- *Corresponding Author:
- R. Chodankar
Department of Pharmaceutical Chemistry, Goa College of Pharmacy, Goa University, Panaji, Goa 403001, India
E-mail: chodankar.rahul@gmail.com
| Date of Received | 04 September 2022 |
| Date of Revision | 19 June 2023 |
| Date of Acceptance | 20 March 2024 |
| Indian J Pharm Sci 2024;86(2):599-605 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The safety of the drug impurities and their admissible limits in the drug formulations have taken centre stage in drug regulatory affairs. Various drug regulatory bodies, like the International Conference on Harmonization and Food and Drug Administration have taken a serious view on drug impurities, and have tried to address them by issuing various guidelines. The research work presented in the manuscript addresses the above mentioned issue for the degradation products of the drug haloperidol by their toxicity profiling. Haloperidol was stressed under the International conference on harmonization specified stress conditions to assess its stability. It degraded under oxidative stress condition to form two degradation products while, being stable under other conditions viz. hydrolytic, thermal and photolytic. The drug and degradation products were separated by using high performance liquid chromatography by a method consisting of HiQ sil C18 column (250 mm×4.6 mm and 5 µm) using acetonitrile, ammonium formate (10 mM, pH adjusted to 3.7 with formic acid) and triethylamine in the ratio of 40:60:0.1 (v/v/v). The flow rate and the detection wavelength used were 1 ml/min and 246 nm, respectively. The degradation products were identified as trans-haloperidol N-oxide and cis-haloperidol N-oxide by using tandem mass spectrometry. The absorption, distribution, metabolism, excretion, toxicity and the mutagenic potential of the degradation products were studied by using in silico tools like the pKCSM webserver, Toxtree and the OSIRIS property explorer. Apart from inhibiting a few specific isoforms of the cytochrome P450 system, both the degradation products were predicted not to pose any health hazard (hepatotoxic and mutagenic potential).
Keywords
Haloperidol, stress studies, degradation products, mass spectrometry, in silico toxicity
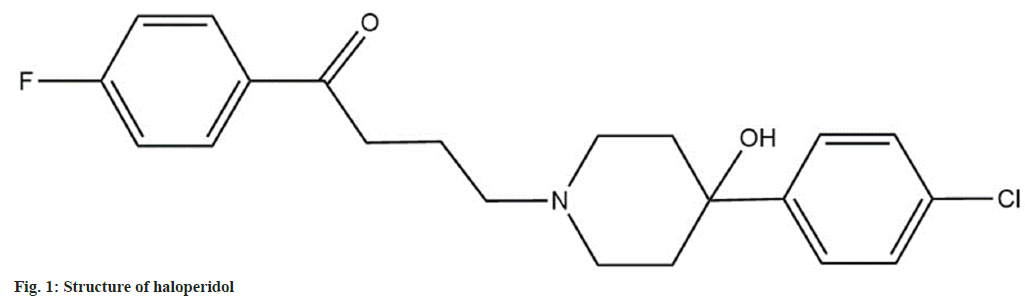
Haloperidol is categorized as a neuroleptic drug and is employed to treat psychotic disorders. Chemically haloperidol is a butyrophenone derivative having the chemical name 4-[4-(4-chlorophenyl)-4- hydroxypiperidin-1-yl]-1-(4-fluorophenyl) butan-1- one. The structure of haloperidol is as seen in fig. 1[1]. Haloperidol is an official drug in the United States Pharmacopeia and National Formulary and British Pharmacopoeia[2,3]. It is also mentioned in Clark’s analysis of drugs and poisons[4]. The most preferred route of administration for haloperidol is intravenous and intramuscular. The bioavailability of the drug after oral administration ranges in between 60 %-65 %. Haloperidol is mainly metabolized by reduction to form reduced haloperidol, which does not exhibit any biological activity[5,6]. A literature survey was carried out to find out the various analytical methods available for the estimation of haloperidol. There were a few High-Performance Liquid Chromatography (HPLC) assay methods (stability-indicating and conventional)[7-10] and bioanalytical methods[11-13] for analysis of haloperidol in dosage forms and plasma respectively. None of the work cited above has performed extensive stability testing on haloperidol under the International Council for Harmonisation (ICH) mandated stress conditions. Further no in silico testing for the Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) of haloperidol or its Degradation products (Dps) have been carried out. The work presented in the manuscript focuses on a systematic approach for developing stability- indicating assay method, identifying Dps by using Liquid Chromatography-Mass Spectrometry (LC- MS) and Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) studies and predicting the in silico ADMET properties of the Dps to establish the safety of the drug.
