- *Corresponding Author:
- J. Soleimani Rad
Stem Cell Research Center, Department of Anatomical Sciences, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
E-mail: Soleimanirj@yahoo.com
| Date of Submission | 10 January 2017 |
| Date of Revision | 07 October 2017 |
| Date of Acceptance | 25 April 2018 |
| Indian J Pharm Sci 2018;80(3):516-524 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Wound healing is critical to the regeneration of various organs and tissues. Traditional medicines, notably mummy, could serve as an alternative stimulator for the treatment of wounds. Till date, mummy has been used for the modulation of inflammation, articular injuries, and bone fractures, as well as wound healing. This study aims to evaluate the effect of mummy on the proliferation and migration of human adipose-derived stem cells and fibroblasts in single or co-culture in vitro conditions. The effective concentration of mummy substance was determined on fibroblasts and adipose-derived stem cells. The cells were treated with mummy separately or co-cultured over a period of 96 h. In vitro scratch assay was used to monitor cell migration rate. Effectiveness of mummy in inducing proliferation was evaluated by measuring the Ki-67 expression using flow cytometry analysis. Based on the data obtained, mummy was found to generate significant increase in the migration of fibroblasts as compared to untreated control in single culture system. On the other hand, an increased migration rate was recorded for adipose-derived stem cells, but it did not reach significant levels. Interestingly, the migration rate of co-cultured fibroblast and adipose-derived stem cells improved after exposure to mummy compared to matched controls. Incubation of adipose-derived stem cells, but not fibroblasts with mummy profoundly increased the expression of Ki-67 as compared to untreated cells. No significant effects were observed in the co-culture system. These data suggested that mummy altered the dynamics of stem cells and mature fibroblasts in vitro. Distinct cell responses could possibly be affected with regard to different cell types.
Keywords
Mummy substance, wound healing, adipose-derived stem cell (ASCs), human fetal foreskin fibroblast (HFFF-2), co-culture, cell migration, cell proliferation
Skin, the external layer covering the body forms 8 % of the total body mass and is the largest body organ [1]. It consists of two main layers, epidermis and dermis. They are derived from different embryological origins and differ anatomically and functionally [2]. The overlying epidermis is mainly composed of ectodermderived keratinocytes that serve as a barrier against environmental insults [3]. The dermis provides support to the epidermis and is responsible for the strength and integration of the skin [4,5]. Any disruption in the normal anatomical structure, with a subsequent loss of functional integrity of the skin, is defined as a wound [6]. Fibroblasts play a key role in the production of dermis component and in wound healing [7].
Wound is a significant biomedical burden; millions of people around the world are affected by it. It is associated with morbidity and its annual cost is estimated to be $ 25 billion in the USA [8]. Cutaneous wound healing is to repair the injured skin, recover the tensile strength, integration, and immunological function of the skin [9]. Skin wound healing is an elaborate process that can be divided into four phases, haemostasis, inflammation, cell proliferation and remodelling [10,11]. During the third phase, fibroblast proliferation and migration into the extracellular matrix of the wound lead to the collagen deposition, thus steadily replacing the blood clots [9,12]. Finally, an intricate reorganization and maturation of newly formed collagen fibres occur in the remodelling phase [13]. In response to the cytokines secreted from neighbouring cells such as keratinocytes, fat cells, and migratory cells fibroblasts stimulate the production of extracellular matrix molecules and also growth factors, which in turn enhance keratinocyte proliferation in a paracrine manner [14].
Mesenchymal stem cells (MSCs) have been reported to play a role in wound healing [15]. Adult MSCs with their unique capacity for self-renewal and differentiation into other cells and tissues can orchestrate the main events occurring during the wound-healing process and emerge as a potential candidate for cell-based therapy in wound treatment [16,17]. Adipose-derived stem cells (ASCs) similar to bone marrow-derived multi-potent stem cells may be differentiated into adipogenic, chondrogenic, myogenic, and osteogenic lineages in response to specific stimuli [18]. ASCs can be obtained from liposuction aspirates with minimal patient discomfort and morbidity [19]. The application of ASCs to accelerate the wound-healing process and tissue regeneration has been investigated in different in vitro and in vivo experiments. Despite the considerable role of fibroblasts and stem cells in the promotion of wound healing, it is important to stimulate the ability of these cells using some enhancers [20-22].
For about 5000 y, nature has been considered as a potential source for the management of different diseases, such as wounds. In the past few years, the revival of interest in traditional medicine prompted investigations for a better understanding of the mechanisms underlying the mysterious effects of various traditional compounds; on the other hand, low-cost, minimal unwanted side effects, and better acceptance by patients make it important to further understand the role of these natural ingredients [23].
