitation
Table 1: Spectral and statistical data for determination of PRQ by proposed HPLC method
Inter-day and intra-day precision were measured regarding %RSD. The %RSD of PRQ was found to be 11.76%, which was well inside the limit according to guideline given by CDER for bio-analytical method validation. The result of the precision showed that the above-proposed method is highly precise. Little variation of PRQ assays was found to be below 15% (according to CDER guidelines). The intra-day, interday assay for PRQ was within the concentration range 5-1000 ng/ml (5, 500, 750 and 1000 ng/ml). The results were summarized in Table 2.
| TC | Day 1 | Day 2 | Day 3 | Intra-day | |||||
|---|---|---|---|---|---|---|---|---|---|
| Plasma | ng/ml | EC | %RSD | EC | %RSD | EC | %RSD | EC | %RSD |
| 1 | 1000 | 1028.81 | 3.83 | 991.91 | 3.00 | 1022.02 | 1.10 | 1021.48 | 1.13 |
| 2 | 750 | 757.19 | 0.53 | 747.80 | 0.43 | 768.45 | 0.87 | 760.31 | 0.91 |
| 3 | 500 | 510.81 | 2.22 | 516.4 | 6.15 | 524.55 | 3.65 | 525.63 | 3.02 |
| 4 | 5 | 5.11 | 6.88 | 4.80 | 9.14 | 5.12 | 11.76 | 5.15 | 3.65 |
TC denotes theoretical concentration and EC denotes experimental concentration
Table 2: Inter-day (n=5) and intra-day (n=5) precision (%RSD) measured for QC points for PRQ in plasma
Accuracy study was performed according to the CDER guidelines for PRQ, which was found to be 100.97 to 109.40%. The results were summarized in Table 3. The results clearly indicate that there was no interference of endogenous plasma components. Inter-day as well as intra-day concentration range was within 5-1000 ng/ml along with a fixed concentration of diazepam 500 ng/ml.
| Nominal concentration (ng/ml) |
Mean concentration founda (ng/ml) |
SD | Precision (%RSD) |
Mean accuracyb (%) |
|---|---|---|---|---|
| Inter-day (n=5) | ||||
| 1000 | 1014.25 | 26.83 | 2.64 | 101.425 |
| 750 | 757.81 | 4.66 | 0.61 | 101.041 |
| 500 | 517.25 | 20.77 | 4.01 | 103.45 |
| 5 | 5.015 | 0.46 | 9.26 | 100.3 |
| Intra-day (n=5) | ||||
| 1000 | 1021.48 | 11.6 | 1.13 | 102.148 |
| 750 | 760.31 | 6.94 | 0.91 | 101.375 |
| 500 | 525.63 | 15.9 | 3.02 | 105.126 |
| 5 | 5.15 | 0.18 | 3.65 | 103 |
aAverage of three and six determinations at three concentration levels for inter-day and intra-day respectively
Table 3: Summary of inter-day (n=5) and intra-day (n=5) precision and accuracy of the method in rat plasma
Mean recoveries from the PRQ spiked plasma samples within the concentration range of 5-1000 ng/ml were found to be more than 90%. The result for the recovery of PRQ from PRQ spiked plasma sample was nearly 100%. This result clearly indicates that there was a lack of interference from the sample preparation procedure.
