- *Corresponding Author:
- A. Chaudhary
Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar-143005, Punjab
E-mail: ashun.chaudhary@gmail.com
| Date of Submission | 04 May 2016 |
| Date of Revision | 12 September 2016 |
| Date of Acceptance | 24 September 2016 |
| Indian J Pharm Sci 2016;78(5):615-623 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The present study was conducted on sprouts (5 and 7 days) of turnip (Brassica rapa), cauliflower (Brassica oleracea) and mustard (Brassica juncea) for bioactivity. Their antioxidant potential was assessed by 2,2-diphenyl-1-picrylhydrazyl, superoxide anion radical scavenging assays at 0.125-2 mg/ml concentration. The extract of turnip, cauliflower and mustard (5 and 7 days) showed a potent antioxidant effect and significant cytotoxic effect at 100 µg/ml concentration of extract. The antiproliferative potential was also evaluated by applying cell cycle and intracellular reactive oxygen species generation assay at IC50 value. Various phytochemicals and hydrolytic products of glucosinolates were observed in a different extract of turnip, cauliflower, and mustard sprouts. Flow cytometric analysis showed that all the extracts caused an increase in a G0 population of the PC-3 cells in cell cycle analysis and an increase in intracellular reactive oxygen species generation as compared to untreated cells. Confocal imaging of the cells stained with 4',6-diamidino-2-phenylindole and 2,7-dichlorodihydrofluorescein diacetate showed DNA fragmentation and increase of fluorescence which supports apoptosis and intracellular generation of the reactive oxygen species as the possible cause of cell death. The reported activity was correlated with the presence of different organosulfur compounds identified by gas chromatography-mass spectroscopy.
Keywords
Brassica sprout, antioxidant assay, cell cycle, ROS, confocal microscopy, MTT assay
In recent years, health protection by natural products or plant derived foods has received a considerable attention [1]. Specific groups of vegetables are particularly rich in potentially protective phytochemicals, especially Brassica contains a high concentration of constituents with antioxidant properties (e.g. carotenoids, vitamin C and folates) as well as glucosinolates precursors of isothiocyanates (ITCs) and indoles that modulate the activity of xenobiotic biotransformation [2]. A diet rich in fruits, cruciferous vegetables has been linked to reduce the risk of many chronic diseases, including cancer. The importance of this family for food crops has led to its selective breeding throughout the history. Cruciferous sprouts of broccoli, alfalfa, buckwheat and bean etc. have received considerable attention due to their rich content of health-promoting phytochemical constituents such as glucosinolates phenolic compounds and ascorbic acid related to cancer prevention as well as having antioxidant properties [3-7].
During sprouting generally, phytonutrient content increases as compared to seeds and consumption of these sprouts is the best way to gain all the health benefits [8,9]. Naturally occurring ITCs, found abundantly in cruciferous vegetables, inhibit tumorigenesis by inducing apoptosis and arresting cell cycle progression. In addition, induction of detoxification enzymes suggested the mechanism through which cruciferous vegetable consumption or the consumption of the glucosinolate breakdown products reduces cancer risk [10]. Due to these protective effects, consumers can find an extraordinary variety of different types of sprouts which represent cruciferous family in the market. The use of seed sprouts as food has spread in the past few decades from far eastern countries to parts of the western world. Therefore, the objectives of the current study are to investigate different extracts for the antioxidant and antiproliferative activity of 5 and 7 days old sprouts from cauliflower, turnip and mustard. In addition, volatile phytoconstituents were also identified by gas chromatography-mass spectroscopy (GC/MS). Moreover, the phytoconstituents of various extracts were examined in order to study their possible relationship with antioxidant and antiproliferative studies.
Materials and Methods
Roswell Park Memorial Institute (RPMI-1640) medium, fetal bovine serum (FBS), penicillin, streptomycin, L-glutamine, 4',6-diamidino-2- phenylindole (DAPI) and the fluorescent probes 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA), 2,2’-diphenyl-1-picryl hydrazyl (DPPH), ribonuclease (RNase) A, and Triton X-100 were obtained from Sigma-Aldrich Corp, St. Louis, MO, USA. Propidium iodide (PI) was purchased from Invitrogen Life Technologies (Carlsbad, CA). Ammonium molybdate, ethylenediaminetetraacetic acid (EDTA), nitroblue tetrazolium (NBT), phenazine methosulphate (PMS), nicotinamide adenine dinucleotide phosphate reduced (NADH), dimethyl sulfoxide (DMSO), ascorbic acid and remaining reagents were of analytical grade. A prostate cancer (PC-3) cell line was obtained from Indian Institute of Integrative Medicine (IIIM), Jammu, India.
