- *Corresponding Author:
- Mangal S. Nagarsenker
Department of Pharmaceutics, Bombay College of Pharmacy, Kalina, Mumbai-400 098
E-mail: mangal.nagarsenker@gmail.com
| Date of Submission | 08 May 2014 |
| Date of Revision | 12 February 2015 |
| Date of Acceptance | 22 September 2015 |
| Indian J Pharm Sci 2015;77(5): 620-625 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Tablets containing metoprolol succinate and Compritol®888ATO in the ratio of 1:2 yielded the desired sustained release profile in phosphate buffer pH 6.8 when evaluated using USP type II paddle apparatus and was selected as the optimized formulation. Robustness of optimized formulation was assessed by studying the effect of factors like varying source of metoprolol succinate and Compritol®888ATO, compression force and hydroalcoholic dissolution medium on the release profile. No significant difference (P >0.05) in release profile was observed when metoprolol succinate from three different sources and Compritol®888ATO from two different batches were used. Release profile of sustained release tablets of metoprolol succinate in media containing various concentrations of ethanol was comparable with media devoid of ethanol as evaluated by f2 test. This indicated that release profile of sustained release tablets of metoprolol succinate was reliable with no significant change due to variation in source of active pharmaceutical ingredient, particularly due to particle size distribution. Sustained release tablets of metoprolol succinate yielded release pattern within specifications irrespective of presence or absence of ethanol in the medium indicating that release properties of Compritol®888ATO matrix are not affected by ethanol. Tablets compressed at compression force of <100 kg/cm2 exhibited low hardness with total porosity of 15.39% and significantly increased (P <0.05) metoprolol succinate release as compared to tablets compressed at 2000 kg/cm2 with 6.90% of total porosity revealing influence of compression force. Compritol®888ATO holds great potential in providing reliable and controlled release profile of highly water soluble metoprolol succinate.
Keywords
Compritol®888ATO, metoprolol succinate, melt granulation, ethanol, compression force
Metoprolol succinate (MPL), a know cardioselective β-1 adrenergic blocker is widely used to treat cardiovascular diseases like angina pectoris, heart failure, myocardial infarction, hypertension, arrhythmias and others [1-3]. MPL is highly water soluble with a half life of 4-6 h which may lead to adverse effects on overdose like severe weakness, fainting, trouble in breathing and very slow heartbeat [4-5]. This resulted in development of sustained release (SR) formulations of MPL like Toprol-XL tablets composed of controlled release pellets which provides slow release of the drug. However, preparation of pellets is a complicated, tedious and time consuming process.
Lipidic and polymeric materials are widely used to modulate the release of drugs from the pharmaceutical dosage forms to achieve greater safety and efficacy. Matrix tablets prepared using lipidic excipients are widely accepted due to their simplicity and ease of formulation. The release of drugs is modified by using and optimizing different ratios of the lipids and drugs. Compritol®888ATO (COM), a lipid excipient with IIG and GRAS status along with excellent tableting properties provides sustained and controlled release of drugs where in release mechanism is based on diffusion and erosion [6-7]. Considering these advantages, matrix tablets of MPL with melt granulation (MG) method using COM which sustained the release of MPL with good stability and IVIVC [3] have been reported.
Release of drug from the formulations is controlled by the physicochemical parameters of the drug molecule and excipients, which may differ from source to source or batch to batch. These parameters have a direct influence on their functionality which in turn plays a critical role in formulation, processing, in vitro release and in vivo performance [8,9]. Specifications provided for the excipients are wide and confer average values of their properties. Sometimes physical evaluation of these excipients suggests that they are not distinguishable; however they may show variation during processing and in the final formulation [10,11]. Compression force (CF) is also a vital control factor in formulating tablets as a small change in CF could affect the disintegration, dissolution, friability and stability of tablets. Variation in the CF may result in polymorphic changes which in turn may lead to differences in the porosity, release profile and consequently change the bioavailability of the drug [12]. Porosity can be viewed as bulk property of the tablets and increase in the CF leads to decrease in the average pore diameter and total porosity of the tablets [13]. Thus, physicochemical properties of the drug and excipients, formulation factors and process parameters like CF could have a significant influence on the release profile of the drug.
