- *Corresponding Author:
- G. Z. Sudo
Programa de Desenvolvimento de Fármacos, Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, Brazil
E-mail: gsudo@icb.ufrj.br
| Date of Submission | 27 September 2013 |
| Date of Revision | 10 November 2014 |
| Date of Acceptance | 24 March 2015 |
| Indian J Pharm Sci, 2015;77(2):237-242 |
Abstract
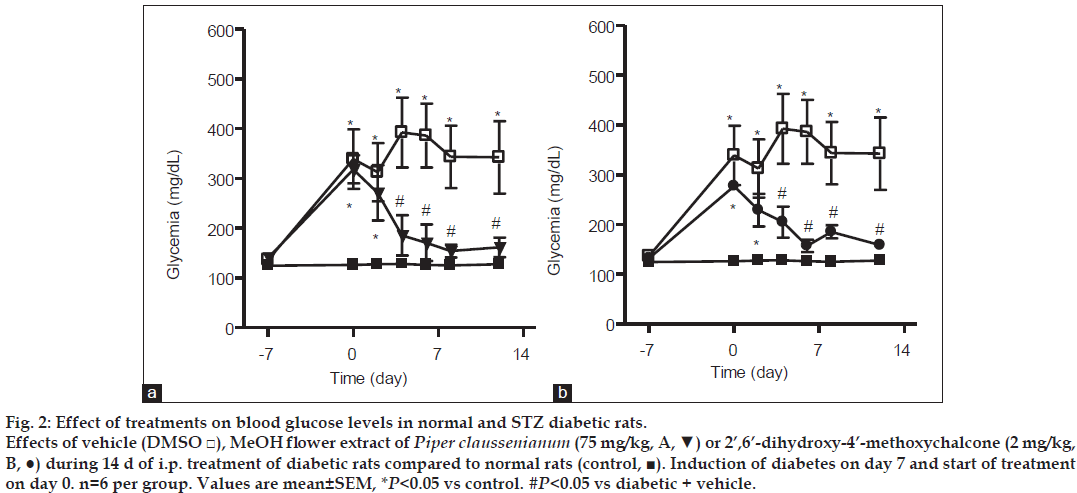
Piper claussenianum inflorescences crude methanol extract was tested for hypoglycemic effect in streptozotocin-induced diabetic rats. The blood glucose levels of rats treated with methanol extract were reduced from 318.4±28.1 mg/dl before treatment to 174.2±38.3 mg/dl after 12 days of treatment (P<0.05). Phytochemical studies were carried out on inflorescences methanol crude extract in order to investigate the possible metabolites responsible for the pharmacological properties of the extract. After chromatographic procedures, three flavonoids were isolated and characterized. The major compound 2',6'-dihydroxy-4'-methoxychalcone was also tested. Rats that received the chalcone content also displayed a reduction in blood glucose levels from 277.4±7.7 mg/dl before treatment to 158.8±9.2 mg/dl after 12 days of treatment (P<0.05). The results suggest this chalcone is one of the metabolite responsible for the blood glucose levels reduction in rats with streptozotocin-induced diabetes. The inflorescence crude extract of P. claussenianum was found to be composed mainly by flavonoids and may be a potential natural source of compounds with hypoglycemic properties.
Keywords
Piper claussenianum, hypoglycemic effect, flavonoids, chalcone
Natural products are usually extracted as complex mixtures containing many constituents in widely different concentrations and representing a broad spectrum of physical and chemical properties [1]. Often found in plants extracts, flavonoids are one of the most studied classes of secondary metabolites. In particular, chalcones have an important role in the flavonoids origin. Naturally occurring chalcones have been documented with different biological properties such as apoptosis induction, antiproliferative action in various cancer cell types, inhibition of proinflammatory mediators, antiplatelet activity, and potential antimalarial activity [2]. These metabolites counteract reactive oxygen species in order to prevent molecular damage and damage by microorganisms, insects, and herbivores [3].
Phytochemical investigation of Piperaceae species has demonstrated a great diversity of flavonoids, specially chalcones, dihydrochalcones, flavanones and flavones many of them isolated from the genus Piper [4-8]. A large variety of flavonoids were found to be effective antihyperglycemic or hypoglycemic agents [9-13]. Ethnobotanical research has identified 1200 plant species throughout the world with antidiabetic potential. There are roughly 450 experimentally proven medicinal plants having antidiabetic properties [14].
