- *Corresponding Author:
- Manami Dhibar
Department of Pharmaceutics, Formulation Development Research Unit, Dr. B. C. Roy College of Pharmacy and Allied Health Sciences, Durgapur, West Bengal 713212, India
E-mail: manamidhibar@gmail.com
| Date of Received | 10 March 2023 |
| Date of Revision | 11 July 2023 |
| Date of Acceptance | 29 March 2024 |
| Indian J Pharm Sci 2024;86(2):703-711 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this present research attempt has been made to enhance the solubility and dissolution of poorly soluble telmisartan by solid dispersion technique using pluronic F127 and implementation of multiple comparison analysis to screen the drug-carrier ratio to optimize the solubility and dissolution. All the solid dispersions were prepared by using various concentrations of drug and carrier and subjected to melting point, solubility and dissolution studies to optimize the drug-carrier ratio. Optimized solid dispersion was also subjected to various analytical studies such as field emission scanning electron microscope, differential scanning calorimetry, X-ray diffraction studies along with stability studies to identify the physico-chemical state of the prepared solid dispersion. It was observed from this research that prepared solid dispersions could able to improve the solubility and dissolution of telmisartan by 6.93 folds and 2.57 folds respectively. Statistical analysis revealed significant difference between telmisartan and prepared telmisartan-pluronic F127 solid dispersion with respect to their solubility and dissolution data. Field emission scanning electron microscope study revealed that distinctive crystalline structure and geometric shape of pure telmisartan was completely disappeared in the prepared solid dispersion which revealed the conversion of crystalline telmisartan to its amorphous form. Differential scanning calorimetry and X-ray diffraction studies confirmed the complete amorphization of drug crystals upon treating with pluronic F127. Stability study also confirmed the stable nature of the prepared telmisartan-pluronic F127 solid dispersion with shelf life of 1.62 y. Finally, it was concluded from the above experimental results that optimized concentration of pluronic F127 could able to improve the solubility and dissolution of telmisartan by solid dispersion technique.
Keywords
Solid dispersion, enhancement in solubility, amorphization, Holm-Sidak multiple comparison analysis, telmisartan
The key factors for assessing the therapeutic efficacy of an Active Pharmaceutical Ingredient (API) are its solubility and dissolution. Most of the newly synthesize drugs possess low solubility and high lipophilicity[1], which may lead several drawbacks like slow dissolution, improper absorption that directly arise sub-therapeutical efficacy and low bioavailability of the APIs[2]. So, it is the major challenge for the researchers to enhance the solubility as well as dissolution of the APIs. As per the literatures, several research works are going on in this field and researchers have tried to improve the solubility by using different techniques such as grinding, use of surfactants, salt formation, pH modulation, prodrugs, complexation, self-emulsifying formulations and cocrystallization[3]. Among the various methods, the most promising, practical and affordable method for potentially improving the solubility and rate of dissolution is solid dispersion (TPF). According to Sridhar, et al. TPF is a class of solid products that made up of at least two components i.e., a hydrophilic matrix and a hydrophobic drug in which the latter may be disseminated either molecularly or in an amorphous form[4]. The entrapment of poorly soluble API within a hydrophilic carrier induces improve wettability, amorphization, reduction in particle size which may leads better dissolution[5].
Telmisartan (TMS), is a non-peptide Angiotensinii Receptor Blocker (ARB), widely used for the treatment of hypertension[6,7]. It is a Biopharmaceutics Classification System (BCS) Class II drug having very low aqueous solubility[8]. The major problem associated with TMS is its low solubility and poor dissolution mainly in the physiological pH from 3-7.
This limited solubility and dissolution revealed a poor and variable bioavailability of 42 %-52 %[9] and suboptimal therapeutic activity[10,11]. To overcome such issues, different formulation strategies were adopted such as use of alkalizers[12,8], solid state manipulation, nanoparticles, mesoporous nanoparticles, cocrystals, immediate release tablets, nano self-emulsifying drug-delivery systems, amorphous formulations[13-19].
Pluronics are hydrophilic Poly(Ethylene Oxide) (PEO) and hydrophobic Poly(Propylene Oxide) (PPO) blocks combined to form an amphiphilic triblock copolymer, PEO-PPO-PEO. These copolymers are understood to inhibit P-glycoprotein (P-gp). Therefore, pluronic polymeric micelles can enhance the bioavailability of poorly water-soluble drugs by inhibiting P-gp efflux and enhancing circulation time in addition to their solubilization impact[20-23].
Many researchers were examined various polymers and other pharmaceutical excipients to enhance the solubility of poorly soluble TMS by TPF technique[24,25], but still there was very less research has been done with Pluronic F127 (PF127). So, in this present research we have tried to improve the solubility and dissolution of poorly soluble TMS by TPF technique using PF127 and investigate the impact of various concentration of PF127 on enhancement of solubility and dissolution.
Materials and Methods
TMS was supplied by Macleods Pvt. Ltd., India as a gift sample. PF127 was procured from Sigma Aldrich, India. Chloroform was procured from Merck, India. All other materials and solvents used were of analytical grade.
Preparation of TMS-pluronic TPFs:
TMS-pluronic TPFs were prepared by solventevaporation technique[26]. TMS was placed in a mortar and grinded with different ratios of PF127 (Table 1). Then chloroform was added drop wise into the drugcarrier mixture and stirred continuous until the drugcarrier blend gets dissolved completely. After that, the solution was transferred into a petridish and kept it overnight for complete drying. Then the resultant dried TPFs were scrapped out by using an ointment spatula and kept in desiccator for further use[27].
| Formulation | Drug: Carrier | Telmisartan (g) | Pluronic F127 (g) | Solvent |
|---|---|---|---|---|
| TPF1:1 | 01:01 | 1 | 1 | Chloroform |
| TPF1:2 | 01:02 | 1 | 2 | Chloroform |
| TPF1:3 | 01:03 | 1 | 3 | Chloroform |
| TPF1:4 | 01:04 | 1 | 4 | Chloroform |
| TPF1:5 | 01:05 | 1 | 5 | Chloroform |
Note: TPF: Telmisartan-Pluronic solid dispersion.
Table 1: Formulation of Tms Solid Dispersion Using Pf127
Methods
Melting point studies:
Melting point of pure TMS, physical mixture and prepared TMS-pluronic TPFs were determined by capillary tube method using melting point apparatus (CL-735, Chemline). The samples were filled in capillary tube (previously sealed at one end) and then inserted in the sample holder of melting point apparatus and observed the reading. The temperature at which powder samples started to melt was examined and shown in Table 2.
| Formulation | Melting point (°C) | Drug content (%) | Solubility (µg/ml) | Solubility enhancement (folds) |
|---|---|---|---|---|
| TMS | 270 ± 3 | - | 5.13 ± 1.05 | - |
| TPM | 266 ± 2 | - | 6.04 ± 2.16 | 1.18 |
| TPF 1:1 | 224 ± 4 | 79.53 ± 3.67 | 13.91 ± 3.61 | 2.71 |
| TPF 1:2 | 193 ± 3 | 85.37 ± 4.08 | 22.16 ± 4.01 | 4.32 |
| TPF 1:3 | 160 ± 3 | 91.02 ± 3.94 | 35.55 ± 4.73 | 6.93 |
| TPF 1:4 | 177 ± 4 | 87.22 ± 2.14 | 27.22 ± 3.69 | 5.31 |
| TPF 1:5 | 209 ± 2 | 89.57 ± 4.26 | 19.07 ± 2.89 | 3.72 |
Note: Mean ± SD; n=3.
Table 2: Melting Point, Drug Content and Solubility Study of Pure Tms, Tpm and Tms-Pluronic Solid Dispersions
Drug content analysis:
An accurately weighted amount of prepared TMSpluronic TPF (i.e., 100 mg) was placed in 100 ml of phosphate buffer pH 7.5. The dispersion was stirred for 6 hours using magnetic stirrer and then sonicated at 125 W for 5 min (Frontline FS 600).
Then the dispersion was filtered and the drug content of the filtrate was determined spectrophotometrically at 296 nm (Ultraviolet (UV)-1800 Shimadzu, Japan). Each determination was made in triplicate and shown in Table 2.
Solubility studies:
An excess amount of sample was placed to 50 ml of distilled water and vigorous stirred for 12 h using a magnetic stirrer. The resulting dispersion was sonicated at 125 W for 10 min (Frontline FS 600) to extract the total drug present in the TPFs. Then the dispersion was filtered using Whatman filter paper (0.45 mm) and the filtrate was analysed spectrophotometrically at 296 nm using UV-visible spectrophotometer (UV-1800 Shimadzu, Japan) to determine the amount of TMS present in the TPFs.
In vitro dissolution studies:
In vitro dissolution study was performed by using United States Pharmacopeia (USP) dissolution rate test apparatus II (DISSO 8000, LAB INDIA, India) at 100 rpm. An accurately weighed quantity of pure TMS (40 mg) and prepared TMS-pluronic TPFs (weight equivalent to 40 mg of drug) were placed in dissolution flask containing 900 ml of phosphate buffer (pH 7.5). At predetermined time intervals, 5 ml aliquots were withdrawn from the dissolution flask and replaced by an equal volume of fresh pre-warmed dissolution medium to maintain sink condition throughout the experiment. After suitable dilution, the samples were analysed for TMS content using UV-visible spectrophotometer at 296 nm. The dissolution study was conducted in triplicate and shown in fig. 1.
