- *Corresponding Author:
- X. Zhao
Department of Pediatrics,
Nantong First People's Hospital,
Nantong,
Jiangsu Province 226001,
China
E-mail: xueyan795533@163.com
| Date of Received | 02 July 2019 |
| Date of Revision | 21 May 2021 |
| Date of Acceptance | 03 July 2022 |
|
Indian J Pharm Sci 2022;84(2):281-286 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of icariin on cell cycle and apoptosis of medulloblastoma cells and its mechanism. Medulloblastoma cell line D341 was cultured and divided into control group, low dose group, medium dose group and high dose group. The number and area of cell clones in low, medium and high dose groups were significantly lower than those in control group, and decreased with the increase of dose (p<0.05). The proportion of synthesis phase in the low, medium and high dose groups was significantly higher than that in the control group and increased with the increase of dose, while the proportion of growth 1 phase in the low, medium and high dose groups was significantly lower than that in the control group and decreased with the increase of dose (p<0.05). The protein expressions of cyclin A, cyclin-dependent kinase 2 and cyclin B1 in low, medium and high dose groups were significantly lower than those in control group and decreased with the increase of dose (p<0.05). The apoptosis rate of low, medium and high dose groups was significantly higher than that of control group and increased with the increase of dose (p<0.05). The expression of Bcl-2-associated X protein in low, medium and high dose groups was significantly lower than that in control group and decreased with the increase of dose. The expression of B-cell lymphoma 2, cleaved caspase-3, cleaved caspase-9 and cleaved poly (ADP-ribose) polymerase protein in low, medium and high dose groups was significantly higher than that in control group and increased with the increase of dose (p<0.05). Icariin can inhibit the proliferation and colony forming ability of medulloblastoma cells, induce cell arrest in synthesis phase by inhibiting the expression of cyclin A, cyclin-dependent kinase 2 and cyclin B1, and induce cell apoptosis by regulating the expression of B-cell lymphoma 2, cleaved caspase-3, cleaved caspase-9 and cleaved poly (ADP-ribose) polymerase protein.
Keywords
Icariin, cell cycle, apoptosis, medulloblastoma
Medulloblastoma (MB) is a kind of cerebellar neuroepithelial tumor, which is the most common intracranial malignant tumor in children. It has the characteristics of rapid growth of tumor cells, high invasion and migration ability [1]. At present, the main therapy method for MB is surgical resection, supplemented by chemoradiotherapy, but it leads to high recurrence rate and poor prognosis [2]. As a result, the search for new targeted drugs for therapeutic MB has become the focus and difficulty of domestic and foreign researchers. Epimedium is a traditional Chinese medicine for tonifying the liver and kidney, dispelling rheumatism, strengthening muscles and bones, anti- aging and other biological activities [3]. Icariin, as the main active ingredient of Epimedium, has many biological activities such as enhancing immunity, regulating endocrine and improving cardiovascular and cerebrovascular diseases [4]. Liu et al. [5] studies have found that icariin can improve cognitive dysfunction in elderly mice by activating neural stem cells. With MB cells D341 as the observation objects, this experiment aims to analyze the effect of icariin on cell cycle and apoptosis of MB cells and its related mechanisms.
Materials and Methods
General data:
Cell line: MB cells D341 provided by Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences were selected.
Experimental reagent: Icariin provided by Chuzhou shinuoda Biological Technology Co., Ltd. Cell Counting Kit-8 (CCK-8) detection kit provided by Jiangxi iBio Biotechnology Co., Ltd.Apoptosis detection kit provided by Beijing Yita Biotechnology Co., Ltd. Bicinchoninic Acid (BCA) protein concentration assay kit provided by Beijing Yita Biotechnology Co., Ltd. Dulbecco's Modified Eagle Medium (DMEM) cell culture medium provided by Shanghai Yiji Industrial Co., Ltd. Fetus calf serum provided by Hanbio (Shanghai) Co., Ltd. Primary antibodies of various proteins (CyclinA, Cyclin-Dependent Kinase 2 (CDK2), Cyclin B1, Bcl-2- Associated X Protein (BAX), B-cell lymphoma 2 (Bcl- 2), cleaved caspase-3, cleaved caspase-9, Cleaved- Poly (ADP-Ribose) Polymerase (PARP-1)) provided by Beijing Beolébo Technology Co., Ltd. Cell cycle and apoptosis detection kit provided by Beijing Beolébo Technology Co., Ltd.
Laboratory apparatus: Microplate reader provided by Molecular Devices (Shanghai) Co., Ltd. Protein electrophoresis instrument provided by Bensheng (Tianjin) Health Technology Co., Ltd. Centrifuge provided by Dexiang Technology Limited. Fluorescence microscope provided by Guangzhou Koster scientific instrument Co., Ltd. Cell incubator provided by Shanghai Thermo Fisher Co., Ltd. Flow cytometry analyzer provided by Amyjet Scientific Inc. Protein concentration meter provided by Guangzhou Bestrun Co., Ltd. Electric heating thermostat provided by Changzhou Gao De Instrument Manufacturing Co., Ltd. Super clean workbench provided by Beijing ASKN Laboratory Equipment Engineering Technology Co., Ltd.
Method:
Cell culture and dosing: MB Daoy cells were inoculated in DMEM medium containing 10 % fetal bovine serum, l×105 U/l penicillin and 1×105 U/l streptomycin and cultured in 37° thermostatic closed incubator containing 5 % Carbon Dioxide (CO2). When the cells were attached to about 85 %, the original medium was sucked out, washed once by Phosphate Buffered Saline (PBS), added 2 ml trypsin digested cells, placed in 37° incubator for about 5 min. After gently blowing the cells, it was then placed in centrifuge tube for 1200 rpm centrifuged for 3 min. The upper layer culture medium was sucked out, 1 ml PBS was added to count the cells; the number of cells required for plate lying was calculated and centrifuged at 1200 rpm for 3 min. PBS was sucked out and hanged in the fresh culture solution. The corresponding number of cells was taken to the required 96 hole plate. After the cells were attached to the wall, they were divided into control group, low dose group, medium dose group and high dose group. The control group did not add any drugs and the cells in low, medium and high dose groups were treated with 10, 20 and 30 μM icariin, respectively.
Observation indictors:
Cell proliferation activity was tested with CCK-8 kit: 10 μl of CCK-8 detection solution was added to each hole after icariin treatment 24 h, 48 h, 72 h and incubated for 2 h, at 37°. The absorptivity at 450 nm was detected by enzyme labeling instrument.
Cell cloning formation test for cell cloning formation: The treated cells were inoculated in 6 cm petri dishes at the density of 1×104 cells/dish for 2 w in 37° incubator. After cloning, the cells were washed in PBS twice and fixed with 4 % paraformaldehyde for 15 min and stained with crystal violet for 30 min. The number of clone formation of cells over 50 was observed and recorded, and the size of clone formation was counted.
Detection of apoptosis rate by flow cytometry: The cell culture medium was collected to the centrifuge tube for 2 ml of trypsin digested cells and placed in 37° incubator for about 5 min. The cell culture solution was added and the anchorage-dependent cells were blown. The cells were collected in the centrifuge tube and centrifuged at 1200 rpm for 5 min. 1 ml ice preconditioning PBS was added and suspended in cells and centrifuged for 5 min at 1200 rpm, leaving the bottom cell mass. 300 μl of binding buffer was added and the cells were oscillated until being mixed. 5 μl of Annexin V-FITC reagents was added and incubated for 15 min at the room temperature. 5 μl of Propidium Iodide (PI) reagents was added and incubated for 5 min at the room temperature after mixing. 200 μl of binding buffer was added. The cell apoptosis rate was tested with flow cytometry.
Cell cycle changes were detected by flow cytometry: The cell culture medium was collected to the centrifuge tube for 2 ml of trypsin digested cells and placed in 37° incubator for about 5 min. The cell culture solution was added and the anchorage-dependent cells were blown. The cells were collected in the centrifuge tube and centrifuged at 1200 rpm for 5 min. 1 ml ice preconditioning PBS was added and suspended in cells and centrifuged for 5 min at 1200 rpm, leaving the precipitation cells. 1 ml of pre-cooling 70 % alcohol was added and fixed at 4° for 24 h. It was centrifuged at 1200 rpm for 5 min, leaving the precipitation cells. 1 m of PBS was added and suspended in cells and centrifuged for 5 min at 1200 rpm, leaving cell dispersing at the bottom of centrifuge tube. 0.5 ml of PI was added for staining and suspended in cells, and incubated at 37° for 30 min. Flow detection and analysis of incubated cell samples were performed. A flow cytometry was used to detect red fluorescence at the excitation wavelength 488 nm and light scattering.
Expression of cell related protein was detected with Western blot: Logarithmic growth of cells was taken and cleaned in PBS for 1 time. Radioimmunoprecipitation assay pyrolysis liquid was added and placed above the ice for 30 min and centrifuged at 1200 rpm for 15 min. Protein lysate was extracted and BCA protein quantitative kit was used to detect protein samples.
12 % Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel was configured. After enclosing and antibody incubation, the protein was colored with color developing solution.
Statistical methods:
The data of this study were analyzed by Statistical Package for the Social Sciences (SPSS) 20.0 software package and all measurement data in accordance with normal distribution were compared by x̄ ±s. One way Analysis of Variance (ANOVA) was used for comparison among groups and Student–Newman– Keuls (SNK)-q test was used for pairwise comparison; the statistical results were statistically significant if p<0.05.
Results and Discussion
At the same time, the cell proliferation activity of low, medium and high dose groups was significantly lower than that of control group and decreased with the increase of dose. The difference was statistically significant (p<0.05) as shown in Table 1.
| Group | 24 h | 48 h | 72 h |
|---|---|---|---|
| Control group | 98.05±1.16 | 98.10±0.98 | 98.46±1.05 |
| Low dose group | 79.64±4.62a | 58.34±3.46a | 39.64±3.85a |
| Medium dose group | 45.61±6.42ab | 26.34±4.87ab | 14.32±3.15ab |
| High dose group | 34.91±4.85abc | 11.26±3.15abc | 8.96±1.89abc |
Note: ap<0.05 compared with control group; bp<0.05 compared with the low dose group and cp<0.05 compared with the medium dose group
Table 1: Comparison of Cell Proliferation Activity at Each Time (x̄±s)
The number and area of cell clones in low, medium and high dose groups were significantly lower than those in control group and decreased with the increase of dose. The difference was statistically significant (p<0.05) as shown in Table 2.
| Group | Number of cloning | Monoclonal area |
|---|---|---|
| Control group | 2364.15±35.64 | 1.02±0.24 |
| Low dose group | 2103.46±24.84a | 0.81±0.16 |
| Medium dose group | 1764.31±20.46ab | 0.46±0.12ab |
| High dose group | 108.64±12.47abc | 0.24±0.08abc |
Note: ap<0.05 compared with control group; bp<0.05 compared with the low dose group and cp<0.05 compared with the medium dose group
Table 2: Comparison of Cloning Forming Ability of Cells in Each Group (x̄±s)
The proportion of Synthesis phase (S phase) in the low, medium and high dose groups was significantly higher than that in the control group and increased with the increase of dose, while the proportion of Growth 1 phase (G1 phase) in the low, medium and high dose groups was significantly lower than that in the control group and decreased with the increase of dose. The difference was statistically significant (p<0.05) as shown in Table 3.
| Group | G2/M | S | G1 |
|---|---|---|---|
| Control group | 17.46±3.84 | 34.28±4.15 | 48.26±3.15 |
| Low dose group | 15.24±4.41a | 38.60±4.78a | 46.16±3.85a |
| Medium dose group | 13.02±3.15ab | 43.80±5.46ab | 43.18±4.16ab |
| High dose group | 10.16±2.46abc | 48.88±7.46abc | 40.96±2.84abc |
Note: ap<0.05 compared with control group; bp<0.05 compared with the low dose group and cp<0.05 compared with the medium dose group, G2: Growth 2 phase; M: Mitotic phase; S: Synthesis phase and G1: Growth 1 phase
Table 3: Comparison of Cell Cycle Ratios in Each Group (x̄±s)
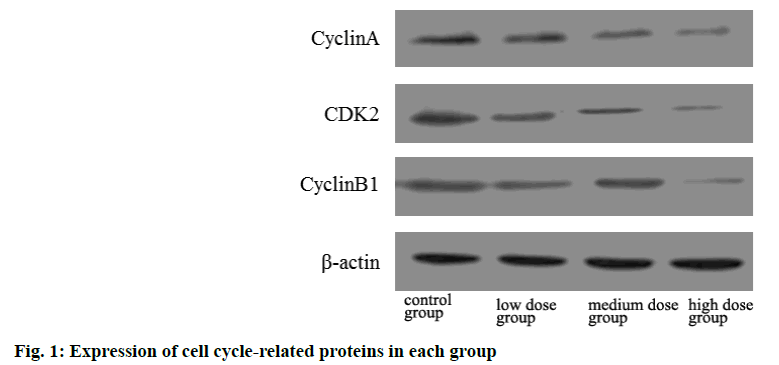
The protein expressions of cyclin A, CDK2 and cyclin B1 in low, medium and high dose groups were significantly lower than those in control group and decreased with the increase of dose. The difference was statistically significant (p<0.05) as shown in Table 4 and fig 1.
