- *Corresponding Author:
- Jianhua Zhang
School of Pharmaceutical Science, Standardization of Chinese Medicine Research Laboratory, Zhejiang Chinese Medical University, Hangzhou 311402, China
E-mail: zhangjh311@163.com
| Date of Received | 10 April 2021 |
| Date of Revision | 18 May 2022 |
| Date of Acceptance | 08 September 2022 |
| Indian J Pharm Sci 2022;84(5):1241-1247 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of the present study was to identify the absorbed components of raw fuzi in a rat adjuvant arthritis model and evaluate the therapeutic effects of three alkaloids of fuzi’s absorbed components, aconitine, hypaconitine and mesaconitine. The adjuvant arthritis model was established by complete Freund’s adjuvant injection in Wistar rats. Then the animal’s body weight, condition of fur, hind paw volume and immunological parameters, nitric oxide and tumor necrosis factor-alpha, were assessed as markers of inflammation and arthritis. Serum samples from rats treated with oral fuzi extracts were analyzed by ultra performance liquid chromatography-mass spectrometric and 12 prototypes of fuzi were identified. Aconitine and mesaconitine could substantially reduce the serum levels of nitric oxide and tumor necrosis factor-alpha in the adjuvant arthritis model, while hypaconitine did not show obvious effects. The method for the identification of absorbed components of raw fuzi was simple and sensitive. Among the alkaloids, aconitine and mesaconitine might be the major compounds from diester-diterpenoid alkaloids for the treatment of rheumatoid arthritis. The current study connected serum pharmacochemistry study of fuzi with pharmacological experiment in adjuvant arthritis model and discovered the effective substance of fuzi, which could provide plausible evidence for its using, a candidate treatment for rheumatoid arthritis.
Keywords
Raw fuzi, serum pharmacochemistry, absorbed components, adjuvant arthritis, screening of effective substances
Rheumatoid Arthritis (RA) is a chronic autoimmune disease, characterized by synovial inflammation, damage to the cartilage and bone erosion in the joints[1-3]. A great number of potent biologic antiarthritic drugs, including Tumor Necrosis Factor-Alpha (TNF-α) antagonist and Janus kinase inhibitors, have been used for the treatment of RA over the past decades[3-5]. However, current drugs are not only expensive, but they have limitations due to severe adverse reactions such as cellulitis, tuberculosis and fungal infection[5,6]. Therefore, a new therapeutic candidate, safer, more effective but cheaper, is urgently needed in the developing country such as China[6].
Recent studies have shown that Traditional Chinese Medicine (TCM) could effectively suppress bone destruction of RA and their effects could be better than those treatment using Western medicine only[6,7]. Fuzi, the lateral root of Aconitum carmichaelii Debx., is such a TCM commonly used to treat chronic arthritis, especially RA[8-12]. It is reported that fuzi could significantly inhibit tissue swelling in RA rat and reduce the gene expression levels of cytokines, like TNF-α, interleukin-1, Nitric oxide (NO) and interferongamma[ 11-13].
Serum Pharmacochemistry (SPC) is a scientific approach to study the effective material basis for TCM[14-17]. The studies of SPC have suggested that only constitutions absorbed in blood could be considered as effective ingredients[15-17]. The metabolic processes of drugs in vivo could be individually different between pathological and normal conditions. In order to make the experimental results reflect the effective material basis of TCM truly, researches on SPC should be carried out under a pathological condition[16,17].
Our previous study has already discovered 12 absorbed components of raw fuzi in the normal rats. Therefore, in the present study, a method of SPC with Ultra Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS) was developed to investigate the absorbed components of fuzi in rats with Adjuvant Arthritis (AA) after oral administration of fuzi extract and therapeutic evaluation of raw fuzi in AA rats was performed.
Materials and Methods
Chemical and Reagents:
The reference standards of Aconitine (AC) (Batch No.:110 720-201 002), Mesaconitine (MA) (Batch No.:110 722-201 006) and Hypaconitine (HA) (Batch No.:110 721-201 009) were supplied by the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The reference standards of Benzoylaconitine (BAC) (Batch No.:BW6989), Benzoylmesaconine (BMA) (Batch No.:BW6990) and Benzoylhypacoitine (BHA) (Batch No. BW6991) were purchased from Yip Reese Biological Products Co. Ltd. (Beijing, China). The purities of the 6 chemicals were over 98 %. Acetonitrile and methanol of High Performance Liquid Chromatography (HPLC) grade were purchased from Merck (Darmstadt, German). Other commercial reagents were of analytical grade. The distilled water was purified using purification system (ELGA, Shanghai, China).
Raw product of fuzi was collected from Jiangyou (Sichuan, China) and authenticated by Professor Zhang Ru-Song from Zhejiang Chinese Medical University (Hangzhou, Zhejiang, China). Complete Freund’s Adjuvant (CFA) was purchased from Sigma (St. Louis, Missouri, United States of America (USA)). TNF-α Enzyme-linked Immunosorbent Assay (ELISA) kit was supplied by Multi Sciences Biotech Co. Ltd (Hangzhou, China). NO ELISA kit was purchased from Nanjing Jiancheng Bioengineering Institution (Nanjing, China).
Animals:
Male Wistar rats weighing 160-200 g were purchased from Shanghai Laboratory Animal Center of Chinese Academy of Sciences (Shanghai, China; Specific- Pathogen Free (SPF) grade; Certificate No. SCXK2008- 0016). The animals were kept in an environmentally controlled breeding room (temperature of 22°±1°, relative humidity of 60 %±10 % and a 12/12 h light/ dark cycle) with at least 1 w acclimatization before experiments. All animals were fed with rodent laboratory chow and tap water ad libitum. All the experimental procedures were done in strict accordance with the China legislation on the use and care of laboratory animals and with the guidelines drawn up by the Institution Animal Ethics Committee and Committee for the Purpose of Control and Supervision of Experiments on Animal in China (Regulations for the Administration of Affairs concerning Experimental Animals).
Analysis of the absorbed components of fuzi in AA rat model:
The induction of AA: 40 Wistar rats were divided randomly into model group and normal control group. Each rat in the model group was injected with 0.1 ml of CFA intradermally into the left hind metatarsal footpad to establish the AA model. Physiological parameters, such as body weight, condition of fur, Hind Paw Volume (HPV) were measured. After 14 d of feeding, 6 rats in the model group were chosen randomly and their blood samples were collected for ELISA analysis of NO and TNF-α to confirm the establishment of the AA rat model[18-21].
Then the AA rats were divided into two groups, model control group and treatment group. Rats in the treatment group were given fuzi extract orally at a daily dose 10 ml/kg for 3 d. The model control group was given an equal volume of distilled water.
Preparation of fuzi extract: The powder of raw fuzi were soaked for 30 min in 50 % alcohol (1:10 w/v) and extracted in an ultrasound for 30 min at 40°. Subsequently, the mixture was filtered. After removing ethanol under reduced pressure at 50°, the residue was diluted with distilled water to a concentration of 0.3 g/ ml.
Preparation of standard solutions: The stock solutions, approximately 1.0 mg/ml, were prepared in 0.05 % Hydrochloric acid (HCl) methanol and further diluted to obtain the appropriate working solutions.
Collection and preparation of serum samples: Blood samples of model control and treatment group were collected at 15 min after the last administration and centrifuged at 3500 rpm for 15 min at 4° to separate serum.
100 μl of fuzi-containing serum or 100 μl blank serum samples were placed in two centrifuge tubes and 300 μl of acetonitrile was added and then vortexes for 30 s. After being centrifuged at 13 000 rpm for 15 min at 4°, the supernatant was separated and evaporated to dryness under a stream of nitrogen. The residue was dissolved in 100 μl of methanol and centrifuged at 13 000 rpm for 15 min at 4°. Finally, the supernatant was used for UPLC-MS analysis.
Instruments and analytical conditions: The UPLC analysis was performed on a waters ACQUITY separation module (Avondale, California, USA) equipped with ultraviolet detector. Chromatographic separation was achieved on an ACQUITY UPLC BEH-C18 column (2.1 mm×100 mm, 1.7 μm i.d) at the column temperature of 30°. The mobile phase was consisted of 0.1 % fomic acid (A)-methanol (B) with a flow rate of 0.2 ml/min. The separation was performed using a gradient program, 10 %-20 % B at 0-0.5 min; 20 %-35 % B at 0.5-10 min; 35 %-50 % B at 10-20 min; 50 %-100 % B at 20-21 min and 100 %-10 % B at 21- 22 min. The detection wavelength was set at 235 nm.
A thermo executive mass spectrometer (Thermo, New York, USA) equipped with an electron spray ionization source was used for mass analysis and detection on a positive-ion mode. Nitrogen was used as desolation gas and the spray voltage was 4.5 kV. The capillary voltage was 35 V for the analytes with capillary temperature at 330°. On the basis of full-scan mass spectra of each analyte, the scanning range was 150-700 m/z.
Screening of the effective substance of fuzi on AA model based on its absorbed components:
Preparation of the administration sample: AC, HA and MA were selected for the in vivo therapeutic efficacy study of fuzi and dissolved in distilled water at concentrations of 0.1828, 0.0755 and 0.0594 mg/ ml analyte, respectively. The concentration levels were about 1/15 of the Lethal Dose 50 % (LD50) of corresponding compounds, respectively.
Experimental design: Another 40 AA Wistar rats were divided into 4 groups, model group, AC group, HA group and MA group. Then each treated group received oral administration of AC, HA and MA at 10 ml/kg, respectively, for 2 w. The model group was given an equal volume of distilled water for 2 w. During this period, physiological parameters were measured again. At the end of the experiment, blood samples were collected for immunological analysis.
Statistical analysis:
The data were expressed as mean±standard deviation and subjected to student t-test. p<0.05 was considered statistically significant. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) 26.0 software (IBM, Chicago, USA).
Results and Discussion
A statistically significant decrease of body weight was observed in the model group, compared with the normal group from 5 d to 14 d after the AA onset (p<0.05; Table 1). This may be due to the fact that the food intake of the model group was less than that of the control rats; the temperature of the inflamed skin rose and the basal metabolism increased, which could also influence the body weight.
7 d after the injection of CFA, the rats in the model group showed symptoms such as mild joint swelling and bruises. During the next 7 d, joint swelling gradually became severe and the right foot (without injection of CFA) of rats started to show secondary joint swelling. The swelling part of the skin became much thicker. HPV is one of the warning signs of arthritis, reflecting both inflammatory and arthritic disorders in AA rats. It was found that from 5 d HPV was dramatically increased and maintained at a high level to the end of the experiment, compared with the normal controls (p<0.05; Table 1).
| Day | Animal number | Body weight (g) | HPV (ml) | ||
|---|---|---|---|---|---|
| Model group | Normal group | Model group | Normal group | ||
| 0 | 40 | 175.6±6.4 | 179.6±9.4 | 1.749±0.133 | 1.128±0.098 |
| 3 | 40 | 181.3±7.5 | 197.3±9.0 | 2.019±0.263 | 1.204±0.069 |
| 5 | 40 | 187.9±7.8* | 206.7±8.2 | 2.032±0.219* | 1.218±0.096 |
| 7 | 40 | 201.0±9.2* | 218.8±7.3 | 2.281±0.328** | 1.351±0.134 |
| 10 | 40 | 201.6±7.7* | 223.5±7.4 | 2.120±0.183* | 1.145±0.117 |
| 14 | 40 | 207.2±5.1* | 237.7±9.3 | 2.222±0.552** | 1.243±0.080 |
Note: Compared with the normal group, *p<0.05 and **p<0.01
Table 1: Comparison of Body Weight and HPV between Normal Group and Model Group (x̄±s)
As shown in Table 2, the serum levels of NO and TNF-α in the model group were significantly higher than that of the normal group (p<0.01). All have proved AA model has been successfully established.