In Brazil, about 200 plant species are used empirically to control diabetes mellitus (DM). Less than 1% of an estimated 2 50 000 plants have been screened pharmacologically for antidiabetic activity [15]. DM is a chronic disease that occurs when the pancreas does not produce enough insulin or the insulin is unable to act on its target tissues. DM is characterized by hyperglycemia which, over time leads to serious damage to many systems, especially nerves and blood vessels [16]. Many plants with hypoglycemic activity have been described as a useful source of new oral antidiabetic compounds [17]. Several Piper species have already been reported to treat many conditions in Brazilian folk medicine [4]. In the present study, we investigated the chemical composition of P. claussenianum inflorescences crude extract and evaluated its application as potentially source of hypoglycemic bioactive compounds.
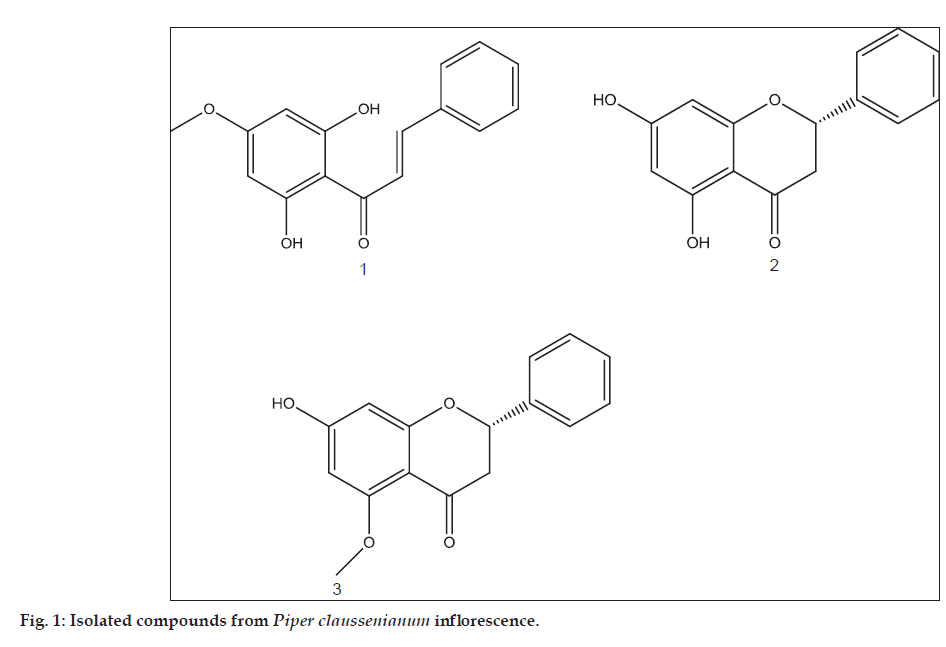
Inflorescences of P. claussenianum (Miq.) C.DC. were collected in São Manoel, Castelo, ES, Brazil in Feb 2009, identified and A voucher sample (RB 489043) has been deposited at the Herbarium of Rio de Janeiro Botanical Garden. Dried and powdered inflorescences were extracted by maceration with methanol (MeOH) at room temperature. The resulting solutions were concentrated in vacuum to yield a crude extract, which was evaluated for antidiabetes properties. This extract was subjected to chromatografic column (CC) over silica gel and eluted with an increasing polarity solvent gradient system from n-hexane to ethyl acetate (EtOAc) and from EtOAc to MeOH. Several flavonoid rich fractions were obtained. The joined fractions 28-34 were further recrystallized with MeOH affording 125.0 mg of pure compound 1 (2’,6’-dihydroxy- 4’-methoxychalcone) while the compound 2 (5,7-dihydroxy-dihydroflavone) was isolated (140 mg) in pure form from the fractions 22-24. The fractions 82-84 were subjected to CC over silica gel and eluted with a gradient of EtOAc in n-hexane, followed by MeOH, yielding 80 mg of compound 3 (5-methoxy- 7-hydroxy-dihydroflavone). The compounds were identified by 1H and 13C NMR spectral analysis and confirmed by comparison with literature data for 1, 2 and 3 [5-8,18].