Materials and Methods
Haloperidol injections (Serenace®) were bought from a local pharmacy. Concentrated Hydrochloric acid (HCL), ammonium formate and 15 % hydrogen peroxide from Molychem, Thane, Mumbai, Maharashtra, India. Acetonitrile (ACN) was bought from Qualigens, Mumbai, Maharashtra, India. Sodium hydroxide (NaOH) and HPLC grade water from Finar, Ahmedabad, Gujarat, India. HPLC grade water was also prepared (in house) by using the double distillation assembly.
Instrumentation:
HPLC instrument (LC-4000) manufactured by Jasco, Japan was used for the HPLC method development. It was made up of the following modules; a column temperature controller (CO-4061), a quaternary pump with in-line degasser (PU- 4180), an autosampler (AS-4050) and a diode array detector (MD-4010). The instrument control and data analysis was handled through the Jasco chromNAV (version 2.01.06) data acquisition software installed on Acer workstation computer running a Windows 7 professional operating system. The LC-MS/MS analysis was done on Agilent’s 1200 series HPLC system coupled with 6200 series Quadrupole Time-of-Flight (QTOF) as well as 6400 series Triple Quadrupole Mass Spectrometer (TQMS) detector.
The other instruments used were a constant temperature water bath (Cintex, 326, Mumbai, India); hot air oven (Universal, Ambala, India); photostability chamber (Newtronics Lifecare Pvt. Ltd., Mumbai, India); bath sonicator (Citizen, Vadodara, India); precision balance (Wensar digital, Chennai, India) and double distillation apparatus (Bhanu scientific instruments, Bangalore, India).
Forced degradation studies:
The drug product was stressed in accordance to the ICH guideline Q1A(R2)[14], Q1B[15] and previous empirical experience[16]. Haloperidol stock solution (1000 µg/ml) was prepared by accurately transferring the drug formulation (injection) equivalent to 25 mg of haloperidol into a 25 ml volumetric flask and making up the volume with ACN.
Hydrolytic degradation:
The acid hydrolysis was first carried out with 0.1 N HCl at room temperature. The concentration of the acid was increased to 1 N for the subsequent trials at room temperature. 1 ml of stock solution was transferred to 10 ml calibrated volumetric flask, 0.1 ml of stressor was added to this and kept aside for 48 h. To facilitate the formation of the Dps, the drug along with the stressor (1 N HCl) was heated on a constant temperature water bath at 70° for 7 h. Following the stress period, the samples were neutralized by adding corresponding strength of NaOH and volume was made up with diluent to get desired concentration. The stress samples were then analyzed using the optimized chromatographic settings. For the base hydrolysis, a similar approach was used. The neutral hydrolysis was conducted by exposing the drug solution to water at room temperature for 48 h. The studies were also repeated by heating the drug with water at 70° for 7 h.
Oxidative degradation:
Oxidative degradation was carried out using 15 % hydrogen peroxide at room temperature. To 1 ml of stock solution, 0.1 ml of 15 % hydrogen peroxide was added and kept aside for 48 h. The drug was also heated with 15 % hydrogen peroxide for 7 h on a constant temperature water bath at 70° to increase the likelihood of forming oxidative Dps. After the stress period, the stressed samples were diluted with the diluent to get desired concentration and analyzed by using optimized chromatographic settings.
Thermal degradation:
Thermal degradation was done in liquid state. The drug solution was placed in a sealed ampoule and exposed to a temperature of 70° for 7 d. The stressed sample was diluted with the diluent to get the desired concentration and analyzed by using optimized chromatographic settings.
Photolytic degradation:
To study the effect of light on the stability, the drug solution was exposed to light amounting to 1.2 million lux hours of overall illumination and 200 wh/m2 of integrated near Ultraviolet (UV) energy. After the exposure period, the sample was diluted with diluent to get the desired concentration and analyzed by using optimized chromatographic settings.
Preparation of samples for HPLC analysis:
Haloperidol stock solution was prepared by taking the drug formulation (injection) equivalent to 25 mg of haloperidol in 25 ml volumetric flask. To this, 10 ml of ACN was added and the contents were subjected to sonication for 5 min and the volume was made up with ACN. The diluent was made by mixing ammonium formate (10 mM, pH adjusted to 3.7 with formic acid) and ACN in the ratio of 50:50 (v/v). The stressed samples were diluted with the diluent to produce a concentration of 100 µg/ml of haloperidol.
HPLC method development:
According to the literature survey, the most preferred column for the HPLC analysis of haloperidol was C18. The organic component of the mobile phase used by most of the methods was ACN while and the pH of the aqueous component (mostly phosphate buffer) of the mobile phase was adjusted in between 3-4. The wavelength used for analysis was in between 210-260 nm.
Based on the information gathered through the literature survey HPLC trials were taken by using ammonium formate buffer (10 mM, pH adjusted to 3.7 with formic acid) and ACN using isocratic elution. The diode array detector was set to scan 200-400 nm to determine the ideal wavelength for analysis. The ammonium formate buffer had the advantage of being volatile hence, the developed HPLC method could be easily adopted for mass analysis. The selected pH of the mobile phase resulted in the best system suitability parameters and was within the buffer capacity of ammonium formate.
HPLC method validation:
HPLC method validation was performed as per the ICH Q2 guideline[17].
Specificity and selectivity:
The specificity of the developed HPLC method was expressed in terms of the separation (resolution) of the drug and the Dps and in between Dps. The percentage peak purity value was calculated by the data acquisition software which was used to obtain the selectivity of the method.
Linearity and range:
Linearity of the developed HPLC method was determined in concentration range of 10-110 µg/ ml at five concentration levels. The samples were injected in triplicate at each concentration level and the average peak area was computed. The data collected was studied by using regression analysis.
Precision:
Intra- and inter-day precision of the HPLC method was studied at 10, 50 and 75 µg/ml in triplicate. The standard deviation and percent Coefficient of Variation (CV) were calculated.
Accuracy:
The recovery studies by the standard addition method were used to establish the accuracy of the method and the percentage recovery was computed. The stressed samples were spiked separately with the drug at 80 % (40 µg/ml), 100 % (50 µg/ml) and 120 % (60 µg/ ml) of the target assay concentration (50 µg/ml) and at each level the samples were injected in triplicate.
In silico toxicity studies:
The safety of the drug impurities has taken a centre stage in the drug regulatory affairs worldwide. The drug impurities formed due to drug degradation can have toxic potential or may possess mutagenicity. In silico toxicity studies are convenient way to access toxicity profile and identify any mutagenic alerts in Dps. The pKCSM webserver[18] was used to predict the ADMET properties of the drug, while the Toxtree[19] and the OSIRIS property explorer[20] was used for secondary screening of any mutagenic alerts.
Results and Discussion
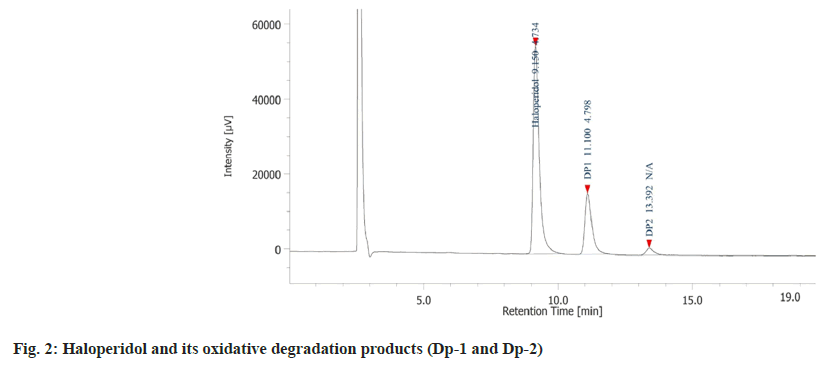
The drug degradation behaviour is depicted in chromatogram (fig. 2). There was formation of a single Dp-1 in presence of 15 % hydrogen peroxide at room temperature for 48 h having retention time of 11.10 min. To further study the formation of the degradation product under oxidative condition, the drug was heated with 15 % hydrogen peroxide at 70° for 7 h. In 2 h, there was formation of another Dp-2 with retention time of 13.3 min. The peak area for Dp-1 was significantly higher than Dp-2 indicating that it was easier to form.