| Group | Animal number | NO (ng/ml–1) | TNF-α (ng/ml–1) |
|---|---|---|---|
| Normal group | 6 | 16.952±0.694 | 130.42±5.40 |
| Model group | 6 | 19.856±0.588** | 141.58±8.64** |
Note: Compared with normal group, **p<0.01
Table 2: Comparison of NO and TNF-Α Expression between Normal Group and Model Group (x̄±s)
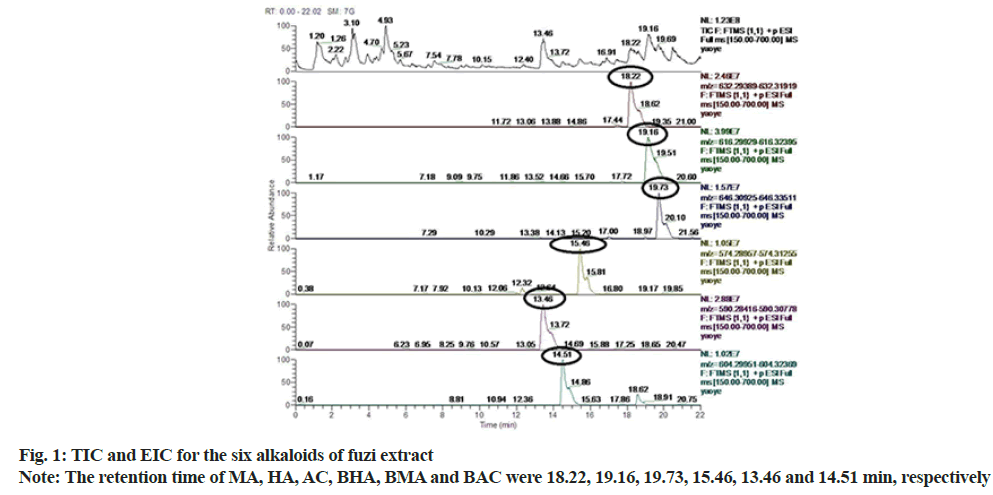
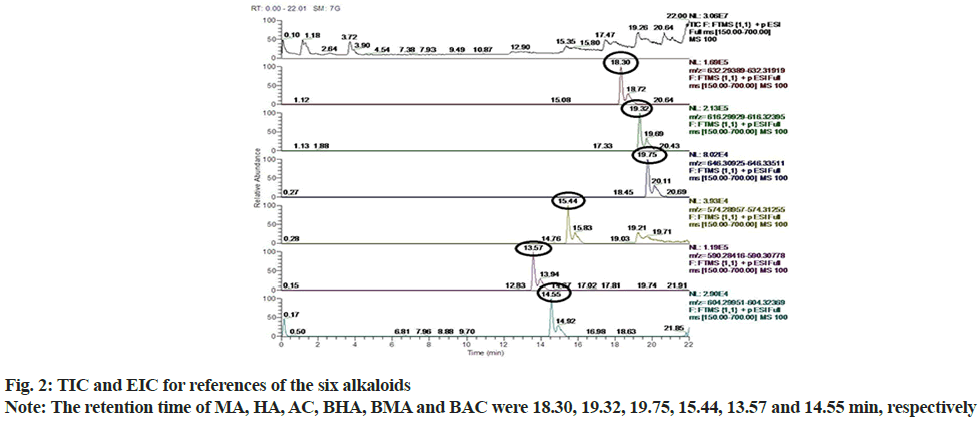
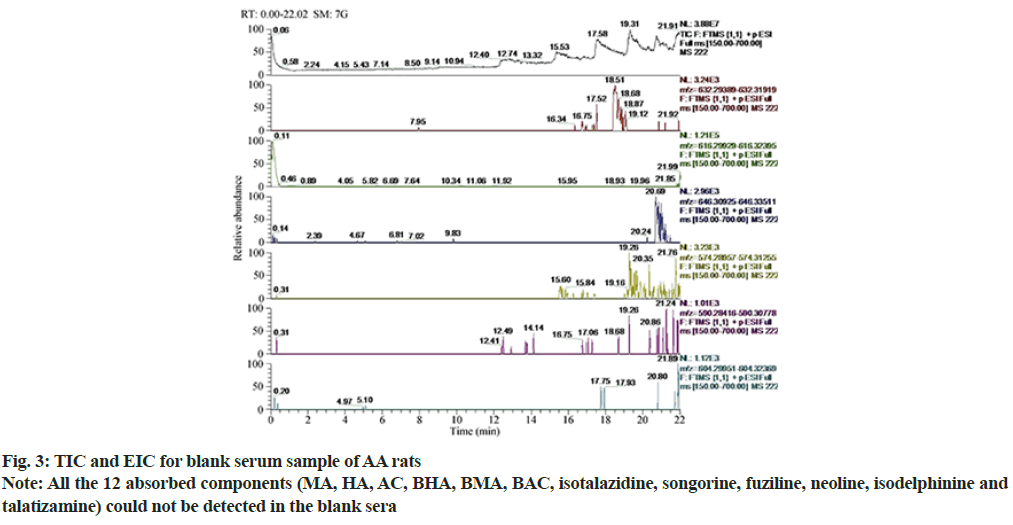
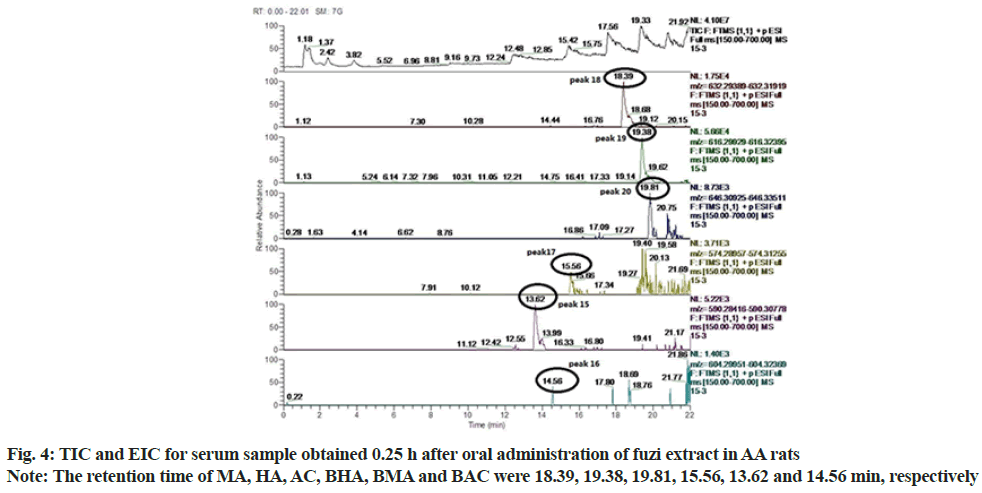
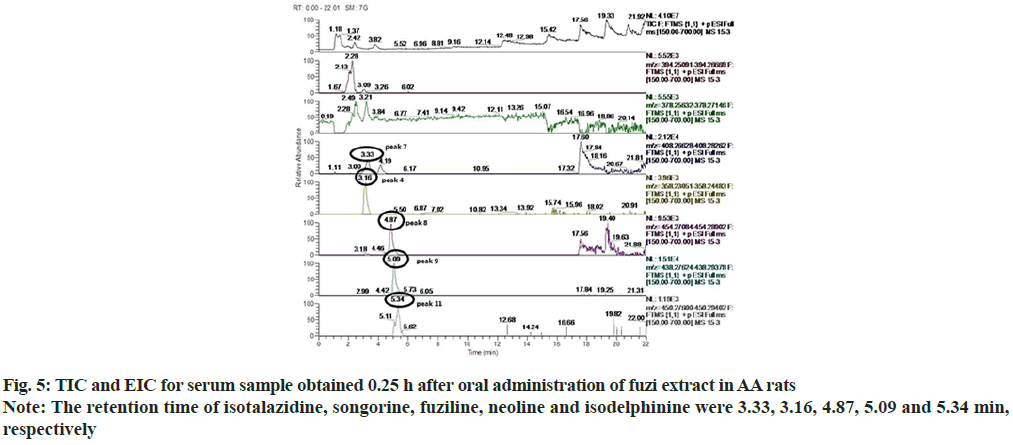
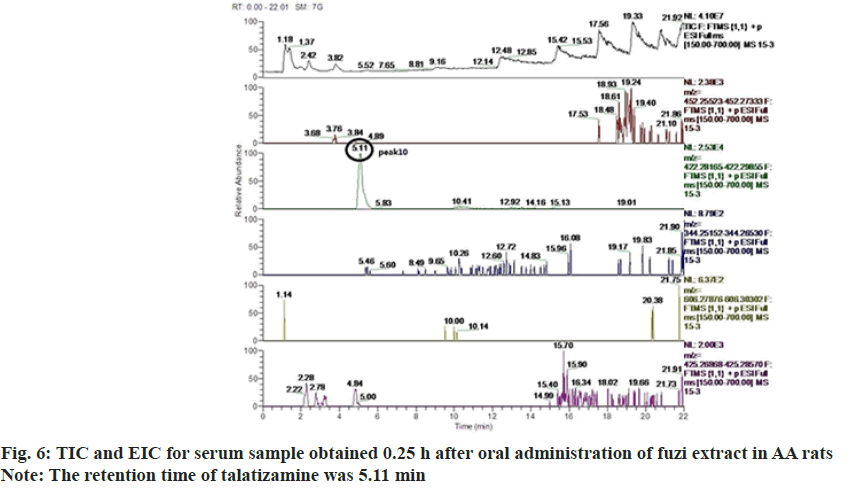
According to Total Ion Chromatogram (TIC) and Extracted Ion Chromatogram (EIC) of fuzi extract, the reference of six alkaloids, the blank serum of AA rats and the fuzi-containing serum of AA rats, 12 prototype components were found in the fuzi-containing serum of AA rats and fuzi extract but not in the blank serum of the AA rats (fig. 1-fig. 6). The absorbed components are shown in Table 3. AC, HA, MA, BAC, BHA, BMA were identified by comparing their retention times and MS spectra with those of the reference compounds. Isotalazidine, songorine, fuziline, neoline, isodelphinine and talatizamine, were identified with reference to the literature data.