Plant material
The seeds of cauliflower (B. oleracea), turnip (B. rapa) and mustard (B. juncea) were procured from Amritsar local market. Seeds were surface sterilized with 70% ethanol for 1 min followed by 1.3% sodium hypochlorite for 15 min and then allowed to germinate in seed germinator. The homogenized plant material was labeled according to their harvested stage like 5 days of mustard (M5D), 7 days of mustard (M7D), 5 days of cauliflower (C5D), 7 days of cauliflower (C7D), 5 days of turnip (T5D) and 7 days of turnip (T7D). All sprouts were grown with a 16 h light and 8 h dark photoperiod at 24° temp, rapidly and gently collected from the trays and homogenized with a grinder.
Extraction procedure
Extraction of plant material was done using the method described by Liang et al. [11] fresh plant material was homogenized in water for 5 min and left for autolysis at room temperature for 30 min. After autolysis, the meal was extracted two times with dichloromethane (DCM), which was combined and then salted with 2.5 g anhydrous sodium sulfate and the fraction was dried at 30° under vacuum on a rotary evaporator.
DPPH radical scavenging assay
The hydrogen donating or a radical scavenging ability of test samples was measured using the stable radical DPPH [12]. The reaction mixture contains 100 μl of different fraction concentrations (0.125 to 2 mg/ml) and 2 ml of DPPH (0.1 mM in methanol solution). The absorbance of the reaction mixture was recorded against the blank at 517 nm. The percent decoloration of the sample in DPPH assay was calculated as radical scavenging activity %=(A0-A1/A0)×100, where, A0 is the absorbance of control (without test sample), A1 is the absorbance of the reaction mixture (with test sample).
Superoxide anion radical scavenging assay
The superoxide anion scavenging activity of different test samples was analyzed by using a method described by Nishikimi et al. [13] Different solutions like 1 ml of 156 μM NBT, 1 ml of 468 μM NADH prepared in phosphate buffer (100 mM, pH 7.4) and various concentration of test samples (0.125 to 2 mg/ml) were mixed and the reaction was started by adding 100 μl of PMS solution (60 μM) prepared in phosphate buffer (100 mM, pH 7.4). The reaction mixture incubated at 25° for 5 min and absorbance was recorded at 560 nm against the control. Radical scavenging activity %=(A0–A1/A0)×100, where A0 is the absorbance of control, A1 is the absorbance of a test sample.
Cell lines and culture
The human PC-3 cell line grown and maintained in RPMI-1640 medium having FBS (10%), penicillin (100 units/ml), streptomycin (100 mg/ml), glutamine (2 mM) were maintained in CO2 incubator at 37°, 90% humidity and 5% CO2 conditions. The cells treated with extracts dissolved in DMSO while the untreated control cultures received only DMSO.
MTT assay
The dimethylthiazolyldiphenyltetrazolium bromide (MTT) assay was performed following the welldescribed procedure with minor modifications [14]. Human PC-3 cells were centrifuged at 1000 rpm for 5 min at 4°. The cell pellet was resuspended in complete growth medium to get 1.5×105 cells/ml and 100 μl of cell suspension/well was seeded in tissue culture plate. Cells were treated with different concentrations of test material incubated for 12 h in a CO2 incubator. Thereafter, 20 μl of freshly prepared MTT solution (5 mg/ml in PBS, sterile filtered) was added to each well and thoroughly mixed. The samples were incubated for 4 h at 37°, to allow metabolization of MTT. The supernatant was aspirated out after centrifuging at 2000 rpm for 10 min. MTT-formazan crystals (MTT metabolic product) were resuspended in 100 μl of DMSO. Thereafter, plates were stirred for 20 min in order to dissolve formazan crystals and absorbance was measured at 570 nm.
Cell cycle analysis using propidium iodide
PC-3 cells incubated in RPMI medium supplemented with 10% FBS in 24 well plates, at 1×106 cells/well with or without different sprouts extract at their respective IC50. After 12 h of treatment, the floating and trypsinized cells were harvested and centrifuged at 1500 rpm for 5 min then washed with PBS twice. For each well, a volume of the cell suspension corresponding to 1×106 cells was centrifuged and the resultant cell pellet was resuspended in ice-cold PBS (1 ml). The cells were fixed in chilled 70% ethanol and incubated for 2 h. The supernatant was discarded and cells were suspended in 500 ml PBS with 15 ml propidium iodide (1 mg/ml). Cells were analyzed in FL-2 channel by BD Accuri C6 flow cytometer (BD Biosciences Immunocytometry Systems, San Jose, CA). DNA content and cell cycle phase distributions were modeled from events by excluding cell aggregates based on scatter plots [15].