In ancient Persian and Egyptian medicine, mummy was used as a healer for inflammation, bone fracture, poisoning, and wounds [24]. Mummy is the substance used for mummifying famous Egyptian kings and is locally called mummy in most parts of Persia; it is a pitch-like substance found in some fractures of earth and also in rare caves. It is dark brown to black in colour and produced as a result of oil oxidation. It contains magnesium, sulphur, nitrogen, oxygen, and polysaccharide. Mummy is found in two types, fatsoluble and water-soluble. For topical application, it is dissolved in boiling water and massaged on to the affected site, such as a wound or an inflamed joint [24,25]. In recent years, various effects of mummy on fracture healing, gastric ulcer treatment, and animal model of wound healing have been investigated by some Persian researchers [25].
Considering the significant effects of mummy on the acceleration of wound healing, as claimed by local people and old Persian books such as Avicenna’s Canon, as well as the role of fibroblasts and ASCs in the process of wound healing, the present study was designed to evaluate the effect of mummy on fibroblasts and ASC proliferation and migration in a two-dimensional culture condition.
Materials and Methods
Adipose-derived mesenchymal stem cell isolation and expansion
Human adipose tissues were obtained from patients undergoing laparotomy surgery. Exclusion criteria included any malignancy and the administration of hormones or chemotherapeutic agents. Written informed consent was obtained from all patients, and the proposal was approved by the medical ethics committee of Tabriz University of Medical Sciences. Adipose tissue samples were transferred to the cell culture lab and washed using phosphate-buffered saline (PBS) containing 100 U/ml penicillin and 100 mg/ml streptomycin (1 % P/S; Gibco BRL). Samples were minced into small pieces using a sterile scalpel blade, weighed, and later digested with 0.2 % collagenase type I (Sigma, cat. no. C9891) per gram in shaking water bath for 60 min at 37°. Enzyme neutralizing was performed using fetal bovine serum (FBS, Gibco). After being passed through a 70 μm cell strainer (Fischer Scientific), cell suspension was centrifuged at 1500 rpm for five minutes. The cells were counted using 0.4 % Trypan-blue exclusion dye test on the haemocytometer slide and then seeded into T25 culture flask in Dulbecco's modified Eagle's medium (DMEM) low glucose (DMEM/LG, Gibco), supplemented with 10 % FBS and 1 % P/S at 37° till confluence. Medium exchanges were done every three days. Cells harvested at the third passage were used for the experiment. Previous studies from our laboratory provided evidence of the same characteristics as mesenchymal cells [26,27]. Human fetal foreskin fibroblast cell line (HFFF-2) was purchased from Pasteur Institute (Tehran, Iran). After thawing, cells were counted, plated at a density of 5×105 in the T75 culture flasks, and used for the experiment.
Mummy preparation
Fresh mummy was purchased from the local market in Kermanshah. Due to the absence of any in vitro study regarding the dosage of mummy in the first step, its effective concentration was determined using 3-[4,5-dimethylthiazoly-2-yl]-2,5-diphenyltetrazolium bromide (MTT, Sigma) assay technique. Since mummy is water-soluble, it was solved in DMEM culture medium completely and filtered through 0.22 μm syringe filter for sterilization.
Cell viability assay by MTT
The cell viability and proliferation were measured using MTT assay technique, in which the yellow tetrazolium MTT was reduced by mitochondrial enzyme namely succinate dehydrogenase to generate intracellular bluish purple formazan, which can be solubilized and quantified [28,29]. For this purpose, fibroblasts (HFFF- 2 cell line)/ASCs (at third cell passage) were plated at a concentration of 1×104 cells/well in a 96-well plate for 24 h, and then cells in the control group were incubated in serum-free DMEM culture medium or treated with different concentrations of mummy (10-5000 μg/ml) for 24, 48, 72 and 96 h. In the next step, MTT reagent solution (5 mg/ml) was added to each well and the cultures were incubated at 37° for four hours while being protected from light. For solving the formazan crystals, dimethyl sulfoxide (Merck) was added to the wells; later, the absorbance was determined at 570 nm with a reference wavelength of 630 nm and measured using ELISA plate reader (Bio Tek).