Due to high rate of alcohol consumption worldwide, the risk for drug-alcohol and excipient-alcohol interactions has increased. Some modified release dosage forms can be expected to exhibit more rapid drug dissolution and increase release rate in the presence of ethanol. Therefore, concomitant consumption of alcoholic beverages along with these products might be expected to have the potential to induce dose dumping [14-16]. Hydromorphone, the opiate drug has been withdrawn from the US market due to its potential interaction with alcohol [17]. Robustness of the formulations when ingested along with alcohol is a matter of concern expressed by regulatory authorities. Draft guidance has been issued by USFDA to industry for dissolution of different extended release formulations in presence of various concentrations of alcohol in the range of 5-40% [18]. Due to high solubility of MPL in water and alcohol, rapid release of MPL may occur from SR tablets leading to increased toxicity and side effects. Release of MPL in vitro in presence and absence of ethanol could help to provide evidence about the ruggedness of SRMPL with reference to ingested alcohol. Objective of the present study was to evaluate the influence of formulation factors, hydro-ethanolic dissolution medium and CF on the release profile of SRMPL.
Materials and Methods
Metoprolol succinate was procured from Aarti Drugs Ltd. (A), Mumbai, India, Polydrug Laboratories Pvt. Ltd. (P), Mumbai and Yanshu Drugs Pvt. Ltd. (Y), Mumbai. Compritol® 888ATO from two different batches was supplied by Gattefosse India Pvt. Ltd., Mumbai, India. Avicel PH 101 and aerosil were obtained as gift samples from Signet Chemical Corporation, Mumbai, India. Magnesium stearate was purchased from Merck, India. Ethanol was procured from S. D. Fine, Mumbai, India. HPLC grade aetonitrile (ACN) and methanol (MeOH) were purchased from Qualigens Fine Chemicals, Mumbai, India. Freshly prepared double distilled (DW) water was used for all the experiments. All other chemicals and reagents used were of analytical grade.
Chromatographic system and conditions for analysis of MPL
MPL was quantified using Shimadzu Corporation (Kyoto, Japan) HPLC system equipped with a PDA detector. Separation was carried out on HIQ sil C18 column (KYA Tech, Japan) with a particle size of 5 μ (250×4.6 mm I.D.) at room temperature (RT). Mobile phase composed of a mixture of ACN and phosphate buffer (pH 3), in a ratio of 30:70 v/v at a flow rate of 1.0 ml/min and detection wavelength of 274 nm.
Particle size analysis of MPL from three different sources
A simple and inexpensive microscopic analytical technique was used to measure the average particle size and to study particle size distribution of drug particles. The analysis was performed using an optical microscope calibrated with a stage micrometer scale. Drug (MPL) particles suspended in liquid paraffin were allowed to settle in one plane such that the particles were sufficiently dispersed to distinguish individual particles under microscope (45X). Mean particle size was determined by counting 100 particles.
Preparation of tablets
Tablets (S1 and S2) were prepared with MPL (A) of two different batches and COM in ratio of 1:2 using MG method as reported earlier [3]. Table 1 lists the composition of SRMPL tablets used in the study. Briefly, MPL and Avicel PH101 were added to molten COM (melted in an oven at 80-85º) with constant stirring to get a uniform dispersion. The dispersion was cooled gradually to R.T. and then passed through B.S.S 40 mesh sieve. The blend was lubricated with aerosil and magnesium stearate which was subsequently compressed using 8.7 mm punch on single station tableting machine (Unimek, India) to obtain tablets. Tablet formulations with MPL from three different sources (A, P and Y) with variations in the drug particle size (S2, S3 and S4) and tablets with COM from two different batches (S5 and S6) were used to prepare SRMPL.
| Composition | Amount (% w/w) |
|---|---|
| MPL | 28.55 |
| COM | 57.11 |
| Avicel pH 101 | 11.42 |
| Magnesium stearate | 1.94 |
| Aerosil | 0.97 |
Table 1: Composition Of Srmpl Tablets
Physical characterization of SRMPL tablets
Tablets were characterized for appearance, weight uniformity, hardness, thickness and friability. Hardness was measured using Monsanto hardness tester (Campbell Electronics, Mumbai, India), thickness by vernier calipers (Mitutoyo Corp., Kawasaki, Japan) and friability using Roche friabilator (F. Hoffmann-La Roche Ltd, Basel, Switzerland).