Qualitative analyses (low resolution electron impact mass spectrometer – LREIMS) were carried out on a GC-QP2010 PLUS Shimadzu with a ZB-5ms fused silica capillary column (30 m×0.25 mm×0.25 mm film thickness). The operating temperatures used were: injector 270°, detector 290° and column oven 60° up to 290° (10° min-1). Helium at 1 ml/min was used as carrier gas for GC-MS.
The inflorescences extract was diluted in MeOH (HPLC grade, Tedia Brazil) and then filtered prior to analyses. HPLC-DAD-UV analyses were performed using a Shimadzu (Shimadzu, Tokyo, Japan) liquid chromatography modular system, consisting of two LC-10AD pumps, UV Shimadzu SPD M10A diode array UV detector (DAD-UV), and an LC Work Station Class LC10 system for data processing. The mobile phase consisted of acetonitrile (A) (HPLC grade, Tedia Brazil) and aqueous trifluoroacetic acid 1% (v/v) (B) in gradient mode:5% (A)/ 95% (B) and then 95% (A)/ 5% (B) in 80 min. The flow rate was 1 ml/min. Chromatograms were generated with UV detection at 240 and 340 nm. LC-MS analyses were conducted on a Shimadzu liquid chromatography modular system consisting of two LC- 10AD pumps coupled to a LC-MS Micromass ZQ 4000 quadrupole detector (Waters). The mobile phase and flow rate were the same as those used for HPLC-DAD-UV.
For LC separations, acetonitrile (Merck, Darmstadt, Germany) and D2O 99.9% (Deutero, Herresbach, Germany) were used. Analyses were performed under ambient conditions using a Varian Prostar module (Varian, Palo Alto, CA, USA) with Prostar 230 Solvent Delivery and a Prostar 330 HPLC DAD. Eluents were acetonitrile and D2O. Separation was performed first with isocratic elution with 40% of acetonitrile (A) and (B) 60% D2O (B) from 2 to 40 min and then with linear gradient from 40% to 55% of acetonitrile in 10 min and then with isocratic gradient with 55% of acetonitrile for 20 min. The flow-rate was maintained at 1 ml/min and elution was monitored by absorbance detection at 340 nm.
NMR spectra were acquired on a VNMRS500 (Agilent Technologies) running VNMRJ software and operating at 1H 499.78 MHz. 1H NMR data were acquired with the following conditions unless otherwise indicated: 1.8194 s acquisition time (at), 0.001 s recovery delay (d1), 9058.0 Hz spectral width, 65 536 complex points (np), 2 steady-state scans, and 264 scans. The pulse sequence Water suppression Enhanced through T1 effects (WET) was used to suppress the signals of the solvent. The NMR analyses were compared with literature data [5-8,18].
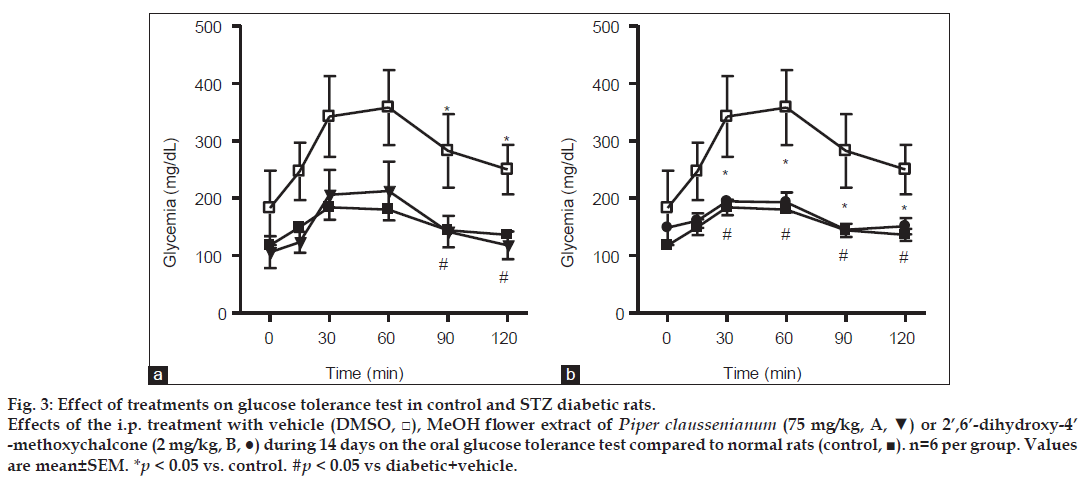
Male Wistar rats (180-220 g) were housed under an artificial 12 h light/dark cycle in controlled temperature (23°) and humidity (60%) conditions with food and water available ad libitum. The Animal Care and Use Committee of the Federal University of Rio de Janeiro approved the study protocol. Each rat received a single intraperitoneal injection of streptozotocin (STZ, 45 mg/kg) to induce diabetes. STZ was dissolved in citrate buffer (pH 4.5) and injected immediately (within a few min) to avoid degradation. STZ-treated rats were randomly divided into three groups (n=6) treated with vehicle (dimethyl sulfoxide, DMSO group 1), MeOH extract (75 mg/kg, i.p. group 2), or chalcone (2 mg/kg, i.p. group 3). These groups were compared with a control group of normal rats. Seven days after the induction of diabetes with STZ, rats with glucose levels >200 mg/dl were treated with vehicle, extract, or the chalcone (2’,6’-dihydroxy-4’-methoxychalcone) for 14 d. Plasma glucose levels were examined using the Accu-Check® Performa monitoring system (Roche, Mannheim, Germany) before and 2, 4, 6, 8, and 12 d after treatment. Blood samples were collected by tail-vein puncture 4 h after the administration of vehicle, extract, or chalcone. At the end of treatment, the animals were submitted to oral glucose tolerance tests. After overnight fasting (14 h), blood glucose was determined before and 15, 30, 60, 90, and 120 min after oral glucose administration (2 g/kg). STZ was purchased from Sigma Chemical (St. Louis, MO, USA). MeOH extract and chalcone obtained from P. claussenianum were dissolved in DMSO. Data are presented as means±standard errors of the mean. Differences among the three groups were determined using analysis of variance followed by the Newman-Keuls test, and were considered significant when P<0.05.
In an effort to investigate chemically unknown Brazilian native Piper species, analysis of the MeOH extract from inflorescences of P. claussenianum was performed. Previous TLC analysis using NP/PEG reagent indicated the presence of free and glycosylated flavonoids in the mixture. As no information is available in the literature regarding the pharmacological properties of this Piper species, evaluation about the antidiabetic effects of the crude MeOH inflorescence extract of P. claussenianum was also performed. LC-UV analysis was performed with the crude MeOH extract in order to confirm the presence of typical flavonoids UV absorptions. The LC-UV chromatogram enabled the detection of characteristic groups, due to the absorption pattern. Thus, LC-UV analysis of the extract showed peaks with UV spectra characteristic for flavonoids (λmax 240, 340 and 360 nm) at 5.87 min and mainly between 38 and 47 min. To confirm these attributions and to obtain more structure information, the same extract from inflorescences of P. claussenianum was submitted to LC-MS analysis in positive ionization mode. This analysis showed a signal at 39.61 min, with [M+H]+ 271 (100%) and fragments with m/z 167 (80%) and m/z 131 (62%). The UV spectrum, the molecular ion (m/z 270) and the fragmentation pattern suggest a dihydroflavone structure with hydroxy and methoxy substitution. Other signal at 44.25 min, showed the same fragments registered to compound, with some differences in the ion percentage [M+H]+ 271 (30%) m/z 167 (100%) and m/z 131 (10%). Considering UV spectrum, the molecular ion (m/z 270) and mass spectra obtained for this compound it was assigned as a chalcone structure also substituted with hydroxyl and methoxyl groups. The LC-MS analysis displayed a signal at 43.55 min, with [M+H]+ 257 (100%) and fragments with m/z 176 (12%) and m/z 148 (78%). These data indicated a dihydroxy-substituted dihydroflavone. The LC-MS analysis showed an additional signal at 5.87 min, peak (3) with [M+H]+ 490 (100%). The LC-UV analysis presented a UV spectra characteristic for flavone O-glycoside with dihydroxyl substitution. Thus, the analysis by LC-MS, suggested the presence of four main compounds, which, include two dihydroflavones, one chalcone and one flavone in the flavonoid fraction. The essential oil of inflorescences of P. claussenianum is basically composed by linalool (55%) and nerolidol (25%) (Marques et al. 2011) but the nonpolar n-hexane extract, was also composed of mono and sesquiterpenes, long chain esters, fatty acids, phytol, tocopherol and proximodiol.