Statistical Analysis:
Solubility and in vitro dissolution data of TMS and all the prepared TPFs were statistically analysed using one way Analysis of Variance (ANOVA) followed by Holm-Sidak multiple comparison analysis. Statistical analysis of the data was performed using the software PRISM software (Graph pad, San Diego, CA, USA). The solubility and dissolution data were subjected to one way ANOVA followed by Holm-Sidak multiple comparison analysis to find out whether any significant difference was present between TMS and each of the prepared TMS-PF-127 TPF or not.
Surface morphology study:
Shape and surface morphology of pure drug, physical mixture and optimized TMS-pluronic TPF were examined using field emission scanning electron microscope (Carl Zeiss, SUPRA 55, Germany). The sample was spread on a stub and then coated with conductive gold with sputter coater attached to the instrument. Then the stub containing sample was placed in the field emission scanning electron microscope chamber at acceleration voltage of 5 kV and chamber pressure of 0.6 mmHg. The photographs were taken using field emission scanning electron microscope and shown in fig. 2.
DSC analysis of pure TMS, PF127, physical mixture and optimized TMS-pluronic TPF were performed using Differential Scanning Calorimeter (DSC) (Diamond DSC, PYRIS, Perkin Elmer, USA). Samples were hermetically sealed in perforated aluminum pans and then heated over a temperature range of 10º-400º. The system was purged with nitrogen gas at the rate of 100 ml/min to maintain inert atmosphere.
X-Ray Diffraction (XRD) studies:
XRD study was performed to assess the physical state of pure drug after incorporation in the prepared TPF. XRD studies of pure TMS, PF127, physical mixture and optimized TMS-pluronic TPF was performed by X-ray diffractometer (X’Pert pro, panalytical, Netherlands) using monochromatized Cu K α-1 radiation (λ=1.54 A) at a voltage of 45 kV and current of 40 mA. Measurements were carried out in the angular scan range from 5°-50° (2θ) at a scan speed of 1°/min.
Stability Studies:
Stability study was performed to observe the physical appearance, drug content as well as shelf life of the formulation. Optimized TMS-pluronic TPF was stored at 40±2°; 75±5 % RH for 6 mo.
After each 30 d interval, sample was evaluated for physical appearance, % drug content and finally determines the shelf life of the formulation. The optimized formulation was also subjected to in vitro dissolution study to observe any possible changes in drug dissolution during storage or not (Table 3).
| Parameters | 40º/75 % RH | |||||
|---|---|---|---|---|---|---|
| Sampling Interval (days) | 30 | 60 | 90 | 120 | 150 | 180 |
| Drug Content (%) | 99.48 | 98.96 | 98.68 | 98.03 | 97.47 | 96.73 |
| Physical appearance | + | + | + | + | + | + |
| Slope | -7.756×10-5 | |||||
| K (days-1) | 1.786 x 10-4 | |||||
| t90 (days) | 590 | |||||
Note: +=No changes in physical appearance; ++ Changes in physical appearance
Table 3: Stability Study of Optimized Tms-Pf127 Solid Dispersion (Tpf1:3)
Results and Discussion
TMS and prepared TMS-pluronic TPFs were subjected to various physico-chemical characterization studies i.e., melting point, drug content and solubility (Table 2). Melting point study revealed that pure TMS showed its melting point at 270±3°. It was noticed from this study that upon physically mixing of TMS with pluronic (without any solvent), a slight decrease in melting point of pure drug was observed (i.e., 266±2°). It was also revealed that when TMS was treated with carrier (i.e., PF127) in presence of solvent, a significant change in melting point was observed and it was found within the range of 160±3°-224±4° (Table 2).
Drug content analysis showed excellent drug content of the prepared TMS-pluronic TPFs. The drug content values of the prepared TMS-pluronic TPFs were found within the range of 79.53±3.67 %-91.02±3.94 % (Table 2) and among all the prepared batches, TPF1:3 showed the maximum drug content value of about 91.02±3.94 %.
Solubility study revealed that pure TMS showed only 5.13±1.05 μg/ml solubility in distilled water at 37° (Table 2). When TMS was physically treated with pluronic F 127 without any solvent, the solubility of TMS was not significantly improved and showed 6.04±2.16 μg/ml solubility. Physical mixture of TMS and pluronic was able to increase the solubility of TMS of about 1.18 folds. It was observed that in presence of solvent, when TMS was treated with pluronic and converted in its TPF form, the solubility of TMS was significantly improved and the solubility was found within the range of 13.91±3.61 μg/ml- 35.55±4.73 μg/ml (Table 2). This may be due to the fact that in the prepared TPF, crystalline TMS was converted to amorphous state which helps to improve the solubility of TMS. It was also observed that the concentration of PF127 has a significant impact on solubility. When the concentration of PF127 was increased from 1:1-1:3 (drug:carrier), the solubility of TMS was significantly increased (13.91±3.61 μg/ ml-35.55±4.73 μg/ml) (Table 2). Further, increasing the concentration PF127 in TPF1:4 and TPF1:5, the solubility of TMS was not further increased. This may be due to the saturated solubility was already achieved with TPF1:3. This saturated solubility might form a saturated layer around the TMS particles which might hinder the drug release in the surrounding media[28-34]. Another reason of this observation is leaching of pluronic from the formulation might also impede TMS release from the prepared TPF. So, after screening it was observed that formulation TPF1:3 showed maximum solubility and could able to increase the solubility of TMS by 6.93 folds.