Measurement of intracellular peroxides
The cells plated in 24 well plates, at 1×106 cells allowed to adhere for 6 h and then grown in the presence or absence of extract. The cells were harvested after 12 h by collecting trypsinized cells together with floating cells in the medium and stained with DCFH-DA for 30 min at the end of treatment. The cell suspension corresponding to 1×106 cells was centrifuged and the resultant cell pellet was resuspended in ice-cold PBS (1 ml). A majority of cellular reactive oxygen species (ROS) were produced by mitochondria during stress conditions that disrupt mitochondrial electron transport. Therefore, a level of intracellular peroxides in PC-3 cells treated with extract at IC50 for 12 h was determined by using DCFH-DA [16].
GC/MS analysis
The GC/MS analysis was carried out on a Shimadzu (QP 2010) series GC/MS (Tokyo, Japan), AOC- 20i auto-sampler coupled and a DB-5MS capillary column, (30 mm×0.25 mm i.d, 0.25 μm). The initial temperature of the column was 70° maintained for 4 min. programmed to 230° at 4°/min. held for 15 min. at 230°; the sample injection volume was 2 μl in GC grade DCM. Helium was used as carrier gas at a flow rate of 1.1 ml/min in split mode (1:50).
Statistical analysis
The superoxide anion radical scavenging assay and DPPH assay was performed in triplicate and the data presented as mean. To compare the statistical difference in means, one-way analysis of variance (ANOVA) was performed with an honestly significant difference (HSD) using Tukey’s test at P≤0.05 (95% level of significance) using Microsoft excel for superoxide anion radical scavenging assay and DPPH assay. The antioxidant capacity and MTT assay calculation done by Microsoft Office excel 2007.
Results and Discussion
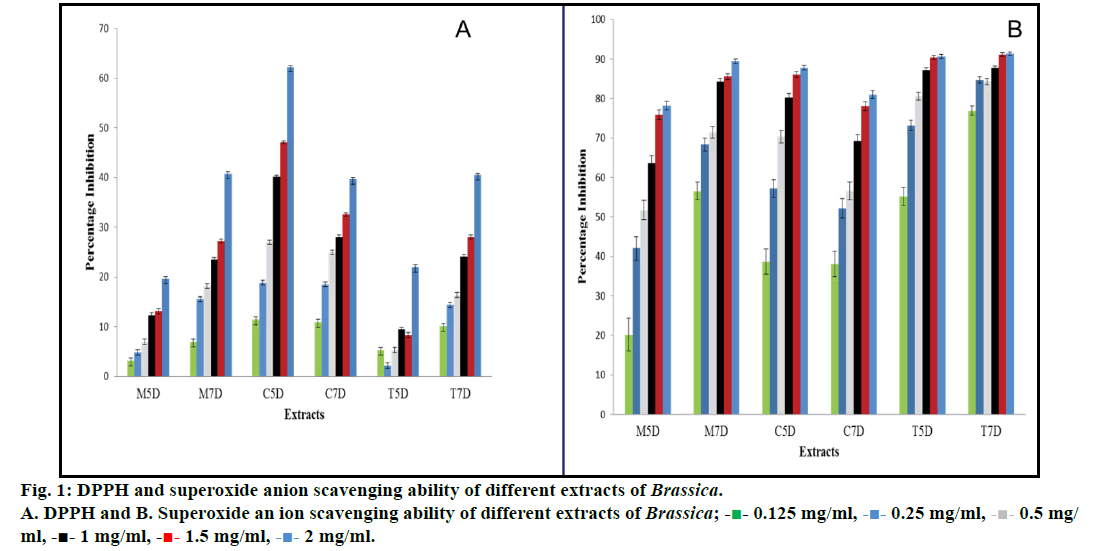
The DPPH radical scavenging activity of all the fractions gradually increased in a concentration-dependent manner. The radical scavenging activities were in the order C5D>M7D>T7D>C7D>T5D>M5D. The M7D extract showed maximum percent inhibition (62.2%) at 2 mg/ml concentration and lowest in T5D (2.19%) at 0.25mg/ml. The extracts C5D, M7D, T7D, C7D, T5D and M5D showed a percent inhibition of 62.20, 40.78, 40.45, 39.63, 21.98 and 19.67%, respectively at 2 mg/ ml concentration (Table 1 and fig. 1a).
| Extracts | IC50 | ||
|---|---|---|---|
| MTT assay(µg/ml) | SAO assay (mg/ml) | DPPH assay (mg/ml) | |
| M5D | 111.6 | 0.463 | 5.79 |
| M7D | 81.11 | 0.059 | 2.76 |
| C5D | 95.57 | 0.170 | 1.51 |
| C7D | 71.58 | 0.26 | 2.75 |
| T5D | 63.5 | 0.053 | 5.92 |
| T7D | 43.61 | 0.00032 | 2.78 |
Table 1: Ic50 Values of Different Extracts Treated With Different Concentration
It was noticed that all the extracts were capable of scavenging hydrogen peroxide in a dose-dependent manner. The scavenging activity was in the order T7D>T5D>M7DC5D>C7D>M5D, where T7D extract showed maximum percent inhibition of 91.30% at 2 mg/ml concentration and lowest in M5D i.e. 20.17% at 0.125 mg/ml concentration. The extracts T7D, T5D M7D, C5D, C7D and M5D showed percent inhibition of 91.30, 90.60, 89.40, 87.70, 80.90 and 78.15%, respectively at 2 mg/ml concentration (Table 1 and fig. 1b).