Content of MPL
Five tablets of SRMPL were crushed in a mortar using pestle. Weight equivalent to one tablet was accurately weighed and dissolved using MeOH in a volumetric flask. After suitable filtration and dilution with MeOH, MPL content of the tablet was estimated using HPLC-UV system (Jasco Corporation, Japan) at 274 nm.
In vitro release
In vitro release studies were carried out in absence or presence of ethanol following the protocol reported earlier [3]. Briefly, the release was performed in 500 ml buffer (pH 6.8) using USP type II paddle apparatus (Electrolab, India) at 37±0.5°. Aliquots of 5 ml were withdrawn at predetermined time intervals of 1, 2, 4, 6, 8, 10, 12 and 20 h followed by replacing with same volume of fresh dissolution medium. Subsequently, samples were filtered and analyzed for MPL content using HPLC-UV method at 274 nm and percent cumulative release was calculated. In vitro release in presence of ethanol was studied by adding ethanol to the dissolution medium to obtain three different hydroalcoholic media with ethanol concentrations of 5, 20 and 40%.
Effect of CF on porosity and release profile of SRMPL
The blend obtained by MG method was compressed on Minipress II multitooling 12 stations Rimek machine at two different CF viz <100 and 2000 kg/cm2 to get SRMPL tablets with low hardness (S7) and high hardness (S8), respectively. The porosity of tablets was determined using high pressure mercury porosimeter (Pascal 440) in the pressure ranging from 0.1–400 MPa.
Statistical analysis
Data was expressed as the mean±S.D. Results were analysed by t test and P<0.05 was considered statistically significant.
Results and Discussion
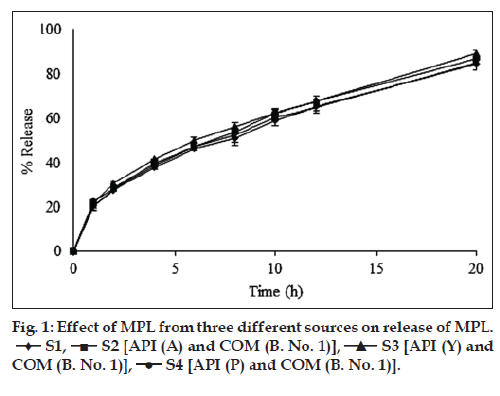
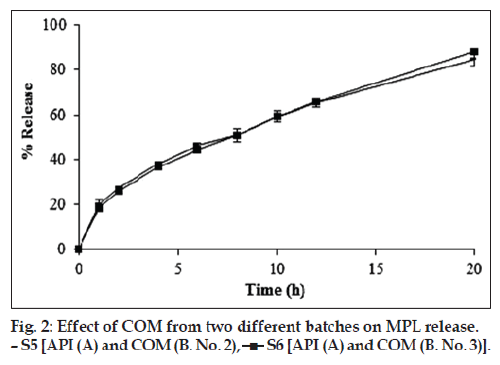
Earlier study reported development and evaluation of SRMPL tablets using COM with regards to formulation, optimization and processing abilities during scale-up using rapid mixer granulator (RMG) and IVIVC [3]. SRMPL matrix tablets with MPL:COM ratio of 1:2 was successfully optimized with desired SR profile for 20 h. In the present study, the source of MPL or the batch of COM was varied in separate experiments by keeping the quantity of ingredients constant. It was observed that the particle size of MPL from source ‘Y’ was highest (~27 m) whereas size from the source ‘P’ was lowest (~14 m) with intermediate size particles from source ‘A’ (Table 2). Similarity factor (f2 value) was employed to evaluate the release profiles of tablets based on the comparison between the experimental results and release profiles. f2 value was found to be above 50 when the release profile of S3 and S4 were compared taking S2 as the reference. Thus, it was observed that there was no significant difference in the release profile of SRMPL tablets indicating that the source of API – particularly particle size distribution of sourced API within the range employed in experimentation did not influence MPL release profile (fig. 1). Further, use of COM from two different batches caused no significant change in the release profile of SRMPL (fig. 2). All the tablets were smooth in appearance, uniform in weight with hardness of 2.0-2.5 kg/cm2 and friability of less than 1%. MPL content of all the formulations was found to be in between 93-96% indicating good distribution and homogeneity of the drug (Table 3).