| Analytic (peak number) | Retention time (min) | m/z [M+H]+ | Chemical formula |
|---|---|---|---|
| Songorine | 3.16 | 358.231 | C22H31NO3 |
| Isotalazidine | 3.33 | 408.266 | C23H37NO5 |
| Fuziline | 4.87 | 454.271 | C24H39NO7 |
| Neoline | 5.09 | 438.276 | C24H39NO6 |
| Talatizamine | 5.11 | 422.282 | C24H39NO5 |
| Isodelphinine | 5.34 | 450.276 | C25H39NO6 |
| AC | 19.81 | 646.309 | C34H47NO11 |
| HA | 19.38 | 616.299 | C33H45NO10 |
| MA | 18.39 | 632.294 | C33H45NO11 |
| BAC | 14.56 | 604.3 | C32H45NO10 |
| BHA (peak 17) | 15.56 | 574.29 | C31H43NO9 |
| BMA (peak 15) | 13.62 | 590.284 | C31H43NO10 |
Table 3: The Retention Time, m/z [M+H]+ and Chemical Formula of the 12 Absorbed Components Containing Serum of AA Rats
A recovery effect was observed for AC and HA treatments in the treated groups; HPV was significantly decreased on 5 d, 9 d and 14 d (p<0.05) (Table 4). However, no significant difference in effect was found for the MA group. The results suggested AC had a rapid absorption and speedy efficacy in AA rats and had significant anti-inflammatory effects; HA, to a certain extent, had anti-inflammatory effect but with slow efficacy and MA showed no obvious effect.
| Group | HPV (ml) | ||||
|---|---|---|---|---|---|
| 1 d | 3 d | 5 d | 9 d | 14 d | |
| Model group | 2.488±0.198 | 2.427±0.099 | 2.574±0.234 | 2.519±0.205 | 2.487±0.387 |
| AC group | 2.109±0.391 | 2.032±0.201 | 1.973±0.139** | 1.910±0.158** | 1.862±0.091** |
| HA group | 2.113±0.391 | 2.314±11.489 | 2.034±0.181** | 2.011±0.081** | 1.928±0.197* |
| MA group | 2.517±0.677 | 2.441±0.367 | 2.396±0.382 | 2.453±0.146 | 2.240±0.210 |
Note: Compared with the model group, *p<0.05 and **p<0.01
Table 4: Effects of AC, HA, MA On HPV of the AA RAT (x̄±s, n=6)
As shown in Table 5, with daily administration of AC, HA and MA, the abnormal serum level of NO decreased significantly and finally attained to normal range. As for serum level of TNF-α, only in AC and HA groups, it had a significant decrease. No significant difference of TNF-α expression was found in MA group. There were no significant differences in anti-inflammatory effects among the three different treatments (p>0.05).
| Group | Animal number | NO (μg/ml-1) | TNF-α (μg/ml-1) |
|---|---|---|---|
| Model group | 10 | 20.29±1.62 | 141.88±5.95 |
| AC group | 10 | 17.07±0.97** | 131.50±7.41* |
| HA group | 10 | 17.58±1.12** | 129.44±11.68* |
| MA group | 10 | 17.83±1.02* | 133.92±8.74 |
Note: Compared with the model group, *p<0.05 and **p<0.01
Table 5: Serum Levels of NO and TFN-α of Treated Groups
TCM are commonly used in the treatment of diseases like RA in China and other Asian countries[6,7]. With the increasing popularity of TCM, it is necessary to find out the effective substance and assure the appropriate use of the herbal medicines in clinic[13,15,16]. Fuzi, as a typical toxic TCM, its therapeutic dose and toxic dose are very close, which attracted great attention and generated a strong research interest for its dual effects, medical efficacy and toxic behavior[8,10,12,17]. SPC is an important approach to discover the pharmacodynamics material basis of TCM herbs. It is believed that the effective substance has to be the ones that can be absorbed into the body. But on the other side, not all the substances absorbed in blood can function effectively[15]. We believe that SPC study should be combined with pharmacological study. So it is necessary to choose the right animal model for SPC study. Because pathological changes in joint tissue and blood of AA rats are quite similar to those of RA patients and the AA model induced by CFA can be easily established, the AA model is used frequently in the studies of therapeutic effects of TCM herbs for the treatment of RA[18,19]. The primary active constituents in fuzi are a series of alkaloids, C19- and C20-diterpenoid alkaloids, which exert the analgesic and anti-inflammatory effects in the treatment of RA[8,10,22]. In the present study, we have detected 12 prototypes in AA rat sera. Compared to our previous study, it is discovered that the number of absorbed components in AA rat serum and normal rat serum are same, both 12. We assume, in spite of the same number of absorbed components in sera of AA rats and normal rats, there may be some differences that we have not noticed yet. Further studies like pharmacokinetic study of the absorbed components should be done.