To investigate sprout extracts (10-100 μg/ml) for antiproliferative potential and to understand their mechanism of action, PC-3 cell line was used. To study the potential of different sprout extracts on proliferation and survival of PC-3 cells, the cells were exposed to 10-100 μg/ml of extracts for 12 h. Sprout extracts induced cell death in a dose-dependent manner. The highest effective IC50 was reported in T7D extract i.e. 43.60 μg/ml whereas lowest effective values of 111.60 μg/ml in M5D extract. The T5D, C7D, M7D and C5D have 63.50, 71.58, 81.11 and 95.57 μg/ml IC50 values, respectively (Table 1). All the extracts showed a very good cytotoxic effect on the PC-3 cells therefore, they were further studied to understand the mechanism involved in cell death.
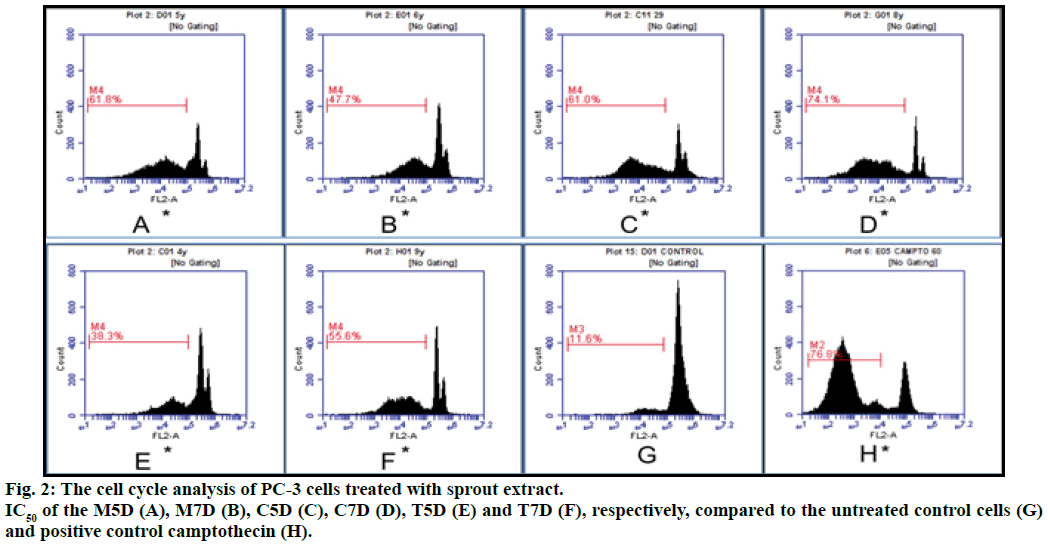
In the present study, PC-3 cells treated with plant extracts at IC50 value for 12 h restricted the cells in hypodiploid (sub G0) phase of the cell cycle in concentration dependent manner as compared to the control. The sub G0 population in M5D, T5D, C5D, M7D, T7D and C7D was 61.80, 38.30, 61.00, 47.60, 55.60 and 74.10%, respectively. The cells treated with positive control camptothecin and untreated cells have 39.40% and 15.60% cells in G0 phase respectively and all extracts an exhibited increase in the sub G0 population. The percentage of G2/M phase cells was increased when cell cycle analysis was done with different extract on PC-3 cells. The cell cycle progression was significantly arrested in G2/M phase after 12 h of treatment with different extracts (fig. 2).