The same extract of P. claussenianum was submitted to on-line LC/1H-NMR analysis, which, was carried out under the HPLC conditions described in the experimental section (see LC-NMR procedures), except for the solvent system. The water used for the LC-NMR gradient system was composed of pure Milli-Q and D2O (9:1) and the sample concentration was increased due to the low sensitivity of NMR. As the crude extract was rather complex, only 20 mg/ml could be injected onto the column without a loss of resolution. In this mode, the HPLC flow was stopped as soon as a UV active constituent of the extract reached the LC/NMR cell. The acquisition of the LC/1H NMR spectra with solvent suppression was then carried out for each constituent until a satisfactory signal-to-noise ratio was obtained for the peak of interest. It was possible to acquire the 1H NMR spectra for the most important peaks in the chromatogram. No on-column diffusion or unacceptable loss of chromatographic resolution was observed in this procedure. The LC-NMR spectrum of compound 1 was typical of chalcones, since it displayed two doublets at δ 7.75 and 7.88 (J=17.1 Hz, 1H), characteristic of hydrogens at α- and β- positions in chalcones. However, the 1H NMR spectrum of 2 and 3 were correlated with chalcone derivatives, dihydroflavone, due to the absence of the doublets with J=17 Hz and doublets at δ 5.33-5.55 and δ 2.69-3.22, in agreement with C ring dihydroflavone hydrogens. The LC-1HNMR analysis did not allow identification of any signal with O-glycosylflavone profile.
Further chromatographic separations of the inflorescences extract on silica gel yielded flavonoid rich fractions. The fractions 28-34 afforded compound 1 as orange needle shaped crystals by recrystallization from MeOH. Fractions 22-24 yielded compound 2 as yellow needle shaped crystals, while the fraction 82-84 afforded compound 3 as colorless needle-shaped crystals. The 1H NMR spectrum showed two doublets at δ 5.87 (J=2.3 Hz, 1H) and 5.92 (J=2.3 Hz, 1H) for compound 1; δ 5.87 (J=2.1 Hz, 1H) and 5.98 (J=2.1 Hz, 1H) for compound 2 and δ 5.97 (J=2.3 Hz, 1H) and 6.04 (J=2.3 Hz, 1H) for compound 3. These are associated to their corresponding carbons (δ 95.25 and 96.90 to 1; δ 92,40 and 96.30 to 2 and δ 83.75 and 96.70 to 3). The chemical shift assignments were based on HSQC and HMBC and were assigned as H-6 and H-8 in the ring A of the flavanones and H-1’ and H-5’ of the chalcone. The related carbons shifts are in agreement to the oxygenated meta substituents. A hydroxyl group chelated to carbonyl group was found in compound 1 and 2 in δ 12.5 and δ 12.2, respectively. Peaks in δ 10.5 and δ 10.3 were observed in the 1H NMR spectra of 2 and 3, respectively. These data associated to the HMBC and 13 C correlations possibly were related to hydroxyl groups in C-5 (δ 165.5) and C-‘6 (δ 164.7) for compounds 2 and 3, respectively. The 1H NMR data were associated to the signals at δ 5.55 (dd, J=13.0 and 2.4 Hz, H-2), 2.77 (dd, J=17.1 and 2.2 Hz, H-3a), 3.22 (dd, J=17.2 and 13.5 Hz, H-3b), δ 5.33 (dd, J=13.0 and 2.9 Hz, H-2), 2.69 (dd, J=17.1 and 2.3 Hz, H-3a), 2.98 (dd, J=17.0 and 13.2 Hz, H-3b) corresponding to the compounds 2 and 3, respectively. Since no substitution was observed in B ring of all compounds, no AB systems were detected as characteristic of a para-dissustituted aromatic rings. LREIMS analyses showed molecular ion-peaks at m/z 270 (52%) for compound 1 (C16H14O4), m/z 256 (100%) for compound 2 (C15H12O4) and m/z 270 (51%) for compound 3 (C16H14O4). The 1H NMR spectrum obtained for these compounds were found in agreement to the compound 1 compound 2, and compound 3, respectively, (fig. 1). These data led us to characterize compound 1 as 2’,6’-dihydroxy-4’-methoxychalcone; 2 as 5,7-dihydroxydihydroflavone (pinocembrin) and 3 as 5-methoxy-7-hydroxydihydroflavone (alpinetin) [5-8,18]. The quantitative analyses of compounds 1, 2 and 3 were carried out using HPLC-UV (HPLC-DAD-UV Shimadzu) instead of LC-MS, since these compounds were present at >0.05% (w/w). The pure isolated compounds were used as standards. Injections of each sample (10 μl) were analyzed in triplicate using the same conditions described for the LC separations. Areas under the curve from experimental peaks in the chromatogram were determined and the percent weight of each compound present was calculated based on a standard curve and amount (μg) of extract injected onto the column. Data were only used if their relative standard deviations (RSD, %; (mean–standard deviation)/mean)×100) were <5%. The three isolated measured flavonoids comprise 6.9% of the weight raw extract of P. claussenianum inflorescences. The compound 1 was found in (2.5%), the compound 2 (2.8%) while the compound 3 was found in smaller amount (1.6%).