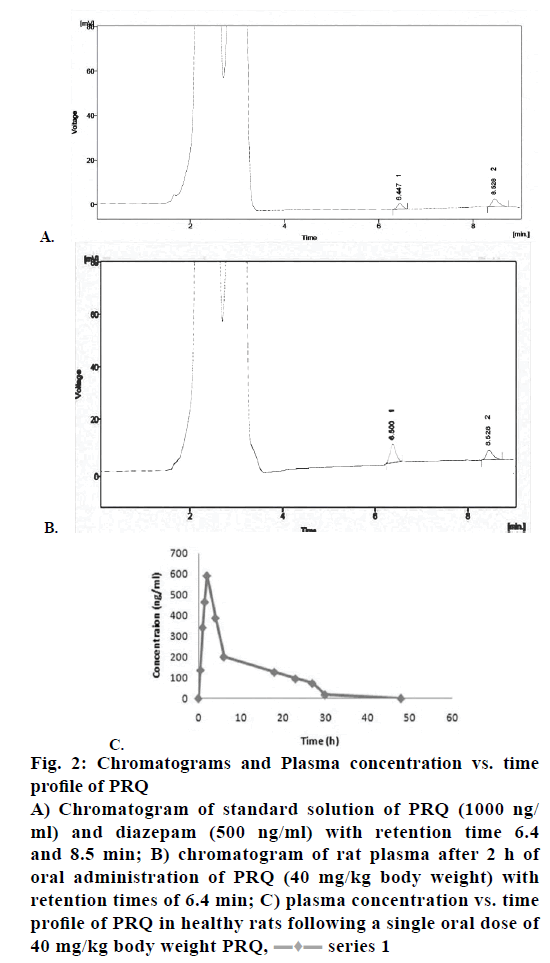
Selectivity of the chromatographic separation was done to check that weather is there any interferences of endogenous peaks in plasma, which is illustrated in the (Figure 1C and Figure 2). The chromatograms for blank plasma spiked plasma with PRQ (1000 ng/ml) and diazepam (500 ng/ml) with retention time of 6.4 and 8.5 min.
In specificity, we found that there was no interference or overlapping of peaks due to endogenous plasma components. Due to presence of plasma endogenous plasma components, which gave peaks within 2-4 min only, which is given in (Figure 2). After the 4 min, there was no significant interference of blank plasma that affected the response of PRQ and diazepam.
Figure 2: Chromatograms and Plasma concentration vs. time
profile of PRQ
A) Chromatogram of standard solution of PRQ (1000 ng/
ml) and diazepam (500 ng/ml) with retention time 6.4
and 8.5 min; B) chromatogram of rat plasma after 2 h of
oral administration of PRQ (40 mg/kg body weight) with
retention times of 6.4 min; C) plasma concentration vs. time
profile of PRQ in healthy rats following a single oral dose of
40 mg/kg body weight PRQ,  series 1
series 1
The LOQ for PRQ in rat plasma was found to be 5 ng using 200 μl plasma given in (Figure 3). PRQ spiked plasma samples having concentrations of 5, 750 and 1000 ng/ml were found to be stable enough at a temperature of –20°. Freezer for a minimum period of 6 mo and noticed no significant decomposition of the drug.
Long time storage of the PRQ spiked plasma samples for six months. This PRQ spiked plasma samples do not affect the LOQ of the analytes. Mean deviation (%) was measured for three different concentrations for (1, 2, 4 and 6 mo), which varied between 4.30 to 8.00% for PRQ as given in Table 4. Freezing thawing for PRQ spiked plasma for three successive cycles did not affect the measured concentrations. The mean deviation was found to be within 0.28 to 3.60 for PRQ as shown in Table 4, from the theoretical value.
