- *Corresponding Author:
- S. H. Lim
Industrial Biotechnology Research Laboratory (IBRL), School of Biological Sciences, Universiti Sains Malaysia, Penang, Malaysia
E-mail: limshehhong77@gmail.com
| Date of Submission | 27 Mar 2015 |
| Date of Revision | 17 Feb 2016 |
| Date of Acceptance | 15 Apr 2016 |
| Indian J Pharm Sci, 2016;78(2):273-277 |
Abstract
Lagerstroemia speciosa bark extract was investigated for its pharmacokinetic and pharmacodynamic parameters against two selected pathogenic bacteria by the time-kill curves assay. The brine shrimp lethality test was carried out to determine the toxic level of the extract towards the eukaryotic cells. The extract showed a concentration-dependent killing effect for both B. spizizenii ATCC 6633 and A. anitratus. The number of bacteria was significantly reduced when they were exposed to 2MIC (minimum inhibition concentration) level. Bacteria B. spizizenii ATCC 6633 was more susceptible to the extract as compared to A. anitratus. The extract was found to be nontoxic during the short term (acute) exposure but it was toxic during the prolonged time (chronic) exposure. The LC50 for acute test and chronic test were 3422.68 and 35.30 µg/ml, respectively. This study indicated that the methanol extract of L. speciosa is a promising candidate for the search of natural antimicrobial and anticancer drugs.
Keywords
Acinetobacter anitratus, Lagerstroimea speciosa, cytotoxicity, time-kill curves, minimum inhibitory concentration
Antimicrobial properties from natural products become an interest among researchers to search for alternatives to combat the rising of global antimicrobial resistance problems. There are two main elements of an antimicrobial therapy usually investigated by researcher. They included in vitro time-kill curves study and in vivo toxicity test. These two studies enable the selection of an appropriate dose and dosing regimen of the antimicrobial agent during the therapy.
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of an antimicrobial agent are the two parameters that quantify in vitro antimicrobial activity.
These two static parameters are very useful to evaluate the factual performance of the antimicrobial agents in vitro, which is the time-kill curves study. This study could provide the descriptive information on the kinetics of bacterial killing effects, whether it is concentration-dependent or time-dependent. The discrepancy in bacterial killing effects is due to the interrelationship between the pharmacokinetic and pharmacodynamic factors [1]. The time-kill curves study provides more accurate description on the pharmacodynamics of antimicrobial agents than the MIC [2,3].
The accumulation of antimicrobial drugs and their metabolic byproducts due to repetitive administration can cause unwanted side-effects to tissues and organs [4]. Therefore, in most antimicrobial studies, the toxicity test is needed to provide an indication of possible toxicity level of the antimicrobial agent towards the normal intestinal flora and other organs of the infected host. The mechanisms of antimicrobial toxicity are including direct effects, hypersensitivity, changes in microbial flora, drug interactions and microbial lysis [4]. The toxicity study of new antimicrobial agents is usually performed via the brine shrimp toxicity test [5]. This method has been successfully practiced to detect toxicity level in many samples; heavy metal [6], plant extract [7], various biocides [8].
Lagerstroemia speciosa is a tropical plant found in many countries of Southeast Asia such as Philippines, Vietnam, Malaysia and southern China. L. speciosa plant has been traditionally used for treatment of diabetes and kidney disease [9] at Philipine. Some researchers have reported the present of corosolic acid, an active ingredient in the extract have played an important role in anti-diabetic activity, antiinflammation and antihypertension properties [10,11]. Due to its broad medicinal values, this study is focuses on antimicrobial property of L. speciosa bark extract against pathogenic bacteria B. spizizenii ATCC 6633 and A. anitratus which can cause infection diseases in human. In this work, we reported the effects of the methanol extract of L. speciosa bark against B. spizizenii ATCC 6633 and A. anitratus, and its toxicity property against brine shrimp A. salina.
The powdered sample of L. speciosa was soaked in 800 ml of 100% methanol (ratio 1:20 w/v) for 3 days at room temperature (30±2º). The filtrate was evaporated to dryness using a rotary evaporator (Heidolph, Korea) at 50º and 150 rpm. The concentrated extract was allowed to dry in a fume hood for several days until dark brown paste was obtained, and kept in a fridge at 4º until further use.
The L. speciosa extract was added into 25 ml of nutrient broth in a 50 ml Erlenmeyer flask to achieve concentration of 0.25 mg/ml (MIC for B. spizizenii) and 0.5 mg/ml (MIC for A. anitratus) after addition of 1 ml inoculum (2.5×106 cells/ml). Another two sets of ½MIC, 2MIC were prepared with the same method to Darah et al. [12]. For the blank set, the extract was replaced with 1.0 ml of 98% methanol. The samples were incubated in an incubator shaker at 37º with the agitation of 150 rpm. An aliquot of 1.0 ml of each set was drawn out from each flask aseptically at 0 h and repeated for every 4 h during the 48 h incubation period. Dilutions were carried out prior to the spread plate method. The plates were incubated at 37º for 24 h. Only the plates with 30 to 300 colonies were counted in order to follow the normality law in statistical tests. The growth profiles of B. spizizenii ATCC 6633 and A. anitratus were analyzed by plotting a logarithm of the average number of the viable cells versus time of incubation.
The method for this bioassay was according to Lim et al. [13] with slight modifications. Dissolving 38 g of sea salt in 1 liter distilled water to obtain artificial sea water (ASW). The ASW was filtered twice with Whatmann No. 1 filter paper before approximately 1.0 g of the brine shrimp eggs was added into the ASW. The eggs were then allowed to hatch for 48 h at 30±2°. Continuous oxygen supply and light source were provided to the hatching set. Mature nauplii with approximately uniform size were used in the test. The extract stock of L. speciosa was prepared at 100 mg/ml in absolute dimethyl sulfoxide (DMSO). A set of test samples with different final extract concentrations (0.01, 0.05, 0.1, 0.5 and 1.0 mg/ml) was prepared from the stock extract in the universal bottles containing appropriate volume of ASW. The final volume for each concentration was 5.0 ml. An aliquot of 0.05 ml DMSO was added to 4.95 ml ASW for the control set (final DMSO concentration was 1%).
Ten to fifteen active matured nauplii were carefully transferred into universal bottles. The toxicity of the extract was determined after 12 h for acute toxicity and 24 h for chronic toxicity. The number of surviving nauplii was observed and counted under a light microscope. Movement of nauplii indicates that the nauplii are still alive. The percentage of lethality of the nauplii was calculated with the equation below.
Percentage of lethality (%) = (Number of dead A. salina/ Total number of A. salina)×100
The LC50 value (lethality concentration that causes 50% lethality) was calculated from the linear equation of the graph. Experiments were performed in triplicate.
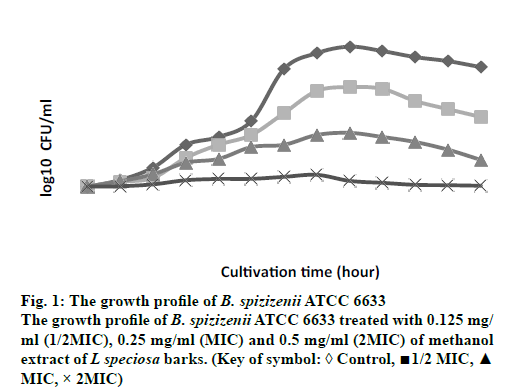
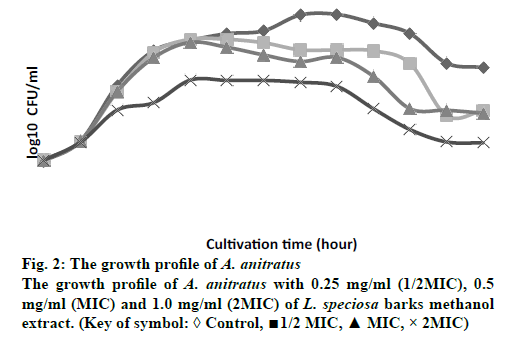
The growth profiles for B. spizizenii ATCC 6633 and A. anitratus that were exposed to the methanol extract at ½ MIC, MIC and 2 MIC for 48 h were determined. In the previous study by Darah et al. [12], these two bacteria were susceptible to the methanol extract of L. speciosa bark. The MIC obtained for B. spizizenii ATCC 6633 was 0.25 mg/ml meanwhile the minimum bactericidal concentration (MBC) was 2.00 mg/ml. As for the A. anitratus the MIC was 0.50 mg/ml and no MBC value was achieved.
Bacteria B. spizizenii ATCC 6633 was treated with the extract showed a dose-dependent pattern in its growth (fig. 1). The graph shows that increment in the extract concentration had resulted in the reduction of the number of viable cells. Generally, the growth pattern for the control, ½ MIC and MIC samples were similar to each other. At the first 20 h, these three samples experienced a gradual increment in the number of viable cells. After the 20 h, the MIC sample started to show slower rise in the bacterial growth. Meanwhile the number of bacterial cells for control and ½ MIC samples continued to grow up steadily until the 28 h. After that the growth was quite stagnant and started to reduce until the end of cultivation period. On the other hand, the 2 MIC sample also showed the presence of viable cells but at a stagnant rate from the 0 h to the 48 h of cultivation time. The number of viable cells at the 48 cultivation hour was 3.95×109 cells/ml, 1.26×107 cells/ml, 8.9×104 cells/ml and 4.5×103 cells/ml for the control, ½ MIC, MIC and 2MIC samples, respectively (data not shown in graph).
Fig. 2 showed the growth profile of A. anitratus was treated with different extract concentrations (½ MIC, MIC and 2MIC). The growth pattern depicted in fig. 2 was also demonstrated a concentration-dependent (time-independent). The graph shows no appreciable change difference in the number of viable cells among all samples during the first eight hours of dosing interval. However, apparent reduction in the number of viable cells was detected at the 12 h for the 2 MIC sample, displayed by the slow growth rate. The killing effect of the extract at ½ MIC and MIC samples started to appear after the 16 h of cultivation as the graph started to decline gradually in relative to the control. The growth profile for 2 MIC sample showed the extract was able to lower the number of cells as compared to the control, ½ MIC and MIC along the cultivation period.
Despite of the concentration-dependent pattern displayed by both bacteria, the gram-positive (B. spizizenii ATCC 6633) and gram-negative bacteria (A. anitratus) have responsed slightly different to the extract treatment. This respond is clearly related with the difference in the structure of the cell wall that each type of bacteria has [14]. In addition to that, the concentration-dependent pattern showed is more likely to be associated with the pharmacokineticpharmacodynamic properties of the plant extract during the antimicrobial therapy [15]. According to Carryn et al. [1] the pharmacokinetics is described as the cellular uptake and disposition of antibiotics whilst pharmacodynamics is related with the intracellular activity of the antibiotics. The killing activity of an antibiotic is initiated with the binding of the drug to its target site of the bacteria. Following the binding at the target site, there will be alterations at the ultrastructural, biochemical or molecular level of the affected bacteria [14].
However, the bactericidal effect of the antibiotic is determined to be either concentration-dependent or time-dependent depends on the concentration or the time of the drug that remains at the binding site during the dosing interval [16]. In the concentration-dependent antibiotic, the rate and extent of bacterial killing of the bacteria will increase with the increment of the drug dosage. In this type of bactericidal effect, the binding sites of the target bacteria will be fully occupied by the drug and subsequently the eradication process will take place. The bacteria will experience a postantibiotic effect (PAE) in which persistent suppression of bacterial growth is achieved in a shorter time [17]. It has been discussed that the efficacy of certain antibiotics could be improved if the bacteria are exposed to the extract concentration that is equal to or greater than the MIC level by ten times [18]. According to Craig [19], the proper dosing regimen of this type of antibiotic is to maximize its concentration with infrequent administration the infected organisms. For example the killing activity of quinolones against some respiratory tract infections (RTI) was proven to be more pronounced when the drug concentration was increased approximately 30 times the MIC level [20]. Antibiotic classes of aminoglycosides and quinolones are both concentration-dependent drugs that elicit their activity against target bacteria by engaging the binding sites and later involve in the alterations to the affected bacteria at the molecular, biochemical or ultrastructural level [19].
In this study, both bacteria B. spizizenii ATCC 6633 and A. anitratus are shown to be effectively eradicated when the concentration of the L. speciosa extract was above the MIC, which was the 2 MIC (fig. 1 and 2). There was discrepancy in the killing kinetics of B. spizizenii ATCC 6633 and A. anitratus. The graph pattern of 2 MIC sample for B. spizizenii ATCC 6633 (fig. 1) was stagnant with no major ascending pattern along the incubation period. However, the pattern for A. anitratus (fig. 2) was different, whereby the line was slightly rose, stagnant and declined. From the results, it can be postulated that B. spizizenii ATCC 6633 was more susceptible as compared to the A. anitratus. The methanol extract possessed instant inhibitory effect against B. spizizenii ATCC 6633 but the effect was delayed for the A. anitratus.
Referring to the previous work by Darah et al., [13] the MBC for B. spizizenii ATCC 6633 was eight times its MIC value. However, in the case of A. anitratus, the MIC result showed no MBC result at 2.0 mg/ml. Therefore, it can be suggested that treating the same B. spizizenii ATCC 6633 and A. anitratus strains with L. speciosa extract with concentrations ≥ 8 MIC could yield a bactericidal result. The bactericidal activity is enhanced as more saturated bioactive compounds could bind and act on the target sites to cause rapid cell death [21]. In addition, the inoculum size of 2 MIC samples for both B. spizizenii ATCC 6633 and A. anitratus at the end of the incubation period were greatly reduced more than 3 log10 as compared to their control sets (data not shown). According to the National Committee on Clinical Laboratory Standards [22], the methanol extract of L. speciosa bark can be categorized as antimicrobial agent with bactericidal effect based on the suppression of bacterial number by or more than 3 log10. The result for concentration-dependent killing effect exerted by L. speciosa extract is corroborates with the study done by Surasak et al. [23] who showed that the bactericidal effect was achieved after the Streptococcus pyogenes NPRC 101 was treated with eight times MIC with the ethanol extract of Rhodomytrus tomentosa leaf. The strain was not completely eliminated when the extract concentration used were at 2 MIC and 4 MIC level.
To evaluate the potential of toxicity level of the methanol extract of L. speciosa barks, the toxicology of the extract were screened with brine shrimp lethality bioassay (BSL). This preliminary screening was carried out by researchers to detect toxins from fungus, heavy metals, pesticide, plant extracts and pesticide [24-26]. In this experiment, the final concentration of 1.0% of DMSO was used as the negative control in order to minimize toxic effect of the DMSO towards the brine shrimp.
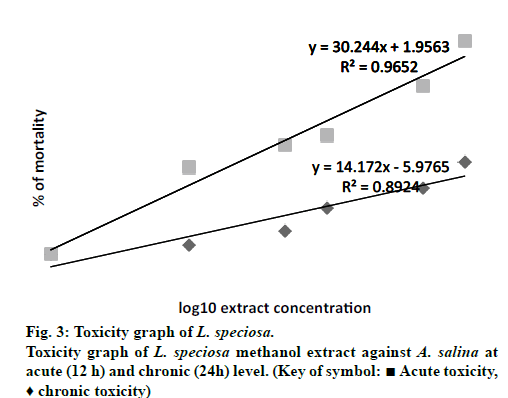
Fig. 3 depicted the acute and chronic toxicity level of L. speciosa methanol bark extract towards the A. salina at different concentrations. In this experiment, a final concentration of 1.0% of DMSO was used as the negative control. The results (data not shown) displayed that 1.0% of DMSO was not lethal towards the brine shrimp as it did not caused any lethality to the brine shrimps for both acute (12 h) and chronic (24 h) tests. From the linear equations, the LC50 for the acute and chronic toxicity was 3422.68 and 35.30 μg/ml, respectively. Based on the results, it was indicated that the time exposure affect the concentration of the methanol extract to exert toxicity affect. According to Sasidharan et al. [27] high toxic effect was achieved during the chronic toxicity test, when the brine shrimps were exposed to the extract for a longer period. Author Simionatto et al. [28] and Déciga-Campos [29] stated that any crude or fractions of plant extracts that possessed LC50 value that is equals to or more than 1000 μg/ml (LC50 ≥ 1000 μg/ ml) is referred as non-toxic towards the brine shrimp. Thus in this study, the LC50 value for acute toxicity is considered as not toxic with LC50 value more than 1000 μg/ml.
The low LC50 (< 1000 μg/ml) value of 35.30 μg/ml obtained for the chronic toxicity (24 h) in this study is in line with the study carried out by Fatema et al. [30] using separated L. speciosa bark extract. The LC50 obtained by Fatema et al. [30] was 72.06 μg/ml. There were also cytotoxic tests for L. speciosa leaves and fruits alcoholic extracts using the brine shrimp as the animal models. The LC50 recorded for the leaves was 9.602 μg/ml whilst the fruits was 60.00 μg/ml [30]. From these collective results, it can be predicted that all parts of the L. speciosa plant including the bark are toxic to living cells. Thus, significant cytotoxic effects of L. speciosa methanol bark exhibited by the present experiment indicates that it can be selected for further cell line assay. Many scientific reports have shown a correlation between cytotoxicity and activity against brine shrimp nauplii [31]. These further studies are needed to warrant the medicinal value of L. speciosa bark extract which could then be used in pharmaceutical industries.
Financial Assistance
This work was supported by a research grant provided by Universiti Sains Malaysia.
Conflict of Interests
None declared.
References
- Carryn S, Chanteux H, Seral C, Mingeot-Leclercq MP, Bambeke FV, Tulkens PM. Intracellular pharmacodynamics of antibiotics. Infect Dis Clin N Am 2003;17:615-34.
- Mueller M, Pena AD, Derendorf H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob Agents Chemother 2004;48:369-77.
- Tam VH, Schilling AN, Nikolaou M. Modelling time-kill studies to discern the pharmacodynamics of meropenem. J Antimicrob Chemother 2005;55:699-6.
- Mandell L, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001;32:S72-9.
- Mclaughlin JL, Rogers LL, Anderson EJ. The use of biological assays to evaluate botanicals. Horsham, PA, ETATS-UNIS, Drug Information Association; 1998.
- Fichet D, Radenac G, Miramans P. Experimental studies of impacts of harbor sediments resuspension to marine invertebrates larvae: Bioavailability of Cd, Cu, Pb and Zn and toxicity. Mar Pollut Bull 1998;36:509-18.
- Krishnaraju AV, Rao TVN, Sundaraju D, Mulabagal V, TsayHs, Subbaraju GV. Assessment of bioactivity of Indian medicinal plants using brine shrimp (Artemiasalina) lethality assay. Int J ApplSciEng 2005;3:125-34.
- Koutsaftis A, Aoyama I. Toxicity of four antifouling biocides and their mixtures on the brine shrimp Artemiasalina. Sci Total Environ 2007;387:166-74.
- Klein G, Kim J, Himmeldirk K, Cao Y, Chen X. Antidiabetes and anti-obesity activity of Lagerstroemia Speciosa. Evid-based Complement Altern Med 2007;4:401-7.
- Shi L, Zhang W, Zhou Y, Li J, Hu L, Li J. Corosolic acid stimulates glucose uptake via enhancing insulin receptor phosphorylation. Eur J Pharmacol 2008;584:21-9.
- Yamaguchi Y, Yamada K, Yashikawa N, Nakamura K, Haginaka J, Kunimoto M. Corosolic acid prevents oxidative stress, inflammation and hypertension in SHR/NDmcr-cp rats, a model of metabolic syndrome. Life Sci 2006;79:2474-9.
- Darah I, Lai KH, Wong CT, Lim SH. In vitro activity of methanol extract from Lagerstroemia speciosa (Linn. Ex Murray) bark against pathogenic bacteria. J ApplPharmaSci 2013;3:25-30.
- Lim SH, Darah I, Jain, K. Assessment of in vivo and in vitro cytotoxic activity of hydrolysable tannin extracted from Rhizophoraapiculata barks. World J MicrobiolBiotechnol 2011;27:2737-40.
- Harold C. NeuTD, Gootz Z. Antimicribial Chemotherapy. In: Medical Microbiology. 4th ed. University of Texas Medical Branch at Galveston; 1996.
- Barger A, Fuhst C, Wiedemann B. Pharmacological indices in antibiotic therapy. J AntimicrobChemother 2003;52:893-8.
- Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin Infect Dis 2004;38:864-70.
- Craig WA, Gudmundsson S. Post antibiotic effect. In, Lorian V, editor. Antibiotics in laboratory medicine. Baltimore; Williams and Wilkins: 1996, p. 296-329.
- Quintiliani R. Using pharmacodynamics and pharmacokinetics concepts to optimize treatment of infectious diseases. Infect Med 2004;21:219-32.
- Craig W. Rationale for antibacterial pharmacokinetic/pharmacodynamic dosing of mice and men. Clin Infect Dis 1998;26:1-10.
- Turnidge J. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Drugs 1999;58:29-36.
- Lacy MK, Nicolau DP, Nightingale CH, quintilanir R. The pharmacodynamics of aminoglycosides. Clin Infect Dis 1998;27:23-7.
- .Barry AL, Craig WA, Nadler H, Reller LB, Sanders CC, Swenson JM. Methods for Determining Bactericidal Activity of Antimicrobial Agent; Approved Guideline. CLSI document M26-A. Wayne, PA; Clinical Laboratoray Standards Institute: 1999.
- Surasak L, Oliver K, Supayang P, Voravuthi K. Antibacterial activity of Rhodomytrustomentosa (Aiton) Hassk. leaf extract against clinical isolates of Streptococcus pyogenes. Evid-Based Compliment Altern Med 2012;1-6.
- Harwig J, Scott PM, Harwig J, Scott PM. Brine Shrimp (Artemia salina L.) larvae as a screening system for fungal toxins. Appl Microbiol 1971;21:1011-6
- Lumor SE, Diez-Gonzalae F, Labuza TP. Detection of warfare agents in liquid foods using the brine shrimp lethality assay. J Food Sci 2011;76:16-9.
- Zhang Y, Mu J, Han J. Gu X. An improved brine shrimp larvae lethality microwell test method. Toxicol Mech Methods 2012;22:23-30.
- Sasidharan S, Darah I, Noordin J. In vitro antimicrobial activity against Pseudomonas aeruginosa and acute oral toxicity of marine algae Gracilaria changii. New Biotechnol 2010;27:390-6.
- Simionatto E, Porto C, Silva UFD, Squizqni AMC, Dalcol II, Morel AF. Composition and antimicrobial activity of the essential oil from Aloysia sellowii. J Brazilian Chem Soc 2005;16:1458-62.
- Déciga-Campos M, Rivero-Cruz I, Arriaga-Alba M, Castañeda-Corral G, Angeles-López GE, Navarrete A, et al. Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine.J Ethnopharmacol 2007;11:334-42.
- Fatema N, Sabir A, Kamrunnahar. Evaluation of antimicrobial, antioxidant and cytotoxic activities of methanol extracts of Lagerstroemia speciosa leaves and barks. J ApplPharmaSci 2012;2:142-7.
- Martin CG, Saenz MT, Ayuso MJ. Cytotoxic activity of Retamaspaerocarpa. Fitotherapia XVI 1995;66:495-8.