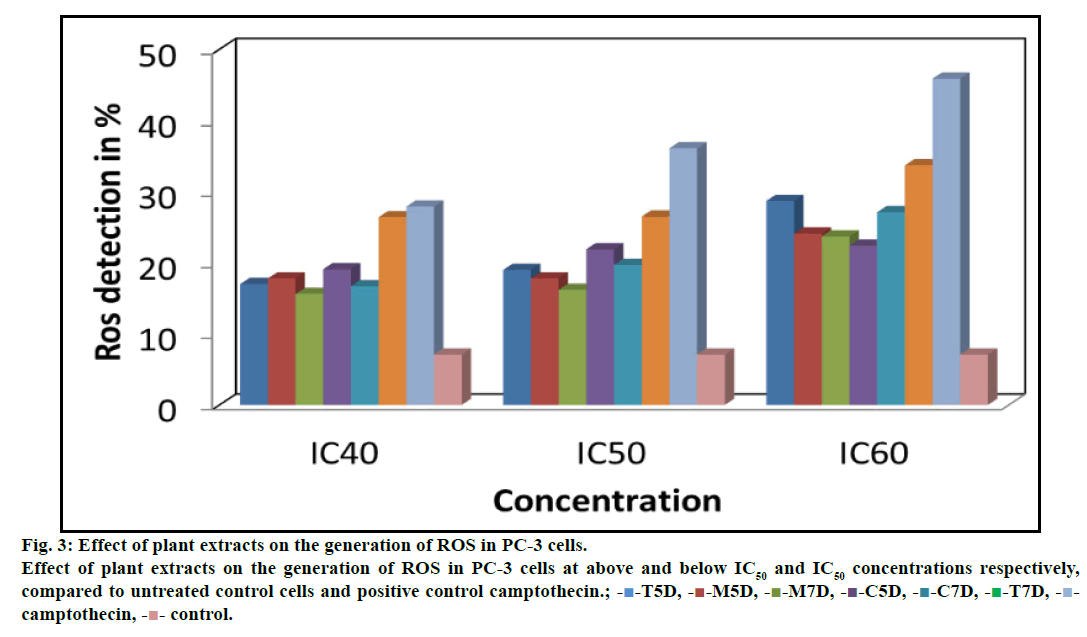
ROS play an important role in depolarizing mitochondria and apoptosis induction. Flow cytometric analysis of PC-3 cells treated with different concentrations of plant extracts was done after staining with DCFH-DA, which penetrate the cells, react with cellular esterases and ROS and then metabolized into fluorescent DCF. A specific fluorescent probe for measuring hydrogen peroxides and hydroxyl radicals revealed a concentration-dependent increase in DCF positive cell population indicating generation of reactive oxygen species. The level of ROS generation was 19.00, 17.80, 16.20, 21.90, 19.70 and 26.50% in sprout extract T5D, M5D, M7D, C5D, C7D and T7D at their respective IC50 after 12 h exposure. The ROS generation was enhanced by 36.10% in treatment with positive control (camptothecin at its respective IC50) as compared to (7.10%) untreated PC-3 cells (fig. 3).
Fig. 3: Effect of plant extracts on the generation of ROS in PC-3 cells.
Effect of plant extracts on the generation of ROS in PC-3 cells at above and below IC50 and IC50 concentrations respectively,
compared to untreated control cells and positive control camptothecin.; - -T5D, -
-T5D, - -M5D, -
-M5D, - -M7D, -
-M7D, - -C5D, -
-C5D, - -C7D, -
-C7D, - -T7D, -
-T7D, - -
camptothecin, -
-
camptothecin, - - control.
- control.
The GC/MS analysis of an individual component in the extract was done by comparison of their mass spectra (MS) with NIST database and Adams libraries [17,18]. It was observed that 3-butenyl isothiocyanate was present in three extracts M7D, T7D and C5D. Cyclopropane, isothiocyanates present in T5D, erucin in T7D and Iberin nitrile in both T5D and C7D (Table 2). Tetradecanal and linoleic acid are the phytochemical which are present in all extracts.
| Phytoconstituents | M7D | M5D | T7D | T5D | C7D | C5D |
|---|---|---|---|---|---|---|
| -butenyl isothiocyanate | P | A | P | A | A | P |
| 5-methylthiazole | P | A | A | A | A | A |
| ,5,5-trimethyl-2-cyclohexene-1-one | P | A | P | A | A | P |
| 4,5-dimethyl-thiazole | P | P | P | A | A | A |
| 1,3-tetradecadien-1-ol | P | A | A | A | A | A |
| Tridecane | P | A | A | A | A | A |
| 1-tetradecanol | P | P | P | A | A | P |
| Tetradecane | P | A | P | A | A | P |
| Hexadecane | P | A | A | A | A | A |
| Tetradecan-3-yl hexanoate | P | A | A | A | A | A |
| 2,4-di-tert-butylphenol | P | A | A | A | P | P |
| Octadecane | P | A | P | P | P | P |
| -octadecane | P | A | A | A | A | A |
| N-octadecane | P | A | A | A | A | A |
| Oleic acid | P | A | P | P | A | A |
| Cyclotetradecane | P | A | A | P | P | P |
| Propyleneoxide | P | A | A | A | A | P |
| 9-eicosene | P | A | A | A | A | P |
| Linoleic acid | P | P | P | P | P | P |
| Tetradecanal DB-5 | P | P | P | P | P | P |
| Cyclohexanoneoxime | A | A | A | A | P | P |
| 1-dodecene | A | A | A | P | A | P |
| Octadecyl ester | A | A | A | A | A | P |
| Heptadecane | A | A | A | A | A | P |
| 1,8-Di(4-nitrophenylmethyl)-3,6-diazahomoadamantan-9-one | A | A | A | A | A | P |
| Eicosene | A | A | A | A | A | A |
| Pentacosane | A | A | A | A | A | P |
| Guai-1(10)-en-11-ol | A | A | A | A | A | P |
| Docosene | A | A | A | P | A | P |
| Cyclopropylisothiocyanate | A | A | A | P | A | A |
| Dodecyl fluoride | A | A | A | A | A | A |
| Iberin nitrile | A | A | A | P | P | A |
| Phenol, 3,5-bis(t-butyl) | A | A | A | P | A | A |
| 1-hexadecanol | A | A | A | P | A | A |
| Neophytadiene | A | A | P | P | A | A |
| Isothiazole, 3-methyl | A | P | P | A | A | A |
| Erucin | A | A | P | A | A | A |
| 1-hexadecene | A | A | P | P | A | A |
| (2R,4S)-2-(2-methyl-3hydroxy-5-hydroxymethylenepyridine-C4) | A | A | P | A | A | A |
| 2H-Benzocyclohepten-2-one,3,4,4a.5,6,7,8,9-octahydro-4a-methyl | A | A | P | A | A | A |
| 1,3,5-Triazine-2,4-diamine,6-chloro-N-ethyl | A | A | P | A | A | A |
| 1,1,4,4-tetradeuteriobutadiene | A | A | P | A | A | A |
| Z,Z-8,10-Hexadecadien-1-ol | A | A | P | A | A | A |
| Methyl 3-oxo-5-(1-nitro-2-oxocyclododecyl)pentanoate | A | A | P | A | A | A |
| 2,5-Furandione,3-(dodecenyl)dihydro | A | A | P | A | A | A |
| ,7,11,15-tetramethyl-2-hexadecen-1-ol | A | A | P | A | A | A |
| Cis-9,10-Epoxyoctadecanoic acid | A | A | P | A | A | A |
| Oxiraneoctanoic acid, 3-octyl-, methyl ester, cis | A | A | P | A | A | A |
| 1,2-benzenedicarboxylic acid, dibutyl ester | A | A | P | A | A | A |
| 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | A | A | P | A | A | A |
| 1-nonadecene | A | A | P | A | A | A |
| 9-tricosene, (z)- | A | A | P | A | A | A |
| Decanoic acid | A | A | A | A | P | A |
| Cyclobutane, methoxy- | A | A | A | A | P | A |
| Trimethylsilyl 2-[(trimethylsilyl)oxy]hexacosanoate | A | A | A | A | P | A |
| Phytolacteate | A | A | A | A | P | A |
| Decyloctylphthalate | A | A | A | A | P | A |
| Dodecenal | A | A | A | A | P | A |
Table 2: Different Phytoconstituents Present In Extracts of Brassica Sprouts
A plant based diet is regarded as one of the potential chemopreventive agents. The extracts of fruits and vegetables and their bioactive components have received particular attention, and many of these have been evaluated as potential nutraceuticals and functional foods [19]. In this paper, we have focused on volatile phytochemicals in sprouts, which are present at significant levels in members of the Brassica family. There was also a report on comparison of bioactive phytochemical content and release of ITCs in selected Brassica sprouts [20]. Their overall protective effect has been generally attributed to the modulation of xenobiotic metabolilizing enzymes and enzymatic antioxidative defense system [21]. However, whether these effects can mainly be ascribed to a single class of molecules, e.g. glucosinolates or ITCs, or to their complex interactions with other phytochemicals present in the whole vegetable, still remains unknown. Therefore, the extracts were profiled for the presence of phytochemicals using GC/MS which showed wide variation. The cruciferous sprouts were found to be rich in many sulfur-containing compounds, such as allyl methyl sulfide and allyl propyl sulfide which are reported in the literature as strong antiproliferative agents [22]. However, till date, no study had considered the degree of variability in antioxidant and antiproliferative potential with respect to the different developmental stage. The DPPH• radical-scavenging activity for samples of sprouts (M5D, M7D, T5D, T7D, C5D and C7D) expressed as percent inhibition from 2.19 to 62.2%. Although all the samples had considerable OH• inhibition activity whereas most of the samples statistically significantly different (P<0.05). In the present study, all sprouts extract showed a concentration-dependent scavenging of DPPH radical with the maximum activity of 62.2% in cauliflower (C5D). The superoxide radicals generated from dissolved oxygen by PMS-NADH coupling can be measured by their ability to reduce NBT. It was noticed that all the extracts exhibited scavenging of hydrogen peroxide in an amount dependent manner. Highest scavenging of hydrogen peroxide was reported by turnip seven day (T7D) i.e. 91.3% at 2 mg/ml concentration of the extract. One way ANOVA statistical analysis showed the significant effect of dose on the free radical scavenging. Our results indicated variations in phytochemicals as well as correlative changes in the antioxidant capacity of different sprouts extracts.
Chemotherapeutic drugs are known to induce cytotoxicity in tumor cells through diverse mechanisms, in which signaling events play an important role depending upon the cell type and stimulus [23,24]. In the current study, antiproliferative activity was detected by the MTT assay, a colorimetric method for determining the number of viable cells in proliferation. The crude sprouts extract (10-100 μg/ml) showed high antiproliferative activity in the human prostate cancer (PC-3) cells (Table 1). A significant dose-dependent antiproliferative effect was observed in human prostate cancer cells (PC-3) in the presence of different sprouts extract in the present findings. The highest antiproliferative activity was found with the turnip seven-day sprout (T7D) with IC50 of 43.6 μg/ml and lowest effective values of 111.6 μg/ml in mustard five-day sprout (M5D) extract. The literature studies revealed the lowest effective dose for HT-29 colon carcinoma cells was 0.25 g eq ww/ml for broccoli and 0.50 g eq ww/ml for cauliflower and brussels sprouts [25].
As all the extracts showed a significant effect on the PC-3 cell line, therefore they were further studies to know the mechanism involved in cell death. Furthermore, the cytostatic effect of the individual biologically active compound is less than the crude plant sprouts extracts [26]. Results of MTT assay and cell morphological changes are supported further by analysis the cell cycle and intracellular measurement of ROS. There are reports which show that sulforaphane exerts its anti-proliferative effect by arresting the cell cycle; this arrest has been documented in the colon, prostate, breast, bladder and T cells [27]. It is well known that cell growth and proliferation of mammalian cells are dependent on cell cycle progression [28]. The fluorescence intensity of sub G0 cell fraction represented the apoptotic cell population. Previous studies revealed that anticancer agents arrest the cell cycle at the G0/ G1, S or G2/M phase and then induce apoptotic cell death [15,24,29]. The cell cycle arrest has become an appreciated target for management and treatment of tumor cells with cytotoxic agents. In the present study, apoptotic cell death increased significantly after treatment with extracts for 12 h, as a gradual increase in sub G0 population and arrest of PC-3 cells in sub G2M phase. It has also been mentioned in the literature that benzyl isothiocyanate induces arrest of cells at G2/M phase and apoptosis in human melanoma A375.S2 cells through ROS [30]. Cancer cells undergo apoptosis either by a generation of free radicals or by depletion of endogenous antioxidants [31]. In the present study, an elevated level of intracellular ROS was observed in the PC-3 cells with an increase in the concentration of sprouts extracts. This clearly indicates that sprout extracts induced cytotoxicity by a generation of ROS. Likewise, the studies in the literature also revealed ROS mediated apoptosis by pomegranate peel extract and in sprouts of crucifers [32,33]. Chemoprevention, a relatively new strategy to prevent cancer, depends on the use of nontoxic chemical substances, to block, reverse or retard the process of carcinogenesis. DNA damage activates the G2/M checkpoint, which prevents damaged cells in G2 phase from entering into mitosis. It is pertinent to mention that the present study report first time that the extraction of sprouts with DCM in vitro cytotoxicity against PC-3 cell lines and induce apoptosis as evidenced by a measurement of several biological endpoints of apoptosis such as the appearance of apoptotic bodies and increase in sub G0 DNA fraction in cells. Although, the extracts of different sprouts exhibited one or the other bioactivity but the C5D extract consistently showed good activity in most of the assays.
In conclusion, this study has shown that plant derived sprout byproducts are a good and cheap source of antioxidants which could be industrially exploited. The extracts showed the highest scavenging activities against superoxide radical followed by and DPPH. Further, in vitro antiproliferative study supports that sprouts are a good source of anticancer agents and the activity can be correlated to phytochemicals high amount of sulfur rich compounds funding.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Foley CM, Kratz AM. Resources and guidelines on buying and using nutraceuticals. In: E Ernst, editor. Nutraceuticals: The complete encyclopedia of supplements, herbs, vitamins, and healing foods. New York: Berkeley Publishing Group; 2000. p. 635-47.
- International Agency for Research on Cancer. Cruciferous vegetables, isothiocyanates and indoles. IARC Handbook of Cancer Prevention, IARC; 2004.
- Kim SL, Kim SK, Park CH. Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Res Int 2004;37:319-27.
- Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA 1997;94:10367-72.
- Tian Q, Rosselot RA, Schwartz SJ. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Biochem 2005;343:93-9.
- Barillari J, Canistro D, Paolini M, Ferroni F, Pedulli GF, Iori R, et al. Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in rocket (Eruca sativa Mill.) seeds and sprouts. J Agric Food Chem 2005;53:2475-82.
- Takaya Y, Kondo Y, Furukawa T, Niwa M. Antioxidant constituents of radish sprout (Kaiware-daikon), Raphanus sativus L. J Agric Food Chem 2003;51:8061-66.