| Time period (months) | Concentration | Concentration measured (ng/ml) | |||||
|---|---|---|---|---|---|---|---|
| a. Long term stability | Added (ng/ml) | ||||||
| Assay 1 | Assay 2 | Assay 3 | Mean | SD | %DEVa | ||
| 1 | 5 | 5.23 | 5.39 | 5.67 | 5.43 | 0.22 | 8.6 |
| 3.93 | |||||||
| 1.6 | |||||||
| 1.73 | |||||||
| 2.15 | |||||||
| 1.19 | |||||||
| 500 | 518.67 | 527.75 | 512.563 | 519.66 | 7.64 | –0.93 | |
| 1000 | 1016.35 | 1014.32 | 1017.36 | 1016.01 | 1.54 | ||
| 2 | 5 | 5.11 | 5.09 | 5.06 | 5.08 | 0.02 | –0.95 |
| 500 | 508.813 | 510.938 | 512.563 | 510.77 | 1.88 | ||
| 1000 | 1011.67 | 1014.56 | 1009.68 | 1011.97 | 2.45 | 0.77 | |
| 4 | 5 | 4.96 | 5.01 | 4.89 | 4.95 | 0.06 | |
| 500 | 496.5 | 499.813 | 489.37 | 495.22 | 5.33 | –3.86 | |
| 1000 | 1016.25 | 1005.67 | 1001.32 | 1007.74 | 7.67 | ||
| 6 | 5 | 4.87 | 4.76 | 4.79 | 4.8 | 0.05 | –4.49 |
| 500 | 488.967 | 478.258 | 465.358 | 477.52 | 11.82 | ||
| 1000 | 988.37 | 980.58 | 985.37 | 984.77 | 3.92 | –1.52 | |
| b. Freeze and thaw stability | |||||||
| 5 | 5.21 | 5.32 | 5.31 | 5.28 | 0.06 | 5.6 | |
| 500 | 500.24 | 501.37 | 504.22 | 501.94 | 2.05 | 0.38 | |
| 1000 | 1024.11 | 1019.32 | 1011.38 | 1018.27 | 6.42 | 1.82 | |
aAverage of three and six determinations at three concentration levels for inter-day and intra-day respectively, ball the mean accuracies were calculated against their nominal concentrations
Table 4: Storage stability data of PRQ in plasma at concentrations 5, 1000, and 5000 ng/ml
Validation was conducted by three analysts in the spiked plasma analysis. In each analysis were included with standard curve QC specimens. Different nominal concentration was taken 5, 500, 750 and 1000 ng/ml of PRQ spiked plasma analysed from the beginning till the end of the analytical run. All the above results were within the acceptable limit (±20% of their respective nominal values).
System suitability test is an important parameter for the chromatographic system. This system suitability test gives the data for theoretical plates, height equivalent to the theoretical plate (HETP) and resolution, which is very much important useful in the chromatographic system to pass the minimum limit [19].
A system suitability test was performed according to USP. The test was on the chromatograms obtained from standard test solutions to check above mentioned parameters. The results were obtained from the six replicate injections of standard solutions were summarized in Table 5.
| Parameters | Praziquantel |
|---|---|
| Retention time, Rt (min) | 6.4 |
| Capacity factor (k) | 3.07 |
| Separation factor (α) | 1.42 |
| Theoretical plates (USP) | 4096 |
| HETP (mm) | 0.061 |
| Resolution (Rs) | 3.48 |
Table 5: System suitability parameters
Robustness of the method was studied by deliberate variations of the analytical parameters such as variation in flow rate (1.1 ml/min and 0.9 ml/min). The results were given in Table 6. The above developed method was then applied to quantify PRQ concentration albino rats by the pharmacokinetic study, which was carried out on three groups each containing 12 Wistar albino rats. HPLC chromatogram of rat plasma after 2 h of oral drug administration (suspension) of PRQ (40 mg/kg body weight) with the retention time of 6.4 given in Figure 2. Plasma concentration versus time profiles of PRQ presented in (Figure 2). Different pharmacokinetic parameters were studied and carried out, which were summarized in Table 7. The Tmax, Cmax were calculated by a single oral dose of 40 mg/kg body weight of PRQ was conducted on three different groups.
| Flow rate (1.1 ml/min) | |||
|---|---|---|---|
| Conc. (ng/ml) | Actual Calc. found (ng/ml) | Statistical analysis | |
| 1000 | 1015.25 | 1014.607 3.125065 0.308008 |
Mean SD %RSD |
| 1000 | 1011.21 | ||
| 1000 | 1017.36 | ||
| 500 | 518.36 | 513.61 4.630065 0.901475 |
Mean SD %RSD |
| 500 | 509.11 | ||
| 500 | 513.36 | ||
| 5 | 5.07 | 4.973333 0.090738 1.824485 |
Mean SD %RSD |
| 5 | 4.96 | ||
| 5 | 4.89 | ||
| Flow rate (0.9 ml/min) | |||
| Conc. (ng/ml) | Actual Calc. found (ng/ml) | Statistical analysis | |
| 1000 | 1018.11 | 1015.437 2.621628 0.258177 |
Mean SD %RSD |
| 1000 | 1012.87 | ||
| 1000 | 1015.33 | ||
| 500 | 504.6 | 507.94 3.315283 0.652692 |
Mean SD %RSD |
| 500 | 507.99 | ||
| 500 | 511.23 | ||
| 5 | 5.01 | 5.02 0.065574 1.306263 |
Mean SD %RSD |
| 5 | 5.09 | ||
| 5 | 4.96 | ||
Table 6: Robustness data of the RP–HPLC method at different flow rates
| Pharmacokinetic parameters | Obtained value |
|---|---|
| Time required for maximum plasma concentration, Tmax (h) | 2 |
| Maximum plasma concentration, Cmax (ng/ml) | 570.4 |
| Plasma half-life, T1/2 (h) | 2.2 |
| Area under curve at 30 h, AUC(0→30) (ng h/ml) | 4007.48 |
| Area under curve at infinite time, AUC(0→∞) (ng h/ml) | 4018.94 |
| Area under momentum curve at 30 h, AUMC(0→30) (ng·h2/ml) | 29231.7 |
| Mean residence time, MRT (h) | 7.29 |
Table 7: Pharmacokinetic parameters of PRQ after a single oral dose of 40 mg/kg PRQ to three groups each containing 12 albino rats
The current method has resulted in promising accuracy, selectivity, sensitivity and less time-consuming actions for the quantitative determination of PRQ in rat plasma than previously reported methods. Moreover, the main advantage of this developed method was the sample preparation step using 8.25% of perchloric acid, which in turns resulted in a clear chromatogram. In addition to this the sample preparation is easy simple (protein precipitation) does not need any evaporation technique, which will not increase the cost of the method.
Inter-day and intra-day precision of PRQ gave a %RSD below 9.00%, which was under the range followed by guideline given by CDER [18]. The pharmacokinetic study clearly indicated that LOQ 5 ng/ml without any interference of endogenous plasma peaks, which serves as an imperative predecessor for further future research.
Acknowledgements
The authors are grateful to the Principal Dr. U. M. Upadhayay as well as Asst. Professors of Q.A. Department of Sigma Institute of Pharmacy, Vadodara, and to the management team as for the support providing necessary chemicals equipment for this research work for which we will remain ever great full to the management team of Sigma Institute of Pharmacy, Vadodara, Gujarat. Also thankful to Micro Labs Ltd., Goa, India for giving PRQ as a gratis sample and Hetero Pharma Hyderabad, India for giving Diazepam as a gratis sample.
Conflict of interest
The authors declare that there is no conflict of interests.
Financial support and sponsorship
Nil.
References
- Reynolds JEF, Parfitt K, Parsons A, Swetman S. Martindale-The Extra Pharmacopoeia. 30th ed. London: The Pharmaceutical Press;1993.
- Patzschke K, Pütter J, Wegner LA, Horster FA, Diekmann HW. Serum concentrations and renal excretion in humans after oral administration of Praziquantel-results of three determination methods. Eur J Drug Metab Pharmacokinet 1979;4:149-56.
- Pütter J, Held F. Quantitative studies on the occurence of Praziquantel in milk and plasma of lactating women. Eur J Drug Metab Pharmacokinet 1979;4(4):193-8.
- Mitsui Y, Nakasaka Y, Akamatsu M, Ueda H, Kihara M, Takahashi M, et al. Neurofibromatosis type 1 with Basilar Artery Fusiform Aneurysm Manifesting Wallenberg''s Syndrome. Intern Med 2001;40(9):948-51.
- Diekmann HW. Quantitative determination of Praziquantel in body fluids by gas liquid chromatography. Eur J Drug Metab Pharmacokinet 1979;4(3):139-41.
- Westhoff F, Blaschke G. High-performance liquid chromatographic determination of the stereoselective biotransformation of the chiral drug Praziquantel. J Chromatogr B Biomed Sci Appl 1992;578(2):265-71.
- Masimirembwa CM, Naik YS, Hasler JA. The effect of chloroquine on the pharmacokinetics and metabolism of Praziquantel in rats and in humans. Biopharm Drug Dispos 1994;15(1):33-43.