Since we have detected 12 prototypes of fuzi in AA rat sera, it raises the question that whether all the 12 absorbed components are the effective substances of fuzi in disease-treating such as RA. To answer this question, we conducted a pharmacological experiment to screen the effective substances of fuzi in AA rats. After AA model was developed, a remarkable paw swelling was found, indicating a severe inflammatory response in the joint of the AA rats. To a certain extent, HPV reflects the severity degree of inflammation[19,20]. Meanwhile, essential proinflammatory cytokines, NO and TNF-α, were measured to evaluate the antiinflammatory effect of fuzi.
NO expression level increases in RA patients synovial fluid[23,24]. TNF-α has been reported to play a pivotal role in the pathogenesis and pathophysiology of RA[1,3,25]. Our data have revealed the anti-inflammatory effect exerted by AC and MA. The inhibition of TNF-α can contribute a lot to the therapy of RA. But there is no significant difference in MA group for its therapeutic effect. This finding revealed that AC and HA are more likely to be the effective substances of fuzi in the treatment of RA.
Further, the dose of fuzi extract was investigated. The final dose was determined according to the survival rate of the animals after administration and the component contents of the drug-containing serum. The results showed that when the dose was 1, 0.50 or 0.40 g/ml, all the rats died within 2 h; when the dose was 0.35 g/ml, all the rats died after 3 d of oral administration; when the dose was 0.25 g/ml, only part of the components could be detected in the obtained drug-containing serum. Therefore, 0.3 g/ml fuzi extract was chosen. During our experiment, we did not examine the therapeutic effects of BAC, BHA and BMA. The reason was that all the AA rats died after administration of these three substances. We believe further study of these three alkaloids should be done.
Acknowledgements:
This work was supported by the National Natural Science Foundation of China under Grant No. 81973868 and No. 81273772; Natural Science Foundation of Zhejiang Province under Grant No. LY17H270 011.
Conflict of interests:
The authors declared no conflict of interests.
References
- Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity 2017;46(2):183-96.
[Crossref] [Google Scholar] [PubMed]
- Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS. Rheumatoid arthritis. Nat Rev Dis Primers 2018;4:18002.
- McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017;389(10086):2328-37.
[Crossref] [Google Scholar] [PubMed]
- Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018;320(13):1360-72.
[Crossref] [Google Scholar] [PubMed]
- Sparks JA. Rheumatoid arthritis. Ann Intern Med 2019;170(1):ITC1-6.
- Cai X, Chen XM, Xia X, Bao K, Wang RR, Peng JH, et al. The bone-protecting efficiency of chinese medicines compared with Western medicines in rheumatoid arthritis: A systematic review and meta-analysis of comparative studies. Front Pharmacol 2018;9:914.
- Shi Y, Shu H, Wang X, Zhao H, Lu C, Lu A, et al. Potential advantages of bioactive compounds extracted from traditional Chinese medicine to inhibit bone destructions in rheumatoid arthritis. Front Pharmacol 2020;11:561962.
[Crossref] [Google Scholar] [PubMed]
- Yang M, Ji X, Zuo Z. Relationships between the toxicities of Radix Aconiti lateralis Preparata (fuzi) and the toxicokinetics of its main diester-diterpenoid alkaloids. Toxins 2018;10(10):391.
[Crossref] [Google Scholar] [PubMed]
- Zeng XZ, He LG, Wang S, Wang K, Zhang YY, Tao L, et al. Aconine inhibits RANKL-induced osteoclast differentiation in RAW264. 7 cells by suppressing NF-κB and NFATc1 activation and DC-STAMP expression. Acta Pharmacol Sin 2016;37(2):255-63.