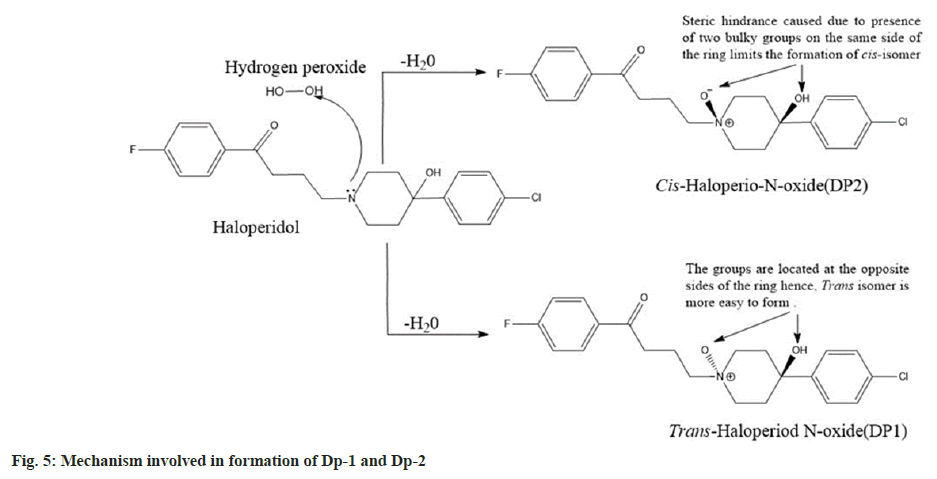
Both the Dps had a molecular mass of 392 which was 16 atomic mass units higher than haloperidol. It is a well-documented that the tertiary nitrogen forms N-oxides[21], and in the case of haloperidol, the piperidine nitrogen gets oxidised to form haloperidol-N-oxide. Further it was clear that there was formation of a pair of geometric isomers (cis and trans haloperidol-N-oxide). In trans isomers the substituents are located on the opposite sides of the rings hence, they are easier to form in comparison to the cis isomers where the substituents are located on the same side of the ring[22] which presents steric hindrance to formation.
Since Dp-1 was formed easily at room temperature it was identified as the trans-haloperidol-N-oxide while, Dp-2 was only produced on heating and hence, it was identified as cis-haloperidol-N-oxide. The drug was found to be stable under other stress conditions like hydrolytic, thermal, and photolytic. The final optimized HPLC method was developed on a HiQsil C18 column (250 mm×4.6 mm and 5 µm) using an isocratic elution technique. The ideal mobile phase combination was found to be ACN:ammonium formate (pH adjusted to 3.7 with formic acid):triethylamine mixed in the ratio of 40:60:0.1 (v/v/v). The flow rate and run time of 1.0 ml/min and 20 min were found to be optimal. Based on the UV scans collected from the trials the ideal wavelength of analysis was found to be 246 nm.
The specificity of the method was ascertained by checking the interference at the retention time of the drug and the Dps from a blank stressed under similar condition to that of the drug. The data acquisition software calculated the peak purity for the individual peaks to be >99 %. The retention time, Relative Retention Time (RRT) and the resolution values are depicted in Table 1.
| Drug/degradation products | Retention Time (RT) (min) | RRT | Resolution |
|---|---|---|---|
| Haloperidol | 9.15 | 0 | 4.7 |
| Dp-1 | 11.1 | 1.2 | 4.7 |
| Dp-2 | 13.39 | 1.5 | 0 |
Table 1: Retention Time, RRT and Resolution of Drug and Dps
The HPLC method developed was found to be linear in concentration range of 10-110 µg/ml. The slope and the correlation coefficient (R2) of the linear regression equation was 25 346 and 0.999 respectively.
The inter- and intra-day precision of the HPLC method was done at 10, 50 and 75 µg/ml in triplicate and the % CV was found to be less than 2 % suggesting that the developed HPLC method possess sufficient reproducibility.
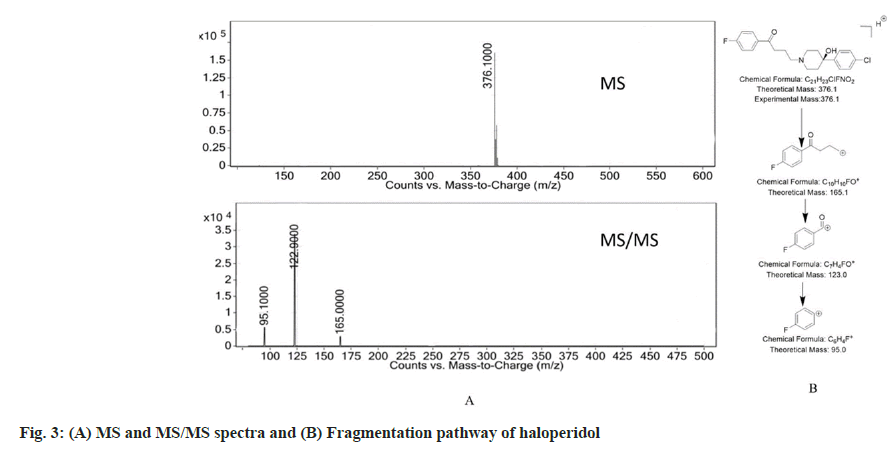
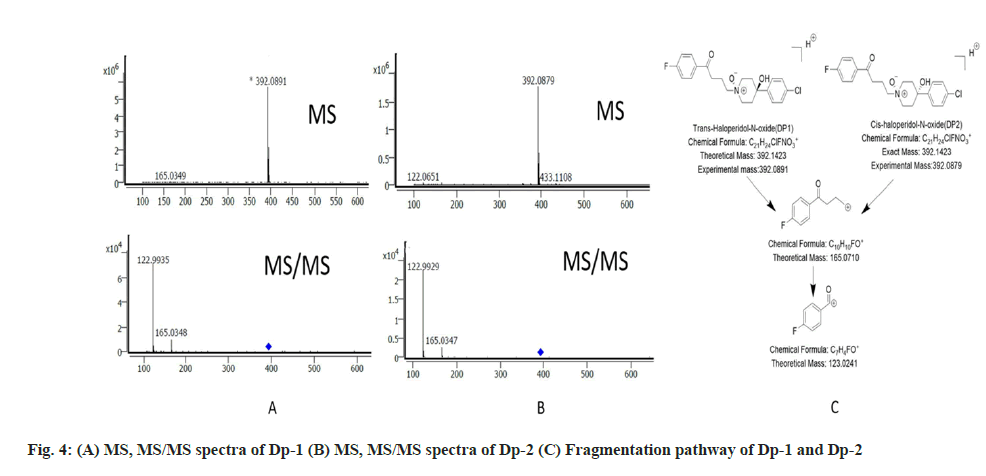
The recovery studies were done at 40, 50 and 60 µg/ ml in triplicate. The recovery at each concentration level as found to be in between 99-101 % with the average recovery of 100.1 %. The drug and the Dps were analysed by using the LC-MS/MS studies and the data thus obtained was used to deduce the most probable structure of the Dps as well as constructing their fragmentation pathway. The LC-MS/MS data for the drug and the Dps are represented in Table 2.
| Compound | Experimental mass | Most likely molecular formulae | Theoretical mass | RDB | Error in mmu | Fragments | Most probable molecular formulae for fragments | |
|---|---|---|---|---|---|---|---|---|
| Experimental mass | Theoretical mass | |||||||
| Haloperidol (TQMS) | 376.1 | C21H23CIFNO2+ | 376.1 | 10 | 0 | 165 | 165.1 | C10H10FO+ |
| 122.9 | 123 | C7H4FO+ | ||||||
| 95.1 | 95 | C6H4F+ | ||||||
| Dp1(QTOF) | 392.0891 | C21H23CIFNO2+ | 392.1423 | 10 | -5.32 | 165.0348 | 165.071 | C10H10FO+ |
| 122.9935 | 123.0241 | C7H4FO+ | ||||||
| Dp2(QTOF) | 392.0879 | C21H23CIFNO2+ | 392.1423 | 10 | -5.44 | 165.0347 | 165.071 | C10H10FO+ |
| 122.9929 | 123.0241 | C7H4FO+ | ||||||
Note: mmu: millimass unit and RDB: Ring Double Bond equivalent
Table 2: LC-MS/MS Data of the Drug and the Dps
Haloperidol has molecular mass of 375.9. The protonated peak of haloperidol captured in positive mode of the electrospray ionization showed a peak at m/z 376.1. The ionization of the piperidine nitrogen followed by an inductive cleavage lead to the formation of fragment with m/z=165 (loss of C11H13ClNO). The fragment with m/z=165 further gets cleaved to produce a secondary carbocation at m/z=122.9 and further loss of CO leads to formation of fragment with m/z=95.1. The MS, MS/MS spectra and the fragmentation pattern of haloperidol is seen
in fig. 3.
The protonated peak of Dp-1 and Dp-2 was seen at m/z value of 392. It had fragmentation like the drug and showed secondary fragments at m/z 165 and 122 respectively. The MS, MS/MS spectra and the fragmentation pathway of Dp-1 and Dp-2 is seen in fig. 4. The mechanism involved in their formation is depicted in fig. 5.
The pKCSM webserver was used to compute the various ADMET properties of haloperidol and its Dps. The drug and the Dps were predicted to possess intestinal absorption above 89 %. It was predicted that haloperidol, Dp-1 and Dp-2 acted as substrate of P-glycoprotein (PgP) which serves as a pump keeping out toxins and xenobiotics out of the cell. Haloperidol was predicted to inhibit CYP2D6 and CYP3A4, while Dp-1 and Dp-2 were predicted to inhibit CYP2C19, CYP2C9 and CYP3A4. Haloperidol was anticipated to possess hepatotoxic potential while Dp-1 and Dp-2 were devoid of it. None of them possessed Ames’s toxicity. The summary of the various ADME properties predicted by the pKCSM is depicted in Table 3.
| Compound | Intestinal absorption (% absorbed) | PgP substrate | BBB permiability (log BB) | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor | Hepatotoxicity | Ames toxicity |
|---|---|---|---|---|---|---|---|---|---|---|
| Haloperidol | 89.5 | 0.012 | 0.112 | No | No | No | Yes | Yes | Yes | No |
| Dp1 | 91.1 | -0.144 | -0.144 | No | Yes | Yes | No | Yes | No | No |
| Dp2 | 91.1 | -0.144 | -0.144 | No | Yes | Yes | No | Yes | No | No |
Table 3: Summary of pKCSM Prediction
The mutagenic potential of the drug and the Dps were further screened through Toxtree and OSIRIS property explorer. Both indicated that neither the drug nor the Dps possess alerts for mutagenicity further OSIRIS property explorer cleared them for any tumorigenic, irritant, and reproductive risks.
Acknowledgments:
The author wishes to thank the Director of the Technical Education, Goa, and the Goa College of Pharmacy for their support.
Conflict of interests:
The authors declare no competing interests.
References
- Hoja H, Marquet P, Verneuil B, Lotfi H, Dupuy JL, Penicaut B, et al. Determination of haloperidol and its reduced metabolite in human plasma by liquid chromatography-mass spectrometry with electrospray ionization. J Chromatogr B Biomed Sci Appl 1997;688(2):275-80.
[Crossref] [Google Scholar] [PubMed]
- United States Pharmacopeia 34 National Formulary 29. The United States Pharmacopeial Convention, Rockville, MD, 2010:3795.