- Xiao SH, Catto BA, Webster LT. Quantitative determination of Praziquantel in serum by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 1983;275:127-32.
- Mandour ME, el Turabi H, Homeida MM, el Sadig T, Ali HM, Bennett JL, et al. Pharmacokinetics of Praziquantel in healthy volunteers and patients with schistosomiasis. Trans R Soc Trop Med Hyg 1990;84(3):389-93.
- Jung H, Sanchez M, Gonzalez-Astiazaran A, Martinez JM, Suastegui R, Gonzalez-Esquivel DF, et al. Clinical pharmacokinetics of albendazole in children with neurocysticercosis. Am J Ther 1997;4(1):23-6.
- Giorgi M, Salvatori AP, Soldani G, Giusiani M, Longo V, Gervasi PG, Mengozzi G, et al. Pharmacokinetics and microsomal oxidation of Praziquantel and its effects on the P450 system in three‐month‐old lambs infested by Fasciola hepatica. J Vet Pharmacol Ther 2001;24(4):251-9.
- Meier H, Blaschke G. Investigation of Praziquantel metabolism in isolated rat hepatocytes. J Pharm Biomed Anal 2001;26(3):409-15.
- Schepmann D, Blaschke G. Isolation and identification of 8-hydroxy Praziquantel as a metabolite of the antischistosomal drug Praziquantel. J Pharm Biomed Anal 2001;26(5):791-9.
- Ridtitid W, Wongnawa M, Mahatthanatrakul W, Punyo J, Sunbhanich M. LC determination of Praziquantel in human plasma. J Pharm Biomed Anal 2002;28(1):181-6.
- Hanpitakpong W, Banmairuroi V, Kamanikom B, Choemung A, Na-Bangchang K. A high-performance liquid chromatographic method for determination of Praziquantel in plasma. J Pharm Biomed Anal 2004;36(4):871-6.
- https://www.fda.gov/downloads/drugs/guidances/ucm073384.pdf.
- https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf.
- https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf.
- Shrestha B, Stephenrathinaraj B, Patel SS, Verma NK, Mazumder R. Simultaneous HPTLC estimation of simvastatin and ezetimibe in tablet dosage form. J Chem 2010;7(4):1206-11.
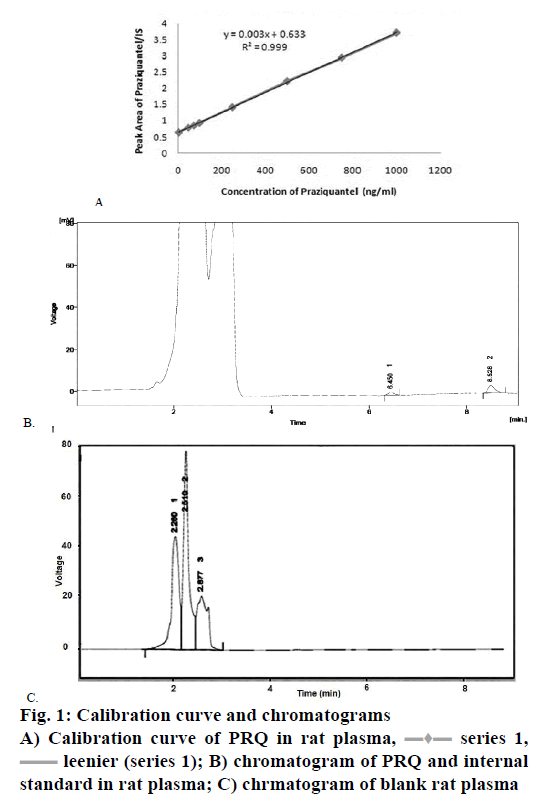
 leenier (series 1); B) chromatogram of PRQ and internal
standard in rat plasma; C) chrmatogram of blank rat plasma
leenier (series 1); B) chromatogram of PRQ and internal
standard in rat plasma; C) chrmatogram of blank rat plasma