The crude methanol extract from inflorescences of P. claussenianum and its major compound 1 were investigated for hypoglycemic activity. In the present study, STZ (45 mg/kg i.p.) produced significant hyperglycemia when compared with blood glucose levels before the induction of diabetes and with the control group. After diabetes induction, rats were treated with vehicle (DMSO), MeOH extract, or chalcone (1) for 14 d. Rats treated with vehicle had glucose levels of 339.1±59.9 and 342.5±72.8 mg/dl before and 12 d after the beginning of treatment, respectively. The blood glucose levels of rats treated with MeOH extract (75 mg/kg i.p.) were reduced from 318.4±28.1 mg/dl before treatment to 174.2±38.3 mg/dl 12 d after the beginning of treatment (P<0.05 fig. 2a). Rats that received chalcone (2 mg/kg i.p.) also showed a reduction in blood glucose levels from 277.4±7.7 mg/ dl before treatment to 158.8±9.2 mg/dl after 12 d of treatment (P<0.05 fig. 2b). The daily intraperitoneal administration of MeOH extract or chalcone for 14 d produced a significant and consistent reduction in blood glucose levels in comparison with vehicle treatment of diabetic rats, indicating the potent hypoglycemic activity of both MeOH extract and chalcone. These results indicate that 2’,6’-dihydroxy- 4’-methoxychalcone contributes to the hypoglycemic activity of the MeOH extract.
The chronic administration of MeOH extract or chalcone for 14 d in diabetic rats resulted in significant improvement in oral glucose tolerance following oral glucose loading, as shown by the significant reduction of glucose levels in rats treated with MeOH extract or chalcone to 118.0±24.2 mg/dl (fig. 3a) and 151.4±14.1 mg/ dl (fig. 3b), respectively, after 120 min of oral glucose administration. In contrast, rats treated with vehicle had glucose levels of 250.0±43.2 mg/dl (fig. 3a and b) after 120 min of oral glucose loading. These results indicate the potent antihyperglycemic activity of both MeOH flower extract and chalcone.
The occurrence of chalcones and dihydroflavones in P. claussenianum species is of particular interest since to date no flavonoid isolation has been described for this species. Until now, the antidiabetic action of 2’,6’-dihydroxy-4’- methoxychalcone has not been reported. Chalcones with proper substitution have been shown to selectively inhibit enzymes such as aldose reductase (AR) [19], protein tyrosine phosphatase 1B (PTP1B) [20], α-glucosidase [11,13] and to increase insulin secretion [12]. In this view, chalcones have emerged as new agents in the treatment of diabetes. PTP1B is thought to function as a negative regulator of insulin and leptin signal transduction [20]. Additionally, AR is a critical component of intracellular signaling, and inhibition of the enzyme prevents high glucose-, cytokine-, or growth factor-induced activation of protein kinase C and nuclear factor-κ-binding protein [21]. Besides, the antioxidant property of chalcones attracted to explore hybrid structures as antidiabetic agents, because oxidative stress also plays an important role in diabetic patients leading to vascular complications.
The present study demonstrated for the first time that both MeOH inflorescence extract from P. claussenianum and its major constituent, 2’,6’-dihydroxy-4’-methoxychalcone exhibited hypoglycemic and antihyperglycemic activities in rats with STZ-induced diabetes. Both the crude extract and the isolated chalcone from P. claussenianum would be of great natural therapeutic benefit in the management of DM. However, further detailed investigation is warranted to determine the mechanism of action and to establish the therapeutic potential of these agents in the treatment of diabetes and diabetic complications.
Acknowledgements
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Universitária Jose Bonifácio (FUJB), Instituto Nacional de Ciência e Tecnologia (INCT), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa de Apoio a Núcleos de Excelência (PRONEX).
References
- Moco S, Bino RS, De Vos RJRC, Vervoot J. Metabolomics technologies and metabolite identification. Trends Anal Chem 2007;26:855-66.
- Zi X, Simoneau AR. Flavokawain: A novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of baxprotein-dependent and mitocondria-dependent apoptotic pathway and suppresses tumor grown in Mice. Cancer Res 2005;65:3479-86.
- Patil CB, Mahajan SK, Katti SA. Chalcone: A versatile molecule. J Pharm Sci Res 2009;1:11-22.
- Parmar SV, Jain SC, Bisht KS, Jain R, Taneja P, Jha A et al . Phytochemistry of the genus Piper . Phytochemistry 1997;46:597-673.
- Torres-Santos EC, Moreira DL, Kaplan MA, Bergmann BR, Meirelles MN, Rossi-Bergmann B. Selective Effect of 2’,6’-Dihydroxy- 4’-methoxychalcone Isolated from Piper aduncum on Leishmaniaamazonensis . Antimicrob Agents Chemother 1999;43:1234-41.
- Facundo VA, Braz-Filho R. C-methylated flavonoids from the roots of Piper carniconnectivum . BiochemSystEcol 2004; 32:1215-7.
- Lago JH, Young MC, Reigada JB, Soares MG, Kato MJ. Antifungical derivatives from Piper mollicomum andP. lhotzyanum. Quim Nova 2007;30:1222-4.
- Portet B, Fabre N, Roumy V, Gornitzka H, Bourdy G, Chevalley S, et al . Activity-guided isolation of antiplasmodialdihydrochalcones andflavanones from Piper hostmannianum var. berbicense , Phytochemistry2007;68:1312-20.
- Alberton EH, Damazio RG, Cazarolli LH, Chiaradia LD, Leal PC, Nunes RJ, et al . Influence of chalcone analogues on serum glucose levels in hyperglycemic rats. ChemBiol Interact 2008;171:355-62.
- Satyanarayana M, Tiwari P, Tripathi BK, Srivastava AK, Pratap R. Synthesis and antihyperglycemic activity of chalcone based aryloxypropanolamines. Bioorg Med Chem 2004;12:883-9.
- Bharatham K, Bharatham N, Park KH, Lee KW. Binding mode analyses and pharmacophore model development for sulfonamide chalcone derivatives a new class of α-glucosidase inhibitors. JMol Graph Model 2008;26:1202-12.
- Damazio RG, Zanatta AP, Cazarolli LH, Mascarello A, Chiaradia LD, Nunes RJ, et al. Nitrochalcones: Potential in vivo insulin secretagogues. Biochimie 2009;91:1493-8.
- Bak EJ, Park HG, Lee C, Lee T, Woo GH, Na Y, et al . Effects of novel chalcone derivatives on α-glucosidasedipeptidyl peptidase-4 and adipocyte differentiation in vitro . BMB Rep 2011;44:410-4.
- Li WL, Zheng HC, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol 2004;92:1-21.
- Barbosa-Filho JM, Vasconcelos TH, Alencar AA, Batista LM, Oliveira RA, Guedes DN. Plants and their active constituents from South Central and North America with hypogycemic activity. Braz J Pharmacogn 2005;14:392-413.
- World Health Organization, Definition Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of WHO Consultation Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva:World Health Organization; 2006.
- Prabhakar PK, Doble M. Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin J Integr Med 2011;17:563-74.
- Marques AM, Paiva RA, Fonseca LM, Capella MA, Guimarães EF, Kaplan MAC. Preliminary anticancer potency evaluation and phytochemical investigation of methanol extract of Piper claussenianum (miq.) C.DC. J Applied Pharm Sci 2013;3:13-8.
- Severi F, Benvenuti S, Costantino L, Vampa G, Melegafi M, Antolini L. Synthesis and activity of a new series of chalcones as aldose reductaseinhibitors. Eur J Med Chem 1998;33:859-66.
- Johnson TO, Ermolieff J, Jirousek MR. Protein tyrosine phosphatase1B inhibitors for diabetes. Nature Rev Drug Discov 2002;1:696-709.
- Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev 2005;26:380-92.