- *Corresponding Author:
- S. Murugan
Department of Biotechnology,
Karunya Institute of Technology and Sciences (Deemed to be University),
Coimbatore-641 114,
India
E-mail: micromurugans@gmail.com
| Date of Received | 03 July 2019 |
| Date of Revision | 18 August 2021 |
| Date of Acceptance | 12 November 2021 |
| Indian J Pharm Sci 2021;83(6):1155-1163 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Sauropus androgynus and Solanum torvum are known to possess antioxidant, anti-cancerous, anti-fungal and antiseptic properties. The current study aimed to evaluate the effect of ethanol extracts of Sauropus androgynus leaves and Solanum torvum fruit in prevention of biofilm formation by methicillin resistant Staphylococcus aureus. The study also assessed the presence of bioactive compounds and their ability to modify the cell surface hydrophobicity of methicillin resistant Staphylococcus aureus biofilm. In this study, methicillin resistant Staphylococcus aureus isolates obtained from tertiary care hospitals in Coimbatore were subjected to antibiotic susceptibility testing and biofilm formation. Among the 20 methicillin resistant Staphylococcus aureus isolates, all were found to be 100 % sensitive to vancomycin and 100 % resistant to cotrimoxazole and tetracycline. Two methicillin resistant Staphylococcus aureus isolates were observed to be strong biofilm producers confirmed by microtiter plate method and only one methicillin resistant Staphylococcus aureus isolate was used for further studies. Phytochemical screening of plant materials was carried out to identify the bioactive compounds potentially responsible for the biological properties. Among the plant extracts tested, extracts of Sauropus androgynus leaves showed a maximum zone of inhibition (18 mm), whereas the zone of inhibition exhibited by Solanum torvum fruit extracts was 16 mm at 200 µg/ml against methicillin resistant Staphylococcus aureus. Minimum inhibitory concentration and minimum biofilm inhibitory concentration testing of extracts of Sauropus androgynus leaves and Solanum torvum fruit were also carried out against methicillin resistant Staphylococcus aureus, in which each extract at a concentration of 5 mg/ml was found to inhibit methicillin resistant Staphylococcus aureus growth and biofilm formation via the microtiter plate method. Moreover, there was an increase in cell surface hydrophobicity of methicillin resistant Staphylococcus aureus biofilm from 33.96 to 61.90 % at 150 µg/ml of extracts of Sauropus androgynus leaves, while Solanum torvum fruit extracts showed 46.80 % hydrophobicity. Similarly, anti-adhesive activity was found to be higher in extracts of Sauropus androgynus leaves (0.008) when compared to Solanum torvum fruit extracts (0.016) at 50 µg/ml. The current study reports for the first time the efficacy of Sauropus androgynus and Solanum torvum extracts as a promising source of anti-biofilm agents, especially against methicillin resistant Staphylococcus aureus.
Keywords
Sauropus androgynus, Solanum torvum, methicillin resistant Staphylococcus aureus, biofilm, cell surface hydrophobicity, anti-adhesive activity
Staphylococcus aureus (S. aureus) causes a broad spectrum of nosocomial and community acquired infections, which lead to increased percentage of hospital stay, antibiotic use and mortality [1,2]. This pathogen causes wound related infections, as well as life-threatening illnesses such as post-surgical infections, septicemia, toxic shock syndrome, purpura fulminans, necrotizing pneumonia, endocarditis and prolonged infections of bones and joints [3,4]. Usage of various antibiotics over many years have led to the world-wide emergence of antibiotic resistant clones such as Methicillin Resistant S. aureus (MRSA) [4,5].
MRSA infections are hard to treat as they acquire resistance to most of the antibiotics such as beta- lactams, aminoglycosides, lincosamide, tetracyclines and macrolides [6].
Resistance can be caused by a variety of mechanisms including biofilm formation. Microbial biofilms are groups of bacterial cells adherent to a biotic or abiotic surface, enclosed by a self-produced extracellular polymeric matrix or Extracellular Polymeric Substances (EPS). The presence of EPS plays an important role in cell adhesion and retention on surfaces, as well as in the transport of microorganisms [7]. Microbial adhesion is mediated by various cell surface interactions such as electrostatic forces, acid-base interactions and van der Waals forces [8]. Resistance towards antibiotics and their side effects has encouraged for the search of a novel plant based bioactive compounds [9-12]. Thus various plant derived compounds were examined for activity against biofilm and their use has gained importance as a natural anti-biofilm agent [13]. Various studies have proven the ability of plant extracts to control biofilm formation and also their inhibition on adherence of pathogenic microorganisms [14,15].