- British Pharmacopoeia. The stationery office, London. 2012:1073-4.
- Moffat AC, Osselton MD, Widdop B, Watts J. Clarke's analysis of drugs and poisons. London: Pharmaceutical press; 2011.
- Froemming JS, Lam YF, Jann MW, Davis CM. Pharmacokinetics of haloperidol. Clin Pharmacokinet 1989;17:396-423.
[Crossref] [Google Scholar] [PubMed]
- Tyler MW, Zaldivar-Diez J, Haggarty SJ. Classics in chemical neuroscience: haloperidol. ACS Chem Neurosci 2017;8(3):444-53.
[Crossref] [Google Scholar] [PubMed]
- Syamala M, Angalaparameswari S, VimalakKannan T, Sumanjali C, Jyotshna T. Stability indicating method development and validation for the determination of haloperidol and benzhexol by RP-HPLC. Int J Res Pharm Chem Anal 2019;1(2):52-8.
- Yasir M, Singh Sara UV, Som I, Singh L. Development and validation of a new HPLC method for in vitro studies of haloperidol in solid lipid nanoparticles. J Anal Bioanal Tech 2016;7:339.
- Gadhavi R, Patel J. Development and validation of stability indicating assay method of haloperidol in oral solution. JPSBR. 2014;4:319-29.
- Wate SP, Borkar AA. RP-HPLC estimation of haloperidol and trihexyphenidyl in tablets. Int J Chem Tech Res 2009;1(6):675-6.
- Jain T, Bhandari A, Ram V, Sharma S, Chaudhary RK, Parakh M. High-performance liquid chromatographic method with diode array detection for quantification of haloperidol levels in schizophrenic patients during routine clinical practice. J Bioanal Biomed 2011;3:1-6.
- Aboul‐Enein HY, Ali I, Hoenen H. Rapid determination of haloperidol and its metabolites in human plasma by HPLC using monolithic silica column and solid‐phase extraction. Biomed Chromatogr 2006;20(8):760-4.
[Crossref] [Google Scholar] [PubMed]
- Ebrahimzadeh H, Dehghani Z, Asgharinezhad AA, Shekari N, Molaei K. Determination of haloperidol in biological samples with the aid of ultrasound‐assisted emulsification microextraction followed by HPLC‐DAD. J Sep Sci 2013;36(9-10):1597-603.
[Crossref] [Google Scholar] [PubMed]
- ICH Q1A, Stability testing of new drug substances and products. In: Proceedings of the International Conference on Harmonisation, Geneva: ICH Harmonised Tripartite Guideline; 1993.
- ICH Q1B, stability testing: photostability testing of new drug substances and products In: Proceedings of the International Conference on Harmonisation, Geneva: ICH Harmonised Tripartite Guideline; 1996.
- Chodankar RS, Mahajan AA. Characterization and In-silico toxicity prediction of degradation products of felbamate. Future J Pharm Sci 2021;7:1-0.
- ICH Q2 (R1) Validation of analytical procedures: Text and methodology. In: Proceedings of the International Conference on Harmonization. Geneva: ICH Harmonised Tripartite Guideline; 2005.
- Pires DE, Blundell TL, Ascher DB. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem 2015;58(9):4066-72.
[Crossref] [Google Scholar] [PubMed]
- Patlewicz G, Jeliazkova N, Safford RJ, Worth AP, Aleksiev B. An evaluation of the implementation of the Cramer classification scheme in the Toxtree software. SAR QSAR Environ Res 2008;19(5-6):495-524.
[Crossref] [Google Scholar] [PubMed]
- Sander T, Freyss J, von Korff, Reich JR, Rufener C. OSIRIS, an entirely in-house developed drug discovery informatics system. J Chem Inf Model 2009;49(2):232-46.
[Crossref] [Google Scholar] [PubMed]
- Baertschi SW, Alsante KM, Reed RA, editors. Pharmaceutical stress testing: Predicting drug degradation. CRC Press; 2016.
- Smith J. Organic chemistry. 1st ed. McGrawHill higher education; 2006.