- Lintschinger J, Fuchs N, Moser H, Jager R, Hlebeina T, Markolin G, et al. Uptake of various trace elements during germination of wheat, buckwheat and quinoa. Plant Food Hum Nutr 1997;50:223-37.
- Martínez-Villaluenga C, Frías J, Gulewicz P, Gulewicz K, Vidal-Valverde C. Food safety evaluation of broccoli and radish sprouts. Food ChemToxicol 2008;46:1635-44.
- Keum YS, Jeong WS, Kong AN. Chemopreventive functions of isothiocyanates. Drug News Perspect 2005;18:445-51.
- Liang H, Yuan QP, Dong HR, Liu YM. Determination of sulforaphane in broccoli and cabbage by high-performance liquid chromatography. J Food Comp Anal 2006;19:473-76.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;26:1199-1200.
- Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazinemethosulfate and molecular oxygen. Biochem Biophys Res Commun 1972;46:849-54.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
- Hu X, Zhang X, Qiu S, Yu D, Lin S. Salidroside induces cell-cycle arrest and apoptosis in human breast cancer cells. Biochem Biophys Res Commun 2010;398:62-7.
- Royall JA, Ischiropoulos H. Evaluation of 2′, 7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys 1993;302:348-55.
- NIST/ EPA/NIH. Mass Spectral Library Database; 1998.
- Adams RP. Identification of essential oil components by gas chromatography/quadruple mass spectroscopy. Carol Stream, Illionis: Allured Publishing Corporation; 2001.
- Moreno DA, Carvajal M, López-Berenguer C, García-Viguera C. Chemical and biological characterization of nutraceutical compounds of broccoli. J Pharm Biomed Anal 2006;41:1508-22.
- De Nicola GR, Bagatta M, Pagnotta E, Angelino D, Gennari L, Ninfali P, et al. Comparison of bioactive phytochemical content and release of isothiocyanates in selected Brassica sprouts. Food Chem 2013;141:297-303.
- Ioannides C, Konsue N. A principal mechanism for the cancer chemopreventive activity of phenethylisothiocyanate is modulation of carcinogen metabolism. Drug Metab Rev 2015;47:356-73.
- Brandt K, Christensen LP, Hansen-Møller J, Hansen SL, Haraldsdottir J, Jespersen L, et al. Health promoting compounds in vegetables and fruits: a systematic approach for identifying plant components with impact on human health. Trends Food Sci Tech 2004;15:384-93.
- Hoshino T, Hara A, Inoue M, Honda J, Imai Y, Oizumi K, et al. Flow cytometric measurement of NK cell cytotoxicity J Clin Lab Immunol 1991;36:39-43.
- Tian Z, Chen S, Zhang Y, Huang M, Shi L, Huang F, et al. The cytotoxicity of naturally occurring styryl lactones. Phytomedicine 2006;13:181-6.
- Ferrarini L, Pellegrini N, Mazzeo T, Miglio C, Galati S, Milano F, et al. Antiproliferative activity and chemoprotective effects towards DNA oxidative damage of fresh and cooked Brassicaceae. Br J Nutr 2012;107:1324-32.
- Vickers A. Botanical medicines for the treatment of cancer: rationale, overview of current data, and methodological considerations for phase I and II trials. Cancer Invest 2002;20:1069-79.
- Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci 2007;64:1105-27.
- Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol 2005;23:9408-21.
- Sun J, Liu RH. Cranberry phytochemical extracts induce cell cycle arrest and apoptosis in human MCF-7 breast cancer cells. Cancer Lett 2006;241:124-34.
- Huang SH, Wu LW, Huang AC, Yu CC, Lien JC, Huang YP, et al. Benzyl isothiocyanate (BITC) induces G2/M phase arrest and apoptosis in human melanoma A375. S2 cells through reactive oxygen species (ROS) and both mitochondria-dependent and death receptor-mediated multiple signaling pathways. J Agric Food Chem 2012;60:665-75.
- Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today 1994;15:7-10.
- Elango S, Balwas R, Padma VV. Gallic acid isolated from pomegranate peel extract induces reactive oxygen species mediated apoptosis in A549 cell line. J Cancer Ther 2011;2:638-45.
- Arimura T, Kojima-Yuasa A, Watanabe S, Suzuki M, Kennedy DO, Matsui-Yuasa I. Role of intracellular reactive oxygen species and mitochondrial dysfunction in evening primrose extract-induced apoptosis in Ehrlich ascites tumor cells. Chem Biol Interact 2003;145:337-47134-43.
 - 0.125 mg/ml, -
- 0.125 mg/ml, - - 0.5 mg/
ml, -■- 1 mg/ml, -
- 0.5 mg/
ml, -■- 1 mg/ml, - - 1.5 mg/ml, -
- 1.5 mg/ml, -