| Group | Cyclin A | CDK2 | Cyclin B1 |
|---|---|---|---|
| Control group | 1.02±0.01 | 1.00±0.02 | 1.01±0.01 |
| Low dose group | 0.85±0.16a | 0.82±0.22a | 0.79±0.11a |
| Medium dose group | 0.61±0.14ab | 0.59±0.12ab | 0.54±0.34ab |
| High dose group | 0.47±0.12abc | 0.32±0.17abc | 0.41±0.21abc |
Note: ap<0.05 compared with control group; bp<0.05 compared with the low dose group and cp<0.05 compared with the medium dose group and CDK2: Cyclin Dependent Kinase 2
Table 4: Comparison of Cell Cycle Related Protein Expression in Each Group (x̄±s)
The apoptosis rate of low, medium and high dose groups was significantly higher than that of control group and increased with the increase of dose. The difference was statistically significant (p<0.05) as shown in Table 5.
| Group | Apoptosis rate |
|---|---|
| Control group | 3.46±2.16 |
| Low dose group | 4.89±2.85a |
| Medium dose group | 12.64±4.62ab |
| High dose group | 26.34±7.64abc |
Note: ap<0.05 compared with control group; bp<0.05 compared with the low dose group and cp<0.05 compared with the medium dose group
Table 5: Comparison of Apoptosis Rate in Each Group (x̄±s)
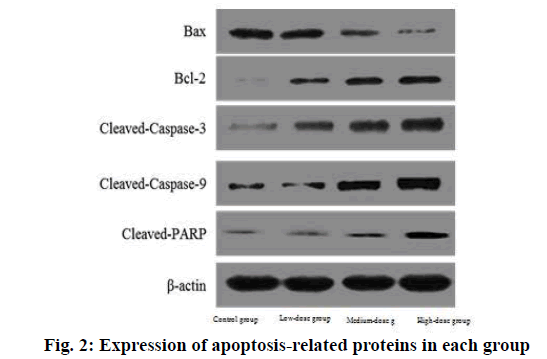
The expression of BAX protein in low, medium and high dose groups was significantly lower than that in control group and decreased with the increase of dose. The expression of Bcl-2, cleaved caspase-3, cleaved caspase-9 and cleaved PARP protein in low, medium and high dose groups was significantly higher than that in control group and increased with the increase of dose.
The difference was statistically significant (p<0.05) as shown in Table 6 and fig. 2.
| Indicator | Control group | Low dose group | Medium dose group | High dose group |
|---|---|---|---|---|
| BAX | 1.01±0.01 | 0.84±0.12a | 0.67±0.15ab | 0.54±0.11abc |
| Bcl-2 | 0.99±0.03 | 1.24±0.16a | 1.47±0.26ab | 1.71±0.31abc |
| Cleaved caspase-3 | 1.02±0.01 | 1.31±0.21a | 1.45±0.31ab | 1.68±0.28abc |
| Cleaved caspase-9 | 1.00±0.02 | 1.28±0.18a | 1.43±0.17ab | 1.75±0.34abc |
| Cleaved PARP | 0.98±0.02 | 1.24±0.14a | 1.42±0.16ab | 1.69±0.28abc |
Note: ap<0.05 compared with control group; bp<0.05 compared with the low dose group and cp<0.05 compared with the medium dose group, BAX: Bcl-2-Associated X Protein and Bcl-2: B-cell lymphoma 2
Table 6: Comparison of Cell Cycle Related Protein Expression in Each Group (x̄±s)
MB is a kind of neuroepithelial tumor often derived from cerebellar vermis or posterior medullary velum, which occurs in children about 8 y old [6]. The main therapy method for MB is surgical resection, supplemented by chemoradiotherapy. Because of the special location of MB lesions and the younger age of the patients, it leads to higher surgical risk and tumor recurrence rate and the poor prognosis of the patients [7].
Surgical adjuvant radiotherapy and chemotherapy can significantly improve the survival rate of patients, but hearing impairment, mental retardation and cognitive dysfunction seriously affect the quality of life of patients [8]. As a result, the search for new therapy of MB is the focus of neurosurgery medical staff.
Icariin is a flavonoid isolated from Epimedium. Previous studies have found that icariin protects nerves by inhibiting inflammatory response, enhancing antioxidant stress and regulating the release of amino acid neurotransmitters. In recent years, it has been found that icariin has a certain anti-tumor effect. Chen et al. [9] studies found that icariin can inhibit the proliferation, invasion and migration of colorectal cancer cells by regulating Nuclear factor kappa10 B (NF-κB) related pathways and delay the occurrence and development of colorectal cancer. Liu et al. [10] studies suggested that icariin can inhibit the progression of hepatocellular carcinoma by inducing apoptosis. Xu et al. [11] studies have shown that icariin can inhibit the proliferation and apoptosis of salivary adenoid cystic cancer cells. Zhang et al. [12] studies have shown that icariin can inhibit the proliferation of ovarian cancer cells by inducing cell cycle arrest and promoting apoptosis. In this experiment, the cell proliferation activity of low, medium and high dose groups was significantly lower than that of control group and decreased with the increase of dose at the same time. The number and area of cell clones in low, medium and high dose groups were significantly lower than those in control group and decreased with the increase of dose. It suggests that icariin can reduce the growth of cancer cells by inhibiting cell proliferation. It is dose-dependent and time-dependent.
The cell cycles include G1 phase, S phase, Growth 2 phase (G2 phase) and Mitotic phase (M phase). Synthetic replication of DNA appeared in S phase and separation of chromosomes appeared in M phase. Cyclin A is the main protein of cell cycle regulation, which can promote cell cycle from G1 phase to S phase [13]. High expression of CDK2 in liver cancer, gastric cancer and other malignant tumors can promote the proliferation and invasion of tumor cells [14]. Cyclin B1 is the main protein that regulates G2 phase and its overexpression can induce the malignant growth of cells [15]. In this experiment, the proportion of S phase in the low, medium and high dose groups was significantly higher than that in the control group and increased with the increase of dose, while the proportion of G1 phase in the low, medium and high dose groups was significantly lower than that in the control group and decreased with the increase of dose. The protein expressions of cyclin A, CDK2 and cyclin B1 in low, medium and high dose groups were significantly lower than those in control group and decreased with the increase of dose. It suggests that icariin can induce cell arrest in S phase by inhibiting the expression of cyclin A, CDK2 and cyclin B1, which is similar to the study results of Wu et al. [16].
Apoptosis is a process in which cells actively choose to die for survival under adverse conditions. Mitochondrial pathway is an important signaling pathway of apoptosis [17]. Apoptosis related factors BAX mainly exist in the cytoplasmic matrix. Mitochondrial damage can make BAX enter mitochondria and activate caspase-9 and caspase-3 for apoptosis. Bcl- 2 is an anti-apoptotic related factor that Bcl-2 co- regulate the apoptosis process with the expression of BAX cells [18]. In this experiment, the apoptosis rate of low, medium and high dose groups was significantly higher than that of control group and increased with the increase of dose. The expression of BAX protein in low, medium and high dose groups was significantly lower than that in control group and decreased with the increase of dose. The expression of Bcl-2, cleaved caspase-3, cleaved caspase-9 and cleaved PARP protein in low, medium and high dose groups was significantly higher than that in control group and increased with the increase of dose. It suggests that icariin can induce cell apoptosis by regulating the expression of Bcl-2, cleaved caspase-3, cleaved caspase-9 and cleaved-PARP.
In conclusion, icariin can inhibit the cell proliferation activity and colony forming ability of MB cells, induce cell arrest in S phase by inhibiting the expression of cyclin A, CDK2 and cyclin B1, and induce cell apoptosis by regulating the expression of Bcl-2, cleaved caspase-3, cleaved caspase-9 and cleaved-PARP.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Zhu D, Osuka S, Zhang Z, Reichert ZR, Yang L, Kanemura Y, et al. BAI1 suppresses medulloblastoma formation by protecting p53 from Mdm2-mediated degradation. Cancer Cell 2018;33(6):1004-16.
[Crossref] [Google Scholar] [Pub Med]
- Duc NM, Huy HQ, Nadarajan C, Keserci B. The role of predictive model based on quantitative basic magnetic resonance imaging in differentiating medulloblastoma from ependymoma. Anticancer Res 2020;40(5):2975-80.