Migration evaluation with in vitro scratch assay
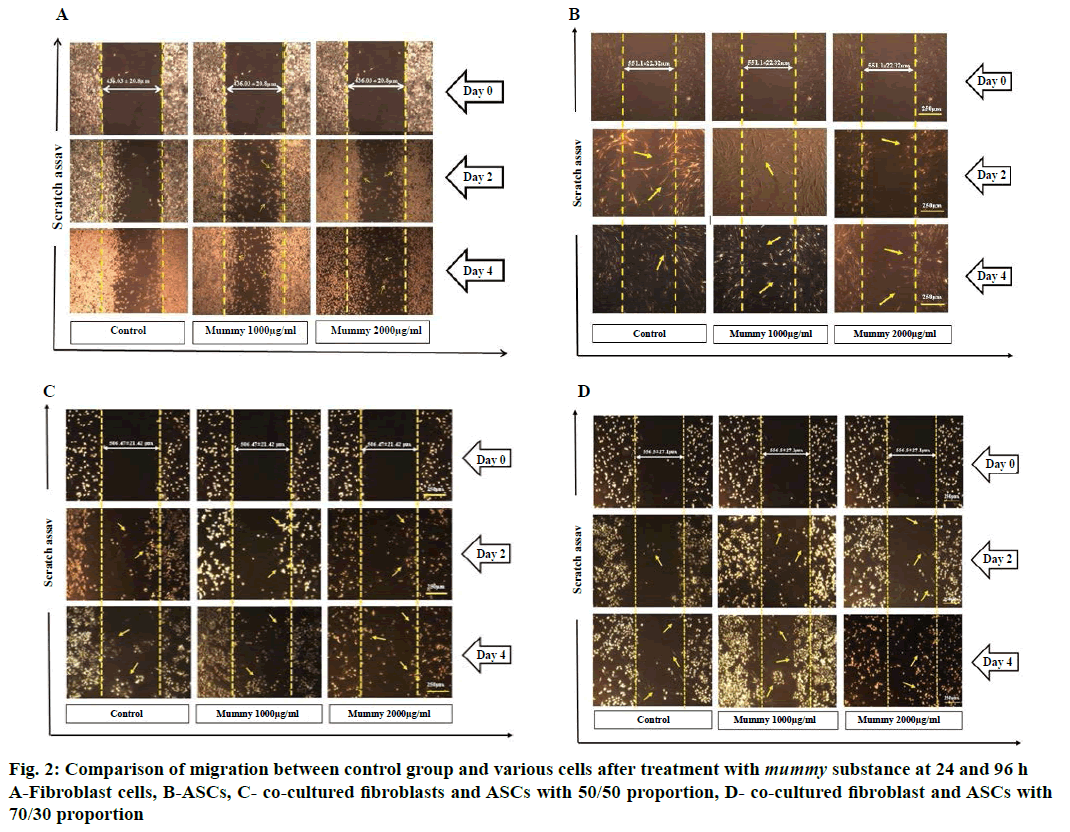
The effect of mummy on the migration of fibroblasts and ASCs was evaluated using in vitro scratch assay. Each well was plated with cells, including fibroblast (5×104/ml), ASCs (5×104/ml), co-culture of fibroblast and ASCs containing equal numbers of each (2.5×104), and their co-culture in a proportion of 30:70 (1.5×104 of ASCs and 3.5×104 of fibroblasts), and then incubated in DMEM supplemented with 10 % FBS for 24 h to allow cell adhesion and formation of a confluent monolayer. Afterwards, three linear scratches were performed in each well using a sterile pipette tip and the cellular debris was immediately removed by washing with PBS. In the next step, cells in control groups received fresh serum-free DMEM or were treated with DMEM containing 1000/2000 μg/ml of mummy. Photographs were taken with 4X magnification using an inverted microscope and digital camera (Olympus CK2, Japan) on days 0, 1, 2, 3, and 4. The acquired images were further analysed with the computing software Image J (ver. 1.49.) and the distances of each scratch closure were evaluated. All scratch assays were performed in triplicate, and the migration rate percent was calculated using the following formula [30], migration rate = average distance between scratch (day 0)–average distance between scratch (day 1 to 4)/average distance between scratch (day 0)×100.
Proliferation assay technique (Ki-67 detection)
Ki-67 protein as a marker for proliferation is expressed in all cell types during cell cycle. To understand the effect of mummy on cell proliferations, fibroblast cell line (HFFF-2) or ASCs alone or in co-culture condition in proportion of 50:50 or 30:70, as previously described, were plated. After adhesion to the culture flask, cells received culture medium as control or were treated with mummy at a concentration of 1000 μg/ml for 24 and 96 h. To preform Ki-67 proliferation assay, cells were trypsinised and neutralized with PBS containing 3 % FBS. Then, cell permeabilization was performed using 0.2 % Triton X-100 solution for 3 min and centrifuged at 800 rpm for 5 min. In the next step, cells were washed with PBS, supernatant was removed, and staining was performed using 5 μl Ki-67 antibody (RFF: 12-5699-41, San Diego, CA) in 100 μl of PBS for 30 min while protected from light and re-suspended using pipette tip. Later, 1000 μl of PBS was added and centrifuged at 800 rpm for 5 min, the obtained supernatant was removed, and the cells were suspended in 400 μl of PBS. In the final step, analysis was done using the flow cytometric method [31,32].
Stastical analysis
Data are expressed as mean ± SD. Statistical analysis was performed using two-way ANOVA and Tukey post hoc test and done with Graph Pad in Stat software version 2.02.
Results and Discussion
Using the MTT assay, the effective concentration of mummy on fibroblasts and ASCs was determined. The mummy material was prepared at different concentrations (0.5, 10, 100, 500, 1000, 2000, and 7000 μg/ml) by dissolving in DMEM culture medium. Figure 1A showed the viability of fibroblasts stimulated with different dosages. The results of ANOVA and Tukey post hoc test showed that mummy at a concentration of 1000 μg/ml generated the highest proliferation rate (p<0.0001). Stimulation of fibroblasts with higher dosages (2000 and 7000 μg/ml) resulted in cell cytotoxicity and death (p<0.0001). Similarly, the most effective dosage of mummy on ASCs was found to be 1000 μg/ml and there was a sharp decrease in cell viability at 2000 and 7000 μg/ml (Figure 1B). This experiment was performed in triplicate.
To study the effect of mummy on fibroblasts and ASCs migration, in vitro scratch assay was used. As described earlier, fibroblasts and ASCs were seeded alone or co-cultured with 50:50 and 30:70 proportions. The length of scratch mark edges between days 0, 2 and 4 decreased. This reduction in length between the scratch was significant in the control group and treated groups (p<0.0001). The migration rate of fibroblasts in the group treated with 1000 μg/ml of mummy increased significantly (p<0.0001) through day 1-4 (Table 1). However, in 2000 μg/ml concentration of mummy, the migration rate of fibroblasts was increased on day 1 (p<0.0001) and decreased with passing time; by day 4, the cells were dead and detached from the bottom of the plate (p<0.0001; Figure 2A).
| Treatment | Control | Mummy | Mummy | |
|---|---|---|---|---|
| Dose (µg/ml) | DMEM alone | 1000 µg/ml | 2000 µg/ml | |
| Distance between edges of scratch (µm) | Day 0 | 436.03 ± 20.8 | 436.03 ± 20.8 | 436.03 ± 20.8 |
| Day 1 | 383.6 ± 6.8 | 259.8 ± 11.8**** | 278.5 ± 10.8**** | |
| Day 2 | 260.09 ± 12.5 | 204.3 ± 22.6**** | 235.8 ± 9.20**** | |
| Day 3 | 257 ± 13.4 | 164.3 ± 8.10**** | 233.5 ± 11.6**** | |
| Day 4 | 227.9 ± 13.09 | 132.1 ± 12.4**** | X**** | |
| Test results for comparing days | **** | **** | **** | |
| % Migration rate of cells | Day 1 | 12 ± 1.5 | 40.4 ± 2.7**** | 36.1 ± 2.4**** |
| Day 2 | 40.3 ± 2.8 | 53.1 ± 5.1**** | 44.7 ± 1.2 | |
| Day 3 | 40.8 ± 3.08 | 62.3 ± 1.8**** | 46.4 ± 2.5 | |
| Day 4 | 47.7 ± 3 | 69.6 ± 2.8**** | X**** | |
Table 1: Effect of the Mummy Substance/Only DMEM on In Vitro Scratch Assay Using Fibroblasts (HFFF-2)
The effects of mummy on the migration rate and the distance between the edges of the scratch on ASCs are presented in Table 2. The length of scratch mark edges between days 0, 2 and 4 decreased. This reduction in length of the scratch was significant in the control group and treated groups at a concentration of 1000 μg/ml (p<0.0001), but not significant at 2000 μg/ml. The migration rate of ASCs in the group treated with 1000 μg/ml of mummy significantly increased on days 1 and 3. However, at 2000 μg/ml, the migration rate of ASCs decreased (p<0.0001; Table 2 and Figure 2B).
| Treatment | Control | Mummy | Mummy | |
|---|---|---|---|---|
| Dose µg/ml | DMEM alone | 1000 µg/ml | 2000 µg/ml | |
| Distance between edges of scratch (µm) | Day 0 | 551.1 ± 22.32 | 551.1 ± 22.32 | 551.1 ± 22.32 |
| Day 1 | 422.49 ± 18.70 | 385.27 ± 14.22 | 454.08 ± 22.94 | |
| Day 2 | 315.33 ± 14.42 | 302.13 ± 16.77 | 366.39 ± 16.48 | |
| Day 3 | 176.64 ± 35.74 | 175.61 ± 11.25 | 348.38 ± 10.82**** | |
| Day 4 | 54.52 ± 6.84 | 46.65 ± 21.46 | 323.91 ± 25.25**** | |
| Test results for comparing days | **** | **** | ||
| % Migration rate of cells | Day 1 | 23.33 ± 3.3 | 30.09 ± 2.75 | 17.60 ± 4.16 |
| Day 2 | 42.66 ± 2.51 | 45.17 ± 3.03 | 32.19 ± 0.59**** | |
| Day 3 | 63.48 ± 0.01 | 68.13 ± 2.04 | 36.78 ± 1.96**** | |
| Day 4 | 88.18 ± 2.62 | 91.53 ± 1.24 | 35.16 ± 2.90**** | |
Table 2: Effect of the Mummy Substance/Only DMEM on In Vitro Scratch Assay Using ASCs
The migration rate of cells in 50:50 proportion of fibroblasts and ASCs at a concentration of 1000 μg/ml did not change in comparison to the control group (Table 3). However, in 2000 μg/ml concentration, the migration rate decreased significantly in comparison to the control group (p<0.0001; Figure 2C). Moreover, the length of scratch mark edges between days 0, 2 and 4 within all of the groups was not significant.
| Treatment | Control | Mummy | Mummy | |
|---|---|---|---|---|
| Dose (µg/ml) | DMEM alone | 1000 µg/ml | 2000 µg/ml | |
| Distance between edges of scratch (µm) | Day 0 | 506.47 ± 21.42 | 506.47 ± 21.42 | 506.47 ± 21.42 |
| Day 1 | 390.78 ± 18.5 | 378.81 ± 6.10 | 378.11 ± 10.4 | |
| Day 2 | 302.44 ± 27.8 | 320.24 ± 12.6 | 350.70 ± 12.6**** | |
| Day 3 | 280.54 ± 15.8 | 283.32 ± 20.0 | 324.7 ± 9.77 | |
| Day 4 | 266.94 ± 12.9 | 237.92 ± 13.3 | 417.37 ± 16.7**** | |
| Test results for comparing days | ns | ns | ns | |
| % Migration rate of cells | Day 1 | 22.8 ± 3.66 | 25.20 ± 1.20 | 25.34 ± 2.06 |
| Day 2 | 38.24 ± 4.51 | 36.77 ± 2.50 | 30.75 ± 2.49 | |
| Day 3 | 44.60 ± 3.13 | 44.05 ± 3.95 | 35.88 ± 1.93 | |
| Day 4 | 47.29 ± 2.54 | 53.02 ± 2.64 | 17.59 ± 3.30**** | |
Table 3: Effect of the Mummy Substance/Only DMEM on In Vitro Scratch Assay Using Fibroblasts+ASCs (50/50)
Table 4 showed the migration rate and distance between edges of scratch at 70-30 proportion of fibroblast and ASCs. At this proportion, the migration rate of cells significantly increased (p<0.0001) at 1000 μg/ml concentration. However, at 2000 μg/ml concentration, the migration rate decreased significantly on day 3 compared to the control group (p<0.0001), and on day 4, the cells died and detached from the bottom of plate (Figure 2D). The distance of scratch lines were significant in the control group and treated groups (p<0.0001). This experiment was performed in triplicate.
| Treatment | Control | Mummy | Mummy | |
|---|---|---|---|---|
| Dose (µg/ml) | DMEM alone | 1000 µg/ml | 2000 µg/ml | |
| Distance between edges of scratch (µm) | Day 0 | 556.5 ± 27.1 | 556.5 ± 27.1 | 556.5 ± 27.1 |
| Day 1 | 482.4 ± 7.4 | 377.4 ± 3.5**** | 480.2 ± 13.9 | |
| Day 2 | 399.8 ± 9.5 | 281.7 ± 10.8**** | 404.3 ± 11.8 | |
| Day 3 | 338.8 ± 15.4 | 216.8 ± 13.9**** | 400.4 ± 7.4 | |
| Day 4 | 311.1 ± 13.7 | 158.6 ± 11.04**** | X**** | |
| Test results for comparing days | **** | **** | **** | |
| % Migration rate of cells | Day 1 | 13.2 ± 1.3 | 32.1 ± 0.6**** | 14.1 ± 2.5 |
| Day 2 | 28.1 ± 1.7 | 49.3 ± 1.9**** | 27.3 ± 2.1 | |
| Day 3 | 39.09 ± 2.7 | 61.02 ± 2.5**** | 28.03 ± 1.3**** | |
| Day 4 | 44.09 ± 2.4 | 71.4 ± 1.9**** | X **** | |
Table 4: Effect of the Mummy Substance/Only DMEM on In Vitro Scratch Assay Using Fibroblast+ASCs (30/70)
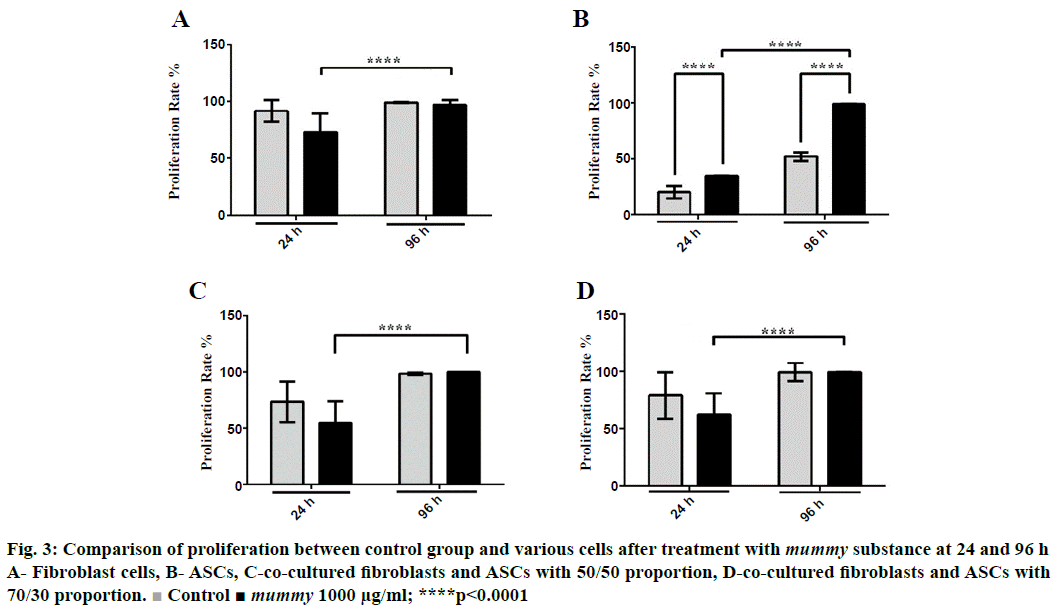
Ki-67 technique was used for the determination of cellular proliferation. The present investigation indicated that Ki-67 expression or proliferation decreased significantly in mummy-treated fibroblasts compared to the control group at 24 h but remained unchanged at 96 h (Figure 3A). Although the exposure of fibroblast to mummy for 96 h did not lead to a marked difference in Ki-67 expression compared to the control, the proliferation rate of the treated cells at 96 h significantly increased in comparison to that of the treated cells at 24 h, as is obvious in Figure 3A. In contrast, data obtained in this study revealed that the proliferation of ASCs, unlike that of fibroblast, increased significantly (p<0.0001) when treated with mummy substance both at 24 and 96 h (Figure 3B). Cellular proliferation of co-culture cells did not increase at any proportion at 24 and 96 h (Figure 3C and D). However, the proliferation rate of treated cells at 96 h was higher than that at 24 h. This experiment was performed in triplicate.
Figure 3: Comparison of proliferation between control group and various cells after treatment with mummy substance at 24 and 96 h
A- Fibroblast cells, B- ASCs, C-co-cultured fibroblasts and ASCs with 50/50 proportion, D-co-cultured fibroblasts and ASCs with 70/30 proportion.  Control ■ mummy 1000 μg/ml; ****p<0.0001
Control ■ mummy 1000 μg/ml; ****p<0.0001
Skin wound healing is a harmonized process activated by injury, consisting of multicellular overlapping and the coordinated steps of inflammation, angiogenesis, formation of granulation tissue, and re-epithelialization [33,34]. Wound healing is still a clinical challenge, especially in older patients, diabetic patients, heavy smokers, or burn patients, despite the current use of a wide array of wound dressings and ointments [35-39]. Novel strategies are required to enhance wound healing and repair.
Proliferation and migration are crucial factors in wound healing. Cell migration fibroblast migration in particular is one of the vital processes in wound healing [40]. Thus, a study of the factors affecting fibroblast migration could improve wound healing [41]. Fibroblasts not only play a major role in epithelialization by providing extracellular matrix, but also contribute to wound contraction by transforming to myofibroblasts [42].
The potential impact of mummy on the migration and proliferation of dermal fibroblasts was studied. In the first step, the effective dosage of mummy on fibroblast survival was determined, showing that the 1000 μg/ml dosage is the optimal dose for fibroblast survival (Figure 1). Studying the effect of mummy on fibroblast proliferation and migration revealed that fibroblast proliferation does not increase under the effect of the 1000 μg/ml dosage of mummy (Figure 3A). However, with regard to the effect of mummy on fibroblast migration, the results showed that fibroblast migration was increased significantly (p<0.0001) in the presence of 1000 μg/ml of mummy; i.e. mummy might improve wound healing by promoting fibroblast migration to the wound site. The scratch assay involved the second phase of wound healing, characterized by the proliferation and migration of keratinocytes or fibroblasts [43-45]. It has been reported that mummy was effective in healing bone fracture [24]. In rats, it has been found that fibroblast migration was regulated by calendula extracts on Swiss 3T3 mouse fibroblast [45,46].
On the other hand, MSCs are more ideal for promoting wound healing [47]. The results regarding the effect of mummy on ASCs showed that ASC migration increased significantly in the presence of 1000 μg/ml of mummy (Figure 2B, Table 2). The current study demonstrated that mummy promoted the proliferation of ACSs at a dose of 1000 μg/ml (p<0.0001; Figure 3B). The MSCs of the skin populate the normal skin niche, remain quiescent, and become active after injury, helping in wound closure [48]. MSCs are applied to contribute to healing by releasing various cytokines and growth factors, by demonstrating therapeutic efficacy and the underlying mechanism of MSCs on wound healing [49]. In fact, the antiinflammatory characteristics of MSCs increase their role in chronic wound treatment. In particular, vasculogenesis and angiogenesis crucial steps in wound healing are stimulated through paracrine factors released by MSCs [50]. At the same time, other studies showed that MSC-CM has little effect on fibroblast (L929) or keratinocyte HaCaT cell proliferation or survival [19,40,51].
The results regarding the effect of mummy on the coculture of fibroblast and ASCs at proportions of 70:30 and 50:50 revealed that migration was enhanced under the effect of 1000 μg/ml mummy (Table 4, Figure 2). However, the effect of mummy on the co-culture of fibroblast and ASCs at both proportions (70:30 and 50:50) did not increase cellular proliferation. However, comparing the effect of mummy on proliferation at 24 and 96 h, the proliferation increase was significant (p<0.0001), while this was not the case in the untreated group (Figure 3). Thus, the stimulatory effect of mummy increased with passing time, confirming the healing effect of mummy [25]. In conclusion, mummy has a positive effect on the promotion of proliferation and migration of fibroblast and ASCs, suggesting that mummy might stimulate the proliferative and reepithelialization phases of wound healing. The results revealed that mummy accelerated wound healing, which was in accordance with public beliefs and ancient medicine.
Acknowledgements
This work originated from a PhD thesis and has been financially supported by Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Chen M, Przyborowski M, Berthiaume F. Stem cells for skin tissue engineering and wound healing. Crit Rev Biomed Eng 2009;37:399-21.
- Yolanda MM, Maria AV, Amaia F, Marcos P, Silvia P, Dolores E, et al. Adult stem cell therapy in chronic wound healing. J Stem Cell Res Ther 2014;4:2.
- Hiraoka C, Toki F, Shiraishi K, Sayama K, Nishimura EK, Miura H, et al. Two clonal types of human skin fibroblasts with different potentials for proliferation and tissue remodeling ability. J Dermatol Sci 2016;82:84-94.
- Flaxman B, Lutzner M, Van Scott E. Cell maturation and tissue organization in epithelial outgrowths from skin and buccal mucosa in vitro. J Invest Dermatol 1967;49:322-32.
- Stern M. The grafting of preserved amniotic membrane to burned and ulcerated surfaces, substituing skin grafts: a preliminary report. J Am Med Assoc 1913;60:973-74.
- Teng M, Huang Y, Zhang H. Application of stems cells in wound healing-an update. Wound Repair Regen 2014;22:151-60.
- Adetutu A, Morgan WA, Corcoran O. Ethnopharmacological survey and in vitro evaluation of wound-healing plants used in South-western Nigeria. J Ethnopharmacol 2011;137:50-6.
- Isakson M, de Blacam C, Whelan D, McArdle A, Clover A. Mesenchymal stem cells and cutaneous wound healing: current evidence and future potential. Stem Cells Int 2015;2015:1-12.
- Lau K, Paus R, Tiede S, Day P, Bayat A. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol 2009;18:921-33.
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol 2007;25:9-18.
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738-46.
- Opalenik SR, Davidson JM. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J 2005;19:1561-3.
- Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C Mater Biol Appl 2015;48:651-62.
- Smithmyer ME, Sawicki LA, Kloxin AM. Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease. Biomater Sci 2014;2:634-50.
- Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, LeRoux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142-9.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7:211-28.
- Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 2010;316:2213-19.
- Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, et al. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology 2016;62:216-25.
- Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007;48:15-24.
- Koenen P, Spanholtz TA, Maegele M, Stürmer E, Brockamp T, Neugebauer E, et al. Acute and chronic wound fluids inversely influence adipose‐derived stem cell function: molecular insights into impaired wound healing. Int Wound J 2015;12:10-16.
- Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem cells 2007;25:2648-59.
- Martin P. Wound healing--aiming for perfect skin regeneration. Science 1997;276:75-81.
- Maver T, Maver U, Kleinschek SK, Smrke DM, Kreft S. A review of herbal medicines in wound healing. Int J Dermatol 2015;54:740-51.
- Dehghan M, Faradonbeh AS. The effect of mummy on the healing of bone fractures. Afr J Pharm Pharmacol 2012;6:305-09.
- Abshenas J, Kheirandish R, Salary AR. Gastroprotective effect of mummy on induced gastric ulcer in rats. Comp Clin Path 2014;23:305-09.
- Shafaei H, Esmaeili A, Mardani M, Razavi S, Hashemibeni B, Nasr-Esfahani M, et al. Effects of human placental serum on proliferation and morphology of human adipose tissue-derived stem cells. Bone Marrow Transplant 2011;46:1464.
- Vahedi P, Soleimanirad J, Roshangar L, Shafaei H, Jarolmasjed S, Charoudeh HN. Advantages of Sheep Infrapatellar Fat Pad Adipose Tissue Derived Stem Cells in Tissue Engineering. Adv Pharm Bull 2016;6:105.
- Green LM, Reade JL, Ware CF. Rapid colormetric assay for cell viability: application to the quantitation of cytotoxic and growth inhibitory lymphokines. J Immunol Methods 1984;70:257-68.