In vitro dissolution studies of pure TMS and TMSpluronic TPFs (TPF1:1-TPF1:5) are depicted in fig. 1. This study was carried out in phosphate buffer (pH 6.8) using 100 rpm at 37±0.5°. It was observed that for pure TMS, only 35.61±3.58 % drug was dissolved within 120 min. It was observed from this study that when TMS was treated with PF127 and converted into TPFs, the dissolution of TMS was significantly increased. TPF TPF1:1, TPF1:2 and TPF1:3 exhibited the percentage drug dissolution of about 62.78±2.79 %, 78.14±3.76 % and 91.61±2.08 % within 120 min respectively, (fig 1). These findings suggested that when TMS was treated with PF127, the crystalline form of TMS was converted to amorphous form and the crystal lattice of crystalline TMS was gradually reduced[35-37]. Increase in dissolution also depend on several factors such as reduction in particle size, reduction in interfacial tension between drug and dissolution medium, improved wettability and micro-environmental solubility of the drug in TPF[38]. In this present research, significant improvement in dissolution may be due to the formation of hydrogen bonds in between drug and carrier, solubilization effect of PF127, micronization of particles and conversion of crystalline TMS to its amorphous state (observed from DSC and XRD studies)[27,28].
As per the literatures, amorphous state of a drug exhibit better solubility as compared to its crystalline state which may be due to the fact that less energy is sufficient to break the crystal lattice during the dissolution process[31,35]. It was also evident from the literature that in TPF after dissolution if the drug precipitates, then it remains in a metastable state, which has higher solubility[29]. So, these findings confirmed that fabricated TMS-pluronic TPFs revealed better dissolution as compared to pure TMS. Further increase in PF127 concentration in TPF1:4 and TPF1:5, decreased drug dissolution was observed and 84.13 %±2.99 % as well as 69.91 %±3.65 % drug was dissolved within 120 min respectively. This may be due to the formation of supersaturated layer around the drug particles and higher concentration of carrier may leach out from the formulation that might hinder TMS dissolution in the surrounding media[34]. These finding justify that proper screening of drugcarrier ratio is very much essential for formulating any TPF. Because continuous increasing in carriers concentration does not give us the guarantee to increase the drug solubility and dissolution in continuous manner. Cumulative drug dissolution from the prepared TPF was found in the following order: TMS<TPF1:1<TPF1:5<TPF1:2<TPF1:4<TPF1:3. Among all the prepared TPFs, TPF1:3 (drug:carrier; 1:3) showed maximum dissolution i.e., 91.61 %±2.08 % as compared to all other prepared TPFs and selected as an optimized formulation for further use.
Solubility and dissolution data of TMS and all the prepared TPFs were statistically analysed using one way ANOVA followed by Holm-Sidak multiple comparison analysis. One way ANOVA suggested a significant difference between TMS and prepared TMS-PF127 TPF with respect to their solubility and dissolution data. Holm-Sidak multiple comparison analysis also suggested significant difference among each of the TPFs with respect to their solubility and dissolution data. Shape and surface morphology of TMS (A), physical mixture (B) and optimized TMS-pluronic TPF1:3 (C) was assessed by using Field Emission Scanning Electron Microscopy (FESEM) (fig. 2). FE-SEM study of pure TMS revealed needle like geometrical shape which confirmed its crystalline structure (fig. 2A) whereas in physical mixture, the crystalline structure of TMS was remaining unchanged (fig. 2B). This suggested that when crystalline TMS was treated with PF127 without any solvent, the crystalline state of TMS was unaltered. FE-SEM photograph of optimized TPF1:3 revealed that the distinctive crystalline structure and geometric shape of TMS was completely disappeared (fig. 2C) in the prepared TMS-pluronic TPF and crystalline drug particles dispersed in the polymer carrier which confirmed the conversion of crystalline TMS to its amorphous form[30]. So, FE-SEM study confirmed the conversion of crystalline TMS to its amorphous state.
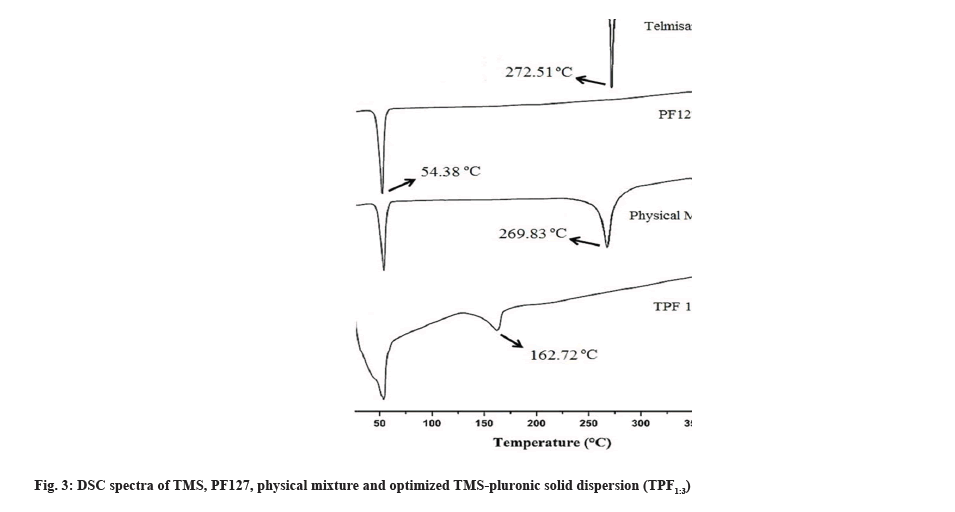
The physicochemical state of TMS, PF127, physical mixture and optimized TMS-pluronic TPF (TPF1:3) was accessed using DSC and shown in fig. 3. DSC study revealed that pure TMS showed a single melting endothermic event at 272.51° whereas PF127 showed its melting endotherm at 54.38°. The physical mixture of TMS and pluronic showed a melting endotherm at 269.83° corresponding to the melting point of TMS (fig. 3). In the optimized TPF1:3, the sharp melting endotherm of pure TMS was disappeared and a new endothermic peak at 162.72° was reappeared (fig. 3). This may be due to the fact that during the preparation process of TPF, continues stirring was applied for uniform mixing of TMS and PF127 and due to this applied shear, TMS molecules has lost their molecular mobility. Furthermore, TMS molecules get entrapped within PF127 and “freeze”. It inhibited the nucleation and absence of any crystal structures[31,32]. So, the disappearance of the characteristic peak of TMS may be due to the molecular level mixing of both drug and carrier[33] which results in the conversion of crystalline TMS to its amorphous form. The amorphization of TMS in the TPF was further confirmed by XRD study.
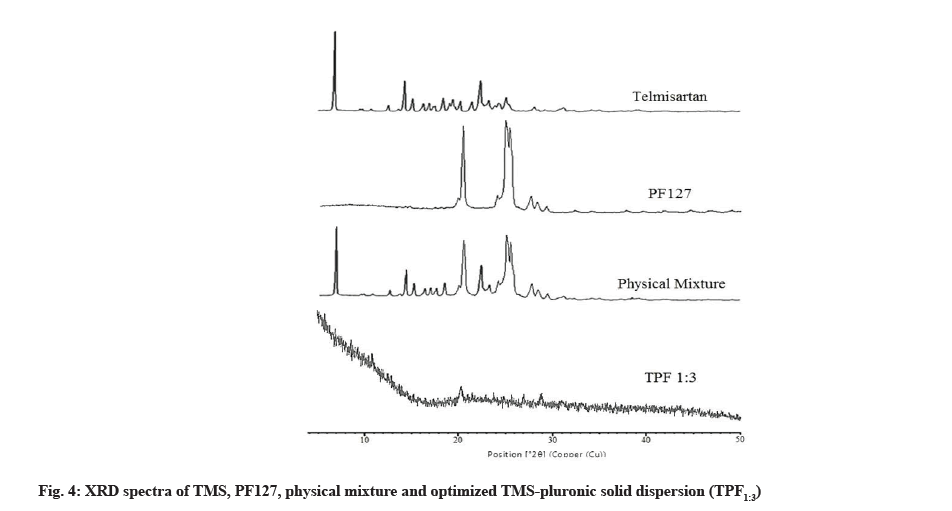
XRD spectra of TMS, PF127, physical mixture and optimized TMS-pluronic TPF1:3 were studied by X-ray diffractometer (X’Pert Pro, Panalytical, Netherlands). XRD pattern of TMS exhibited the diffraction peaks at 6.81°, 14.23°, 15.06°, 19.03°, 22.31° etc. (fig. 4). In the physical mixture all the characteristic peaks of TMS and PF127 were intact. This suggested that in this physical mixture, TMS was remaining in its crystalline state. In the optimized TMS-pluronic TPF1:3, no diffraction peaks of TMS and pluronic was observed (fig. 4). The disappearance of characteristic peaks indicates amorphization of drug crystals[8]. This revealed that when crystalline TMS was treated with PF127, it was converted in its amorphous state[12]. This amorphization of TMS in the prepared TPFs justifying the significant enhancement in solubility as well as dissolution of pure TMS.