| Source of MPL | Mean particle size (µ) | ||
|---|---|---|---|
| By calculation | By graph | ||
| Aarti Drugs Pvt. Ltd. (A) | 20.23 | 19.99 | |
| Yanshu Chemicals Pvt. Ltd. (Y) | 27.54 | 25.70 | |
| Polydrug Laboratories Pvt. Ltd (P) | 13.86 | 14.45 | |
Table 2: Mean particle size of mpl from Different sources
| Parameters | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Hardness (kg/cm2) | 2.0–2.5 | |||||
| Uniformity of weight (mg) | 174.6 ± 5.9 | 174.8 ± 4.2 | 174.2 ± 5.1 | 174.4 ± 6.3 | 175.9 ± 4.9 | 175.3 ± 6.5 |
| MPL content# | 93.62 ± 0.27 | 94.16 ± 0.71 | 95.21 ± 0.21 | 93.41 ± 0.44 | 94.11 ± 0.07 | 94.01 ± 0.09 |
| Percentage of drug release at 20th h# | 91.35 ± 0.89 | 85.22 ± 0.85 | 89.16 ± 1.44 | 87.06 ± 0.44 | 84.44 ± 2.66 | 85.78 ± 0.26 |
Table 3: Physical Characterization Of Srmpl
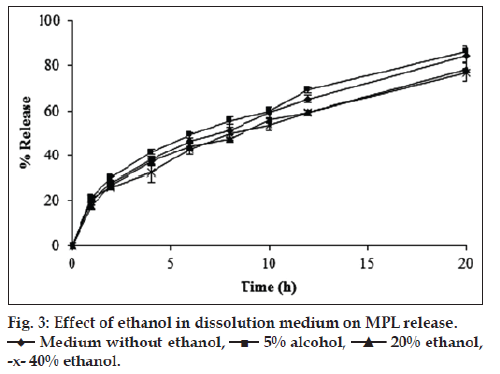
It is been observed that informing patients and labeling the formulations regarding the potential drug-alcohol interactions is not always helpful. Hence, USFDA acknowledges development of formulations that are insensitive to alcohol [19]. In view of this fact, influence of hydroalcoholic dissolution medium at three different concentration levels of ethanol on release profile of SRMPL was studied. Use of 5% ethanol in the dissolution medium, though resulted in small apparent increase in the percent release of MPL, did not produce a significant difference in the release profile when compared to release profile in medium devoid of ethanol (fig. 3). Slight reduction in the MPL release at later time points was observed in medium with 20 and 40% ethanol as compared to dissolution medium containing 5% ethanol and devoid of ethanol. However, f2 values were found to be above 50 when release profiles in media containing various concentrations of ethanol were compared with release profile devoid of ethanol. This indicated that presence of ethanol in dissolution media did not significantly influence release pattern of MPL from SR tablets. In previous studies it has been observed that concentration of ethanol upto 40% in dissolution medium did not cause increase in the release of oxymorphone from extended release tablets or crush resistant tablet formulation. Infact, oxymorphone release was slower in dissolution media containing 40% ethanol compared to dissolution medium devoid of ethanol and medium with 4% of ethanol [20]. Another study on in vitro dissolution of oral modified release tablets and capsules in ethanolic media suggested that the high release may be dependent on the dosage form, excipients and properties of the drug. Many tested modified release capsules showed increase in the in vitro release whereas modified release tablets demonstrated decrease in the release with increase in the percentage of ethanol from 5 to 40%. Further, it was observed that difference in the release profile between the dissolution medium devoid of ethanol and medium containing 5% ethanol was not significant [21]. Oral modified release tablets of aspirin prepared using hydroxypropyl methylcellulose (HPMC) showed increase in the aspirin release with increase in the ethanol concentration from 0 to 40% [15]. Thus, in vitro release in ethanol containing medium depends on different factors and could be used to evaluate the effect of ethanol on drug release from the modified/controlled release dosage formulations [22].