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev 2005;11:127-52.
- Balekar N, Katkam NG, Nakpheng T, Jehtae K, Srichana T. Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. J Ethnopharmacol 2012;141:817-24.
- Palutke M, KuKuruga D, Tabaczka P. A flow cytometric method for measuring lymphocyte proliferation directly from tissue culture plates using Ki-67 and propidium iodide. J Immunol Methods 1987;105:97-105.
- Siavashi V, Sariri R, Nassiri SM, Esmaeilivand M, Asadian S, Cheraghi H, et al. Angiogenic activity of endothelial progenitor cells through angiopoietin-1 and angiopoietin-2. Animal Cells Syst (Seoul) 2016;20:118-29.
- Arno A, Smith AH, Blit PH, Shehab MA, Gauglitz GG, Jeschke MG. Stem cell therapy: a new treatment for burns? Pharmaceuticals (Basel) 2011;4:1355-80.
- Blit PH, Arno AI, Jeschke MG. Stem cells and tissue engineering in burns and wounds. In: Sell S, editor. Stem Cells Handbook. New York: Humana Press; 2013. p. 399-09.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736-43.
- Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci 2013;70:2059-81.
- Jorgensen LN, Kallehave F, Christensen E, Siana JE, Gottrup F. Less collagen production in smokers. Surgery 1998;123:450-55.
- Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol 2007;25:19-25.
- Wasiak J, Cleland H, Campbell F, Spinks A. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev 2013;(3):CD002106.
- Walter M, Wright KT, Fuller H, MacNeil S, Johnson WEB. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res 2010;316:1271-81.
- Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res 2010;316:48-54.
- Germain L, Jean A, Auger FA, Garrel DR. Human wound healing fibroblasts have greater contractile properties than dermal fibroblasts. J Surg Res 1994;57:268-73.
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314-21.
- Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 2007;4:413-37.
- Salama SM, Gwaram NS, AlRashdi AS, Khalifa SA, Abdulla MA, Ali HM, et al. A Zinc Morpholine Complex Prevents HCl/Ethanol-Induced Gastric Ulcers in a Rat Model. Sci Rep 2016;27:29646.
- Fronza M, Heinzmann B, Hamburger M, Laufer S, Merfort I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J Ethnopharmacol 2009;126:463-67.
- Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant 2011;20:205-16.
- Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns 2009;35:171-80.
- Laverdet B, Micallef L, Lebreton C, Mollard J, Lataillade J-J, Coulomb B, et al. Use of mesenchymal stem cells for cutaneous repair and skin substitute elaboration. Pathol Biol (Paris) 2014;62:108-17.
- Borena BM, Martens A, Broeckx SY, Meyer E, Chiers K, Duchateau L, et al. Regenerative skin wound healing in mammals: state-of-the-art on growth factor and stem cell based treatments. Cell Physiol Biochem 2015;36:1-23.
- Karimian H, Fadaeinasab M, Moghadamtousi SZ, Hajrezaei M, Zahedifard M, Razavi M, et al. The Chemopreventive Effect of Tanacetum polycephalum Against LA7-induced Breast Cancer in Rats and the Apoptotic Effect of a Cytotoxic Sesquiterpene Lactone in MCF7 Cells: A Bioassay-Guided Approach. Cell Physiol Biochem 2015;36:988-1003.
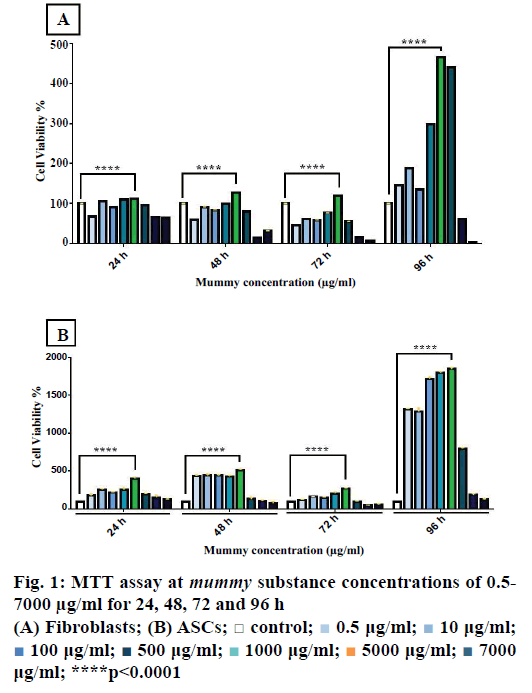
 0.5 μg/ml;
0.5 μg/ml;  10 μg/ml;
10 μg/ml;  100 μg/ml;
100 μg/ml;  500 μg/ml;
500 μg/ml;  1000 μg/ml;
1000 μg/ml;  5000 μg/ml;
5000 μg/ml;  7000 μg/ml; ****p<0.0001
7000 μg/ml; ****p<0.0001