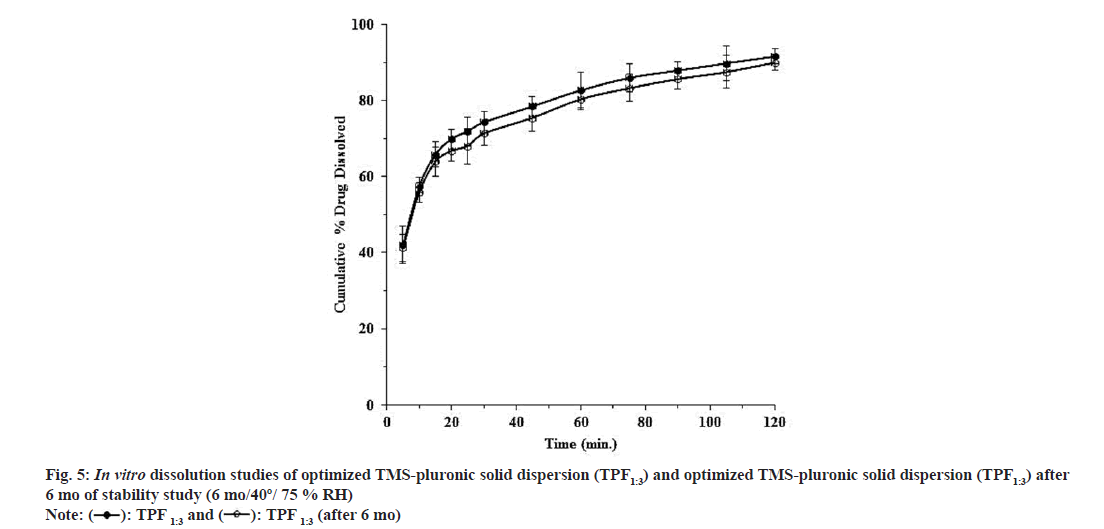
Optimized TMS-PF127 TPF1:3 was subjected to stability studies and % drug content, shelf life as well as physical appearance of the optimized TPF was examined (Table 3). The optimized batch was also subjected to in vitro dissolution study to observe any possible changes in drug dissolution during storage or not (fig. 5). From the experimental data, physical evaluation suggested the stable nature of the optimized TMS-PF127 TPF1:3 and no changes were observed with respect to its colour and odour. The shelf life of the optimized TPF1:3 at 40º/ 75 % RH was found to be 590 d (i.e., 1.62 y) (Table 3). In vitro dissolution studies also suggested no significant difference in drug dissolution of the stored TPF sample (fig. 5). This study confirmed that on storage the TPF was stable in nature.
In this present research solubility as well as dissolution of poorly soluble TMS was tried to be improve by TPF technique using various concentration of PF127. The experimental results revealed that prepared TMSPF127 TPF significantly improved the solubility as well as dissolution of TMS by 6.93 folds and 2.57 folds respectively. DSC and XRD studies confirmed the complete amorphization of drug crystals upon treating with PF127. Stability study also confirmed the stable nature of the prepared TMS-PF127 TPF with shelf life of 1.62 y. Finally, it was concluded that optimized concentration of PF127 could be able to improve the solubility and dissolution of TMS by TPF technique.
Acknowledgements:
Authors are grateful to Dr. B. C. Roy College of Pharmacy and A.H.S., Durgapur for providing all the facilities for this research work.
Conflict of interest:
The author(s) confirm that this article content has no conflicts of interest.
References
- Ku MS, Dulin W. A biopharmaceutical classification-based right-first-time formulation approach to reduce human pharmacokinetic variability and project cycle time from first-in-human to clinical proof-of-concept. Pharm Dev Technol 2012;17(3):285-302.
[Crossref] [Google Scholar] [PubMed]
- Blagden N, de Matas M, Gavan PT, York P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv Drug Deliv Rev 2007;59(7):617-30.
[Crossref] [Google Scholar] [PubMed]
- Frizon F, de Oliveira EJ, Donaduzzi CM, Mitsui ML, Marchetti JM. Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 TPFs by solvent methods. Powder Technol 2013;235:532-9.
- Sridhar I, Doshi A, Joshi B, Wankhede V, Doshi J. TPFs: an approach to enhance solubility of poorly water soluble drug. J Sci Innov Res 2013;2(3):685-94.
- McFall H, Sarabu S, Shankar V, Bandari S, Murthy SN, Kolter K, et al. Formulation of aripiprazole-loaded pH-modulated TPFs via hot-melt extrusion technology: In vitro and in vivo studies. Int J Pharm 2019;554:302-11.
[Crossref] [Google Scholar] [PubMed]
- Kumar AS, Ghosh S, Mehta GN. Efficient and improved synthesis of telmisartan. Beilstein J Org Chem 2010;6(1):25.
[Crossref] [Google Scholar] [PubMed]
- Cagigal E, Gonzalez L, Alonso RM, Jimenez RM. pKa determination of angiotensin II receptor antagonists (ARA II) by spectrofluorimetry. J Pharm Biomed Anal 2001;26(3):477-86.
[Crossref] [Google Scholar] [PubMed]
- Tran PH, Tran HT, Lee BJ. Modulation of microenvironmental pH and crystallinity of ionizable telmisartan using alkalizers in TPFs for controlled release. J Control Release 2008;129(1):59-65.
[Crossref] [Google Scholar] [PubMed]
- Stangier J, Su CA, Roth W. Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients. J Int Med Res 2000;28(4):149-67.
[Crossref] [Google Scholar] [PubMed]
- Wienen W, Entzeroth M, van Meel JC, Stangier J, Busch U, Ebner T, et al. A review on telmisartan: A novel, longâ€acting angiotensin IIâ€receptor antagonist. Cardiovasc Drug Rev 2000;18(2):127-54.
- Patel BB, Patel JK, Chakraborty S, Shukla D. Revealing facts behind spray dried TPF technology used for solubility enhancement. Saudi Pharm J 2015;23(4):352-65.
[Crossref] [Google Scholar] [PubMed]
- Zhong L, Zhu X, Yu B, Su W. Influence of alkalizers on dissolution properties of telmisartan in TPFs prepared by cogrinding. Drug Dev Ind Pharm 2014;40(12):1660-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Jiang T, Zhang Q, Wang S. Inclusion of telmisartan in mesocellular foam nanoparticles: Drug loading and release property. Eur J Pharm Biopharm 2010;76(1):17-23.
[Crossref] [Google Scholar] [PubMed]
- Dhibar M, Chakraborty S, Khandai M. Solid state manipulation of telmisartan via mechanochemical activation with kaolin: An assessment of biopharmaceutical properties of amorphous phase and its physical stability. Int J Pharm Sci Res 2021;12(9):5030-9.
[Crossref]
- Dhibar M, Chakraborty S, Basak S. Assessment of Effects of Solvents on cocrystallization by computational simulation approach. Curr Drug Deliv 2021;18(1):44-53.
[Crossref] [Google Scholar] [PubMed]
- Sekar V, Chellan VR. Immediate release tablets of telmisartan using superdisintegrant-formulation, evaluation and stability studies. Chem Pharm Bull 2008;56(4):575-7.
[Crossref] [Google Scholar] [PubMed]
- Lepek P, Sawicki W, Wlodarski K, Wojnarowska Z, Paluch M, Guzik L. Effect of amorphization method on telmisartan solubility and the tableting process. Eur J Pharm Biopharm 2013;83(1):114-21.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zhi Z, Jiang T, Zhang J, Wang Z, Wang S. Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J Control Release 2010;145(3):257-63.
[Crossref] [Google Scholar] [PubMed]
- Patel J, Kevin G, Patel A, Raval M, Sheth N. Design and development of a self-nanoemulsifying drug delivery system for telmisartan for oral drug delivery. Int J Pharm Investig 2011;1(2):112.
[Crossref] [Google Scholar] [PubMed]
- Francis MF, Cristea M, Winnik FM. Polymeric micelles for oral drug delivery: Why and how. Pure Appl Chem 2004;76(7-8):1321-35.
- Lavasanifar A, Samuel J, Kwon GS. Poly (ethylene oxide)-block-poly (L-amino acid) micelles for drug delivery. Adv Drug Deliv Rev 2002;54(2):169-90.
[Crossref] [Google Scholar] [PubMed]
- Aliabadi HM, Shahin M, Brocks DR, Lavasanifar A. Disposition of drugs in block copolymer micelle delivery systems: From discovery to recovery. Clinical Pharmacokinetics 2008;47:619-34.
[Crossref] [Google Scholar] [PubMed]
- Wei Z, Hao J, Yuan S, Li Y, Juan W, Sha X, et al. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: Formulation, optimization and in vitro characterization. Int J Pharm 2009;376(1-2):176-85.
[Crossref] [Google Scholar] [PubMed]
- Cao Y, Shi LL, Cao QR, Yang M, Cui JH. In vitro characterization and oral bioavailability of organic solvent-free TPFs containing telmisartan. Iran J Pharm Res 2016;15(2):385.
[Crossref] [Google Scholar] [PubMed]
- Zhong L, Zhu X, Luo X, Su W. Dissolution properties and physical characterization of telmisartan–chitosan TPFs prepared by mechanochemical activation. AAPS PharmSciTech 2013;14:541-50.
[Crossref] [Google Scholar] [PubMed]
- Dubey A, Kharia AA, Chatterjee DP. Enhancement of aqueous solubility and dissolution of telmisartan using TPF technique. Int. J Pharm Sci Res 2014;5(10):4478.
- Ahuja N, Katare OP, Singh B. Studies on dissolution enhancement and mathematical modeling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur J Pharm Biopharm 2007;65(1):26-38.
[Crossref] [Google Scholar] [PubMed]
- Craig DQ. The mechanisms of drug release from TPFs in water-soluble polymers. Int J Pharm 2002;231(2):131-44.
[Crossref] [Google Scholar] [PubMed]
- Vasconcelos T, Sarmento B, Costa P. TPFs as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today 2007;12(23-24):1068-75.
[Crossref] [Google Scholar] [PubMed]
- Giri BR, Kwon J, Vo AQ, Bhagurkar AM, Bandari S, Kim DW. Hot-melt extruded amorphous TPF for solubility, stability, and bioavailability enhancement of telmisartan. Pharmaceuticals 2021;14(1):73.
[Crossref] [Google Scholar] [PubMed]
- Baghel S, Cathcart H, O'Reilly NJ. Polymeric amorphous TPFs: A review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J Pharm Sci 2016;105(9):2527-44.
[Crossref] [Google Scholar] [PubMed]
- Theil F, Anantharaman S, Kyeremateng SO, van Lishaut H, Dreis-Kühne SH, Rosenberg J, et al. Frozen in time: kinetically stabilized amorphous TPFs of nifedipine stable after a quarter century of storage. Mol Pharm 2017;14(1):183-92.
[Crossref] [Google Scholar] [PubMed]
- Repka MA, Bandari S, Kallakunta VR, Vo AQ, McFall H, Pimparade MB, et al. Melt extrusion with poorly soluble drugs-an integrated review. Int J Pharm 2018;535(1-2):68-85.
[Crossref] [Google Scholar] [PubMed]
- Kwon J, Giri BR, Song ES, Bae J, Lee J, Kim DW. Spray-dried amorphous TPFs of atorvastatin calcium for improved supersaturation and oral bioavailability. Pharmaceutics 2019;11(9):461.
[Crossref] [Google Scholar] [PubMed]
- Park J, Cho W, Cha KH, Ahn J, Han K, Hwang SJ. Solubilization of the poorly water soluble drug, telmisartan, using Supercritical Snti-Solvent (SAS) process. Int J Pharm 2013;441(1-2):50-5.
[Crossref] [Google Scholar] [PubMed]
- Otsuka M, Matsumoto T, Kaneniwa N. Effect of environmental temperature on polymorphic solid-state transformation of indomethacin during grinding. Chem Pharm Bull 1986;34(4):1784-93.
[Crossref] [Google Scholar] [PubMed]
- Savolainen M, Kogermann K, Heinz A, Aaltonen J, Peltonen L, Strachan C, et al. Better understanding of dissolution behaviour of amorphous drugs by in situ solid-state analysis using Raman spectroscopy. Eur J Pharm Biopharm 2009;71(1):71-9.
[Crossref] [Google Scholar] [PubMed]
- Bley H, Fussnegger B, Bodmeier R. Characterization and stability of TPFs based on PEG/polymer blends. Int J Pharm 2010;390(2):165-73.
[Crossref] [Google Scholar] [PubMed]
 ): TMS; (
): TMS; ( ): TPF 1:1; (
): TPF 1:1; (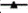 ): TPF 1:2; (
): TPF 1:2; ( ): TPF 1:3; (
): TPF 1:3; ( ): TPF 1:4 and (
): TPF 1:4 and ( ): TPF 1:5
): TPF 1:5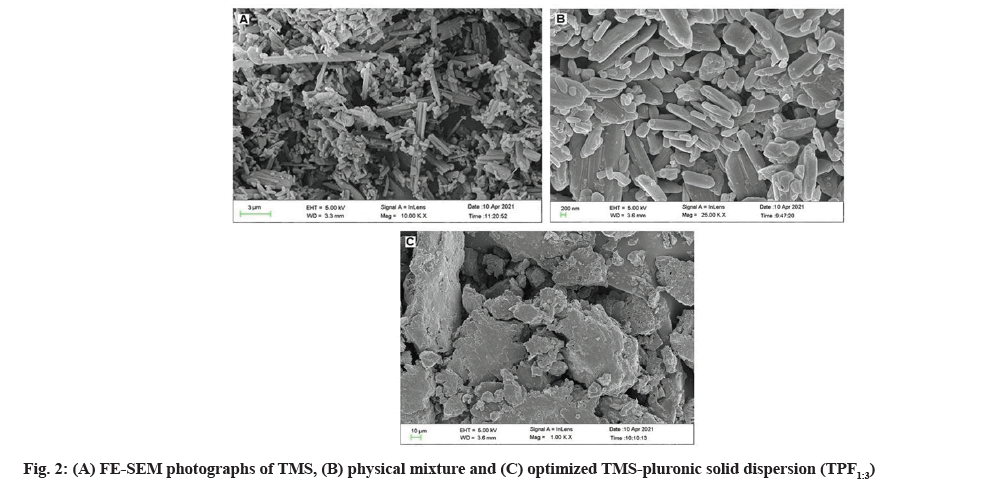
 ): TPF 1:3 and (
): TPF 1:3 and ( ): TPF 1:3 (after 6 mo)
): TPF 1:3 (after 6 mo)