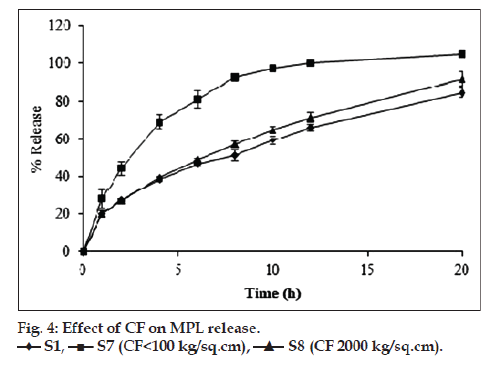
The hardness of the tablets increased with increase in the CF as the tablet becomes denser and less porous. However, the value of hardness reached a constant level at CF of 2000 kg/cm2 above which chipping and sticking was observed. Compression was smoother and uniform up to CF of 2000 kg/cm2 with good appearance and hardness of 3 kg/cm2. At CF less than 100 kg/cm2, tablets were compressed easily with uniform hardness but with less shine and slight roughness on surface and sides. Based on these observations tablets were compressed at two different CF of less than 100 kg/cm2 and 2000 kg/cm2. The hardness, thickness and porosity parameters of tablets at the two different CF are shown in Table 4. As can be observed, the variation in the thickness of S7 and S8 was nominal. Release profile of tablets compressed at high CF of 2000 kg/ cm2 (S8) was comparable with S1 (fig. 4). However, S7 (CF<100 kg/cm2) showed significant increase (P<0.05) in the release compared to S1 and S8 with ~90% release at the end of 8 h suggesting influence of CF on the release profile of MPL.
| Batch number | CF (kg/cm2) | Thickness (mm)# | Hardness (kg/cm2) | Porosity parameters | |
|---|---|---|---|---|---|
| Average pore diameter (µ) | Total porosity (%) | ||||
| S7 | <100 | 3.81 ± 0.02 | ~1.7–2.0 | 0.571 | 15.392 |
| S8 | 2000 | 3.61 ± 0.02 | 3.0 | 0.080 | 6.907 |
Table 4: Thickness, hardness and porosity Parameters of tablets at two different cf
Hardness and porosity parameters have correlation with the release profile of tablets. The pore diameter and total porosity of S7 was found to be significantly high (P<0.05) compared to S8. Penetration of dissolution medium into the tablet matrix is strongly influenced by the pore size and porosity of the tablets. At low CF there is decrease in the hardness with increase in the pore diameter and total porosity of tablets which contributes to faster penetration of the dissolution medium [13,23]. Thus, low hardness with high pore diameter and total porosity of S7 tablets resulted in high release of MPL compared to S8.
Thus, the study revealed that variation in the source of MPL or the batch of COM and presence of ethanol in the dissolution medium has no significant influence on release profile of SRMPL. CF was found to be the critical factor for predicting the release of MPL from tablets. SRMPL formulated using COM as retarding lipid matrix holds a great prospective in providing consistent and sustained release of MPL.
Acknowledgements
Authors would like to thank Rubicon Research Pvt. Ltd., Mumbai for compressing the tablets.
Financial support and sponsorship
The project was funded by Gattefosse India Pvt. Ltd., Mumbai.
Conflicts of interest
There are no conflicts of interest.
References
- Walle PO, Westergren G, Dimenäs E, Olofsson B, Albrektsen T. Effects of 100 mg of controlled-release metoprolol and 100 mg of atenolol on blood pressure, central nervous system-related symptoms, and general well being. J Clin Pharmacol 1994;34:742-7.
- Falkner B, Kushner H. Treatment with metoprolol succinate, a selective beta adrenergic blocker, lowers blood pressure without altering insulin sensitivity in diabetic patients. J Clin Hypertens (Greenwich) 2008;10:51-7.
- Patere SN, Desai NS, Jain AS, Kadam PP, Thatte UM, Gogtay N, et al. Compritol®888 ATO a lipid excipient for sustained release of highly water soluble active: Formulation, scale-up and IVIVC study. Curr Drug Deliv 2013;10:548-56.
- Rasool F, Ahmad M, Murtaza G, Khan HM, Khan SA, Khiljee S, et al. Hydrophobic plastic polymeric tableted microparticles loaded with metoprolol tartrate: Development of a validated in vitro in vivo correlation. Int J Med Med Sci 2010;1:211-4.
- Abrahamsson B, Lücker P, Olofsson B, Regårdh CG, Sandberg A, Wieselgren I, et al. The relationship between metoprolol plasma concentration and beta 1-blockade in healthy subjects: A study on conventional metoprolol and metoprolol CR/ZOK formulations. J Clin Pharmacol 1990;30 2 Suppl:S46-54.
- Ke WT, Hsu TT, Ho HO, Sheu MT. Physical and clinical characterization of ambroxol SR matrix tablets containing melt coated granules of ambroxol with compritol 888. Asian J Pharm Sci 2006;1:35-42.
- Sharma N, Sharma A, Kohli K, Arora S. Development and evaluation of release equivalent sustained release formulation of dextromethorphan HBr using simple technology. Int J Pharm Sci 2009;1:121-7.
- Sriamornsak P, Thirawong N, Korkerd K. Swelling, erosion and release behavior of alginate-based matrix tablets. Eur J Pharm Biopharm 2007;66:435-50.
- Landin M, Martinez Pacheco R, Gomez Amoza JL, Souto C, Concheiro A, Rowe RC. Influence of microcrystalline cellulose source and batch variation on the tableting behavior and stability of prednisone formulations. Int J Pharm 1993;91:143-9.
- Chatlapalli R, Rohera BD. Study of effect of excipient source variation on rheological behavior of diltiazem HCl-HPMC wet masses using a mixer torque rheometer. Int J Pharm 2002;238:139-51.
- Dahl TC, Calderwood T, Bormeth A, Trimble K. Influence of physicochemical properties of hydroxypropyl methylcellulose on naproxen release from sustained release matrix tablets. J Control Release 1990;14:1-10.
- Santos JV, Batista de Carvalho LA, Pina ME. The influence of the compression force on zidovudine release from matrix tablets. AAPS PharmSciTech 2010;11:1442-8.
- Riippi M, Yliruusi J, Niskanen T, Kiesvaara J. Dependence between dissolution rate and porosity of compressed erythromycin acistrate tablets. Eur J Pharm Biopharm 1998;46:169-75.
- Johnson F, Wagner G, Sun S, Stauffer J. Effect of concomitant ingestion of alcohol on the in vivo pharmacokinetics of KADIAN (morphine sulfate extended-release) capsules. J Pain 2008;9:330-6.
- Roberts M, Cespi M, Ford JL, Dyas AM, Downing J, Martini LG, et al. Influence of ethanol on aspirin release from hypromellose matrices. Int J Pharm 2007;332:31-7.
- Fadda HM, Mohamed MA, Basit AW. Impairment of the in vitro drug release behaviour of oral modified release preparations in the presence of alcohol. Int J Pharm 2008;360:171-6.
- FDA. FDA Alert for Healthcare Professionals. Hydromorphone Hydrochloride Extended Release Capsules (Marketed as Palladone); 2005. Available from: http://www.fda.gov/Drugs/DrugSafety/ Postmarket Drug Safety Information for Patients and Providers/ucm129288. htm [Last accessed on 2015 Feb 12].
- FDA. Draft Guidance; 2007. Available from: http://www.fda.gov/cder/ guidance/bioequivalence. [Last accessed on 2015 Feb 12].
- Meyer RJ, Hussain AS. Awareness Topic: Mitigating the Risks of Ethanol induced Dose Dumping from Oral Sustained/ Controlled Release Dosage Forms. FDA’s Advisory Committee for Pharmaceutical Services Meeting; 2005. p. 1-4.
- Fiske WD, Jobes J, Xiang Q, Chang SC, Benedek IH. The effects of ethanol on the bioavailability of oxymorphone extended-release tablets and oxymorphone crush-resistant extended-release tablets. J Pain 2012;13:90-9.
- Smith AP, Moore TW, Westenberger BJ, Doub WH. in vitro dissolution of oral modified-release tablets and capsules in ethanolic media. Int J Pharm 2010;398:93-6.
- Emeje MO, Nwabunike PI, Isimi CY, Kunle OO, Ofoefule SI. Hydroalcoholic media: An emerging in vitro tool for predicting dose dumping from controlled released matrices. J Pharmacol Toxicol 2008;3:84-92.
- Selkirk AB, Ganderton D. An investigation of the pore structure of tablets of sucrose and lactose by mercury porosimetry. J Pharm Pharmacol 1970;22:79S-85S.