[Crossref] [Google Scholar] [PubMed]
- Nyirimigabo E, Xu Y, Li Y, Wang Y, Agyemang K, Zhang Y. A review on phytochemistry, pharmacology and toxicology studies of Aconitum. J Pharm Pharmacol 2015;67(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Park G, Lee SH, Han JY, Oh DS. Altered TNF-α response by Aconibal® and methotrexate in a lipopolysaccharide-induced setting of inflammatory conditions: Potential on a synergistic combination. J Ethnopharmacol 2018;213:191-7.
[Crossref] [Google Scholar] [PubMed]
- Tong P, Wu C, Wang X, Hu H, Jin H, Li C, et al. Development and assessment of a complete-detoxication strategy for fuzi (lateral root of Aconitum carmichaeli) and its application in rheumatoid arthritis therapy. J Ethnopharmacol 2013;146(2):562-71.
[Crossref] [Google Scholar] [PubMed]
- Xie YF, Feng WW, Liu MC, Xie J, Yu L, Gong XH, et al. Investigation of efficacy enhancing and toxicity reducing mechanism of combination of Aconiti lateralis Radix preparata and paeoniae radix alba in adjuvant-induced arthritis rats by metabolomics. Evid Based Complement Alternat Med 2019;2019:9864841.
[Crossref] [Google Scholar] [PubMed]
- Tashino S. “Serum pharmacology” and “serum pharmaceutical chemistry”: From pharmacology of Chinese traditional medicines to start a new measurement of drug concentration in blood. Ther Drug Monit Res 1988;5:54-64.
- Wang XJ. Progress and future developing of the serum pharmacochemistry of traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi 2006;31(10):789-92, 835.
[Google Scholar] [PubMed]
- Han Y, Sun H, Zhang A, Yan G, Wang XJ. Chinmedomics, a new strategy for evaluating the therapeutic efficacy of herbal medicines. Pharmacol Ther 2020;216:107680.
[Crossref] [Google Scholar] [PubMed]
- Zhang K, Liu C, Yang T, Li X, Wei L, Chen D, et al. Systematically explore the potential hepatotoxic material basis and molecular mechanism of Radix Aconiti lateralis based on the concept of toxicological evidence chain (TEC). Ecotoxicol Environ Saf 2020;205:111342.
[Crossref] [Google Scholar] [PubMed]
- Choudhary N, Bhatt LK, Prabhavalkar KS. Experimental animal models for rheumatoid arthritis. Immunopharmacol Immunotoxicol 2018;40(3):193-200.
[Crossref] [Google Scholar] [PubMed]
- Dekkers JS, Schoones JW, Huizinga TW, Toes RE, Van Der Helm-Van Mil AH. Possibilities for preventive treatment in rheumatoid arthritis? Lessons from experimental animal models of arthritis: A systematic literature review and meta-analysis. Ann Rheum Dis 2017;76(2):458-67.
[Crossref] [Google Scholar] [PubMed]
- Huh JE, Hong JM, Baek YH, Lee JD, Choi DY, Park DS. Anti-inflammatory and anti-nociceptive effect of Betula platyphylla var. japonica in human interleukin-1β-stimulated fibroblast-like synoviocytes and in experimental animal models. J Ethnopharmacol 2011;135(1):126-34.
[Crossref] [Google Scholar] [PubMed]
- Gupta S, Mishra KP, Singh SB, Ganju L. Inhibitory effects of andrographolide on activated macrophages and adjuvant-induced arthritis. Inflammopharmacology 2018;26(2):447-56.
[Crossref] [Google Scholar] [PubMed]
- Wu JJ, Guo ZZ, Zhu YF, Huang ZJ, Gong X, Li YH, et al. A systematic review of pharmacokinetic studies on herbal drug fuzi: Implications for fuzi as personalized medicine. Phytomedicine 2018;44:187-203.
[Crossref] [Google Scholar] [PubMed]
- Thornadtsson A, Lind A, Weitoft T, Högman M. Altered levels of exhaled nitric oxide in rheumatoid arthritis. Nitric Oxide 2018;76:1-5.
[Crossref] [Google Scholar] [PubMed]
- Gul A, Kunwar B, Mazhar M, Faizi S, Ahmed D, Shah MR, et al. Rutin and rutin-conjugated gold nanoparticles ameliorate collagen-induced arthritis in rats through inhibition of NF-κB and iNOS activation. Int Immunopharmacol 2018;59:310-7.
[Crossref] [Google Scholar] [PubMed]
- Huang CC, Chiou CH, Liu SC, Hu SL, Su CM, Tsai CH, et al. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J Pineal Res 2019;66(3):e12560.
[Crossref] [Google Scholar] [PubMed]