Sauropus androgynus (S. androgynous) also known as star gooseberry or sweet leaf, belonging to the family Phyllanthaceae is a multi-vitamin plant with high nutritive value since it is a good source of Vitamins A, B, C, K as well as carotenoids [16]. S. androgynus leaves, a typical Indian vegetable dish has got the name sweet leaves due to its sweet taste after cooking. The leaves of S. androgynus are traditionally used to treat genitor- urinary diseases, cardiovascular diseases, skin problems and also for good vision in India [17-19]. Solanum torvum (S. torvum), also known as Turkey Berry, is a small shrub which belongs to the family Solanaceae. Pharmacological studies on S. torvum have shown that the stem, root, leaves and fruits of S. torvum have anti-bacterial, anti-tumour, anti-viral, analgesic, antiinflammatory, anti-oxidant, immunosecretory, anti- ulcerogenic activities [20-23]. As the fruits of S. torvum are rich in minerals, traditionally they have been used to treat cough, anemia and conditions such as liver and spleen enlargement [24,25].
To the best of our knowledge, so far no studies have reported the hydrophobicity and anti-adhesive effects of S. androgynus and S. torvum. Also, this is the first report on the anti-biofilm activity of S. torvum. With the above-stated information, the present work was aimed to determine the antimicrobial, Minimum Inhibitory Concentration (MIC), anti-biofilm, Cell Surface Hydrophobicity (CSH) and anti-adhesive effects of S. androgynus Leaves (SALE) and S. torvum Fruit (STFE) against MRSA biofilm.
Materials and Methods
Collection and processing of plant samples:
Fresh SALE and STFE were collected in and around Palakkad and Coimbatore regions, South India. The collected plants with complete herbarium was identified and authenticated from Botanical Survey of India, Coimbatore. All the collected plant parts (leaves and fruits) were washed thoroughly and dried under shade for a few days. The dried plant parts were powdered and stored in airtight containers for further studies.
Preparation of crude extract:
Ethanol was used for the preparation of crude extract, since our preliminary tests showed a better yield compared to other solvents. The powdered plant materials (25 g) were continuously extracted with ethanol using the Soxhlet extraction apparatus based upon the polarity of solvent [26]. Different extracts were concentrated under reduced pressure using rotary vacuum evaporator. The ethanolic extracts of SALE and STFE were dried and stored at low temperature. Dimethyl Sulfoxide (DMSO) was used to prepare stock solutions of extracts (10 mg/ ml).
Phytochemical screening:
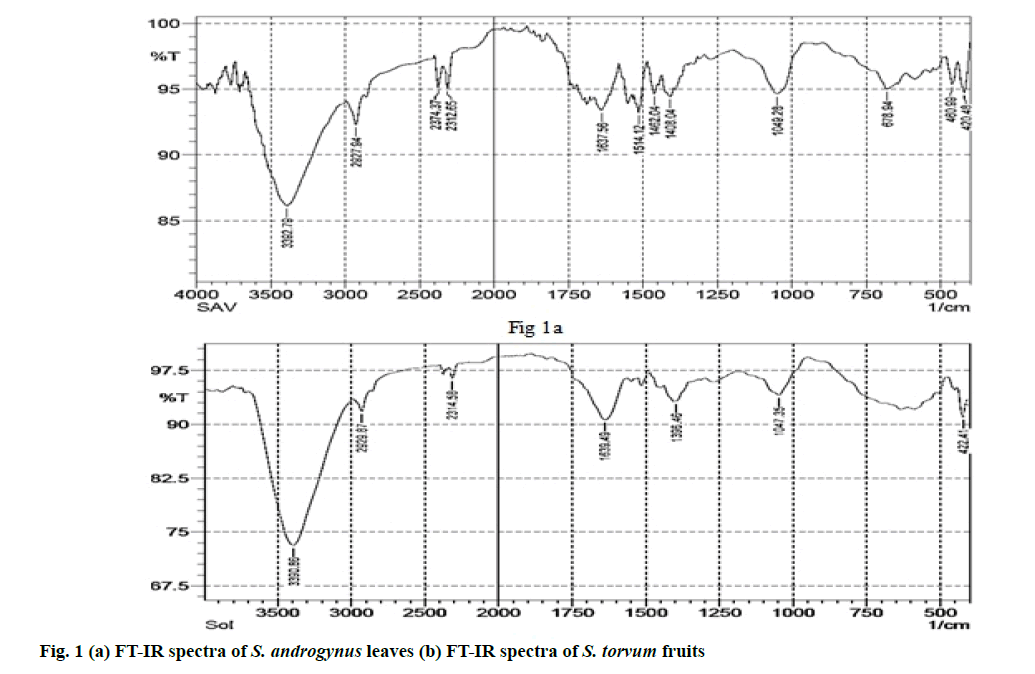
The presence of bioactive compounds including alkaloids, flavonoids, phenols, tannins etc. present in the plant extracts were identified using Fourier- transform infrared (FT-IR) spectral analysis.