[Crossref] [Google Scholar] [Pub Med]
- Huang Z, Cheng C, Cao B, Wang J, Wei H, Liu X, et al. Icariin protects against glucocorticoid-induced osteonecrosis of the femoral head in rats. Cell Physiol Biochem 2018;47(2):694-706.
[Crossref] [Google Scholar] [Pub Med]
- Jing X, Yin W, Tian H, Chen M, Yao X, Zhu W, et al. Icariin doped bioactive glasses seeded with rat adipose-derived stem cells to promote bone repair via enhanced osteogenic and angiogenic activities. Life Sci 2018;202:52-60.
[Crossref] [Google Scholar] [Pub Med]
- Liu HJ, Liu XY, Jing DB. Icariin induces the growth, migration and osteoblastic differentiation of human periodontal ligament fibroblasts by inhibiting Toll-like receptor 4 and NF-κB p65 phosphorylation. Mol Med Rep 2018;18(3):3325-31.
[Crossref] [Google Scholar] [Pub Med]
- Fan Q, Gong T, Zheng C, Ng JM, Chen J, Myers C et al. Statins repress hedgehog signaling in medulloblastoma with no bone toxicities. Oncogene 2021;40(12):2258-72.
[Crossref] [Google Scholar] [Pub Med]
- Rao S, Uppar AM, Santosh V. Sonic hedgehog activated large cell/anaplastic medulloblastoma with myogenic differentiation. World Neurosurg 2020;135:16-8.
[Crossref] [Google Scholar] [Pub Med]
- Paulino AC, Suzawa HS, Dreyer ZE, Hanania AN, Chintagumpala M, Okcu MF. Scoliosis in children treated with photon craniospinal irradiation for medulloblastoma. Int J Radiat Oncol Biol Phys 2021;109(3):712-7.
[Crossref] [Google Scholar] [Pub Med]
- Chen SX, Rao H, Chen DS. Effects of icariin on androgen receptor signaling in androgen-dependent prostate cancer in balb/c-nu nude mice. J Changchun Univ Chin Med 2018;34(3):436-8.
- Liu XJ, Zhang MZ, Chen D. Progress on the mechanism of Epimedium in prevention and treatment of malignant tumor. Hebei J Tradit Chin Med 2018;40(11):149-53.
- Xu W, Liu X, Li P. Effects of icariin combined ly294002 on proliferation and apoptosis of salivary adenoid cystic carcinoma cells. Guangxi Med J 2018;40(4):430-4.
- Zhang ZG, Wang X, Zai JH, Sun CH, Yan BC. Icariin improves cognitive impairment after traumatic brain injury by enhancing hippocampal acetylation. Chin J Integr Med 2018;24(5):366-71.
[Crossref] [Google Scholar] [Pub Med]
- Niwa T, Akaike Y, Watanabe K, Chibazakura T. Hyperactivation of cyclin A-CDK induces centrosome overduplication and chromosome tetraploidization in mouse cells. Biochem Biophys Res Commun 2021;549:91-7.
- Lee S, Kim S, Chung H, Moon JH, Kang SJ, Park CG. Mesenchymal stem cell-derived exosomes suppress proliferation of T cells by inducing cell cycle arrest through p27kip1/Cdk2 signaling. Immunol Lett 2020;225:16-22.
[Crossref] [Google Scholar] [Pub Med]
- Liu B, Liu L, Zang A, Song Z, Yang H, Wang Z, et al. Tanshinone IIA inhibits proliferation and induces apoptosis of human nasopharyngeal carcinoma cells via p53-cyclin B1/CDC2. Oncol Lett 2019;18(3):3317-22.
[Crossref] [Google Scholar] [Pub Med]
- Wu T, Xu JC, Nan KH, Pei GX. Icariin enhances proliferation and osteogenic differentiation of goat bone marrow mesenchymal stem cells. J Clin Rehabilitative Tissue Eng Res 2009;13:3725-9.
- Liu CS, Shen MJ, Han XF. Effects of salidroside on UVB-radiated HaCaT cell activity and caspase-3/9 protein expression. J Tradit Chin Med Univ Hunan 2019;8:952-6.
- Zheng ML, Luo DL, Ruan SW. Protective effect of Yishen Jiangzhuo granule on kidney of chronic renal failure and its effect on renal free radical, renal tubular caspase-3 and caspase-9 expression. Chin J Tradit Chin Med Pharm 2019;34(8):118-22.