FT-IR spectral analysis of S. androgynus and S. torvum: FT-IR spectrum analysis was performed for the identification of different types of chemical bonds (functional groups) present in the phytochemicals. Phytochemical analysis of both plant powders were carried out using FT-IR spectrum [27,28]. The infrared spectrum was recorded on a Bruker Tensor 27 spectrometer. The spectrum was scanned in the 4000- 40° cm-1 range using the potassium bromide (KBr) pellet technique. KBr was dried under a vacuum at 100° for 48 h and 100 mg of KBr with 1 mg of each plant powder (S. androgynus or S. torvum) was taken separately to prepare the KBr pellet. The absorbance spectrum of plant extracts was plotted as intensity versus wave number.
MRSA isolates:
A total of 20 MRSA isolates (from body fluids, wound, pus, blood or urine) were obtained from tertiary care hospitals in and around Coimbatore, South India. The MRSA isolates were cultivated in Trypticase Soy Broth (TSB) and reconfirmed for Methicillin resistance based on Kirby-Bauer disk diffusion method using cefoxitin discs (30 mcg) the isolates were considered methicillin resistant, if the zone of inhibition was 14 mm or less [6].
Antibiotic discs:
For antimicrobial susceptibility testing, 18 antibiotics, namely Amoxycillin/Clavulanic acid (AC-20/10 μg), Ampicillin (A-10 μg), Ampicillin/Sulbactam (AS- 10/10 μg), Chloramphenicol (C-30 mcg), Ciprofloxacin (CF-30 μg), Clindamycin (CD-2 μg), Cotrimoxazole (COT-25 mcg), Fusidic acid (FC-30 μg), Gentamicin (G-10 mcg), Levofloxacin (LE-5 μg), Linezolid (LZ- 30 μg), Minocycline (MIN-30 μg), Mupirocin (MU-5 μg), Ofloxacin (OF-5 μg), Rifampicin (RIF-5 mcg), Teicoplanin (TEI-30 μg), Tetracycline (TET-30 μg) and Vancomycin (V-30 μg) (all from Hi-Media Laboratories, Mumbari, India)) were used.
Biofilm formation using microtiter plate method:
All the MRSA isolates (n=20) were subjected to quantitative biofilm screening by the Microtitre Plate method. Quantitative screening of MRSA isolates was performed according to the method of Stepanovic et al. [29]. MRSA isolates from fresh agar plates were inoculated into Brain Heart Infusion (BHI) broth which is supplemented with 2 % sucrose. After incubation for 18 h at 37° in a stationary condition, the isolates were diluted 1 in 100 with fresh medium. About 0.2 ml aliquots of the diluted MRSA cultures were added to the wells of sterile 96-well flat-bottom microtitre plates.
The plates were gently tapped to remove the content of wells after incubated for 18-24 h at 37°. The plates were washed four times using 0.2 ml of Phosphate Buffered Saline (PBS) to remove the free floating ‘planktonic’ bacteria. The biofilm which was produced by the adherent ‘sessile’ organisms in the plates were fixed using sodium acetate (2 %). After staining the plates with crystal violet (0.1 % w/v), the excess stain was thoroughly washed off using deionized water and the plates were dried. Biofilm formation by the adherent cells were seen as uniform staining on the sides of the wells and Optical Density (OD) was determined using a micro Enzyme-Linked Immunosorbent Assay (ELISA) auto reader (Model 680, Bio-Rad) at a wavelength of 570 nm (OD 570 nm). The experiment was performed in triplicates. The OD values of each well were considered as an index of bacterial adherence to their surface and their ability to form biofilm. The OD value of control, fixative and dye were averaged and subtracted from all test values, in order to compensate for background absorbance. The mean OD value obtained from control wells containing un-inoculated medium was deducted from all the test OD values.
Anti-MRSA activity of extracts:
The activity of SALE and STFE against MRSA Microbial Type Culture Collection (MTCC) 1430 was determined by the agar well diffusion method and by MIC assay.
Agar well diffusion:
In order to study whether the plant extracts possessed any inhibitory effect on MRSA, agar well diffusion was performed as previously described using Mueller- Hinton Agar (MHA) medium [30]. Overnight culture of MRSA was sub cultured in TSB until a turbidity of 0.5 McFarland’s (1×108 Colony Forming Unit (CFU)/ ml) standard was attained. Stock was prepared using plant extract and DMSO at a concentration of 10 mg/ ml. Using a sterile cotton swab, the cultures were uniformly spread over the surface of the agar plate. Excess moisture absorption was allowed to occur for 10 min. In the swabbed plate, four wells were created with a diameter of 6 mm and different concentrations (50, 100, 150 and 200 µg/ml) of SALE and STFE extracts were added to respective wells. DMSO- containing well served as the Control. The MHA plates were then incubated at 37° for 24 h and the plates were observed for zone of inhibition.
Minimum inhibitory concentration:
Serial micro dilution assay method was performed to determine the MIC values of SALE and STFE against MRSA [31]. About 100 µl of SALE (or STFE) extracts from stock solution (10 mg/ml) was added to the first wells in the each row of a sterile 96-well polystyrene flat-bottom microtiter plate. After the addition of 50 µl of TSB to the remaining wells of each row, two- fold serial dilution was performed. Overnight MRSA culture developed from storage were resuspended in TSB and incubated at 37° for 3 h. About 50 µl of MRSA (1-1.5×106 CFU/ml) was added to the wells of microtiter plate to make the final volume of each wells 100 µl. Likewise gentamycin was diluted serially and kept as positive control. MRSA along with TSB devoid of SALE and STFE were used as negative control. Wells containing only TSB were kept as blank reference. After incubation at 37° for 24 h, 40 µl of 0.2 mg/ml p-iodonitrotetrazolium violet salts solution was added to each well and the microtiter plates were allowed to stand at 37° for 30 min [32]. Blue color formation on the wells indicates the presence of viable cells [33]. The lowest concentration at which the plant extract inhibited the bacterial growth was determined visually and the bioassay was carried out in triplicate.
Anti-biofilm properties of SALE and STFE:
Effect of SALE and STFE on MRSA biofilm inhibition was carried out by using the most common and reliable, Microtitre plate method. Further, the extracts were analyzed for their activity on cell surface hydrophobicity and surface adhesion of biofilm.
Inhibition of biofilm formation:
Activity of SALE and STFE on inhibition of MRSA biofilm was performed according to the standard microtitre plate method [29]. Two-fold dilutions of SALE and STFE (10 mg/ml) were made using BHI broth (supplemented with 2 % sucrose) on 96 well flat bottom microtiter plate. MRSA along with BHI broth serve as growth control whereas only BHI containing wells kept as media control. Plant extracts along with BHI were maintained as blank.
The plates were kept for incubation at 37° for 24-48 h. After incubation, the plates were stained with 150 µl of crystal violet (0.1 %) and allowed to stand for 15 min at room temperature. The plates were then washed to remove excess stain and then left to dry. The Minimum Biofilm Inhibitory Concentration (MBIC) of SALE and STFE is defined as the lowest concentration of extracts required for the inhibition of MRSA biofilm. The MBIC of SALE and STFE was determined by observing the growth inhibition on microtitre plates compared to control wells. The assay was carried out in triplicates.
Bacterial Adhesion To Hydrophobicity (BATH) assay:
The effect of SALE and STFE on CSH of MRSA was determined by using microbial adhesion to hydrocarbons assay as a measure of their adherence to hydrophobic hydrocarbons (toluene) [34]. One ml of MRSA culture (OD 530 nm=1.0) was placed in a glass tube and 100 µl of toluene along with SALE and STFE at a concentration of 40 or 50 mg/ml were added on to the tubes. The mixture was vigorously vortexed for 2 min and incubated for 10 min at room temperature to allow phase separation and then the OD 530 nm of the aqueous phase was recorded. MRSA incubated with toluene devoid of SALE and STFE served as controls. The percentage of hydrophobicity was calculated using the equation;
Percentage hydrophobicity = 1-(OD 530 after vortex/ OD 530 before vortex)×100
Anti-adhesive activity of SALE and STFE against MRSA biofilm:
The inhibitory effect of SALE and STFE at varying concentrations on glass dependent adherence of MRSA was studied [35]. MRSA was grown aerobically at 37° at an angle of 30° in boiling tubes containing 10 ml of BHI broth with 5 % sucrose along with or without SALE and STFE at a concentration of 40 and 50 mg/ml. Tubes containing MRSA devoid of SALE and STFE were the controls. After 24 h of incubation, the planktonic cell suspensions were discarded and the adhering cells were washed with 0.5 M of NaOH followed by agitation. The adhering cells were washed and suspended in saline. Finally, the adherence was quantified using a spectrophotometer at OD 600 nm, wherein the untreated BHI tubes with MRSA served as control.
Results and Discussion
Phytochemical screening of SALE and STFE revealed the presence of various bioactive compounds which are known to possess properties like antimicrobial, anti- oxidant, anti-allergic, anti-cancer and antifungal [36, 37].
The functional groups of the compounds present in plant can be identified by FT-IR spectra. FT-IR spectrum of SALE powder (fig. 1a) showed prominent bands at 420.48 cm-1, 460.99 cm-1, 678.94 cm-1,1049.28 cm-1, 1408.04 cm-1, 1462.04 cm-1, 1514.12 cm-1, 1637.56 cm-1, 2312.65 cm-1, 2374.37 cm-1, 2927.94 cm-1, 3392.79 cm-1. Similarly, the prominent bands in the FT-IR spectrum of STFE powder (fig. 1b) are observed at 422.41 cm-1, 1047.35 cm-1, 1396.46 cm-1, 1639.49 cm-1, 2314.58 cm- 1, 2929.87 cm-1 and 3390.86 cm-1.
The anti-biogram pattern suggests that 20 MRSA isolates exhibited different resistant patterns against the 18 conventional antibiotics. Clindamycin, linezolid, rifampicin, teicoplanin and vancomycin were found to be the most effective drugs against MRSA and none of the tested isolates exhibited resistance to them. However, tetracycline and cotrimoxazole were found to have 100 % resistance, rendering it incapable to act as an effective drug against MRSA. The percentage of resistance to antibiotics such as amoxicillin/clavulanic acid, chloramphenicol, ampicillin/sulbactam, minocycline, ampicillin, gentamicin, levofloxacin, ofloxacin, fusidic acid, ciprofloxacin and mupirocin were found to be in the order of 85, 83.3, 73.3, 71.6, 70, 63.3, 56.6, 56.6, 55, 53.3 and 48.3 % respectively.
In the standard MTP assay, only 2 (10 %) isolates were strong biofilm producers, 8 (40 %) isolates were moderate biofilm producers whereas remaining 10 (50 %) isolates were biofilm negative phenotype in TSB medium.
Different concentrations (50, 100, 150, 200 µg/ml) of SALE and STFE exhibited different degrees of antibacterial activity against MRSA. The results showed that extracts of S. androgynus and S. torvum were found to be effective against MRSA with inhibition zones ranging from 9.66±0.58 to 17.33±1.00 mm (Table 1). When the ethanolic extracts of S. androgynus (SALE) tested against MRSA, maximum zone of inhibition of 17.33±1.00 mm was observed at 200 µg/ml whereas minimum zone of inhibition (11.66±0.58 mm) was observed at 50 µg/ml. Similarly, the zone of inhibition of ethanolic extract of STFE was found to be 16.00 ±0.58 mm at 200 µg/ml. The minimum zone of inhibition of ethanolic STFE extract was found to be 9.66±0.58 at 50 µg/ml. The inhibitory effect exhibited by SALE and STFE are comparable to the zone of inhibition showed by standard drugs (clindamycin, linezolid, rifampicin, teicoplanin and vancomycin). The control wells containing DMSO did not show any inhibitory activity against MRSA. Thus, the studies shows that ethanolic extract of SALE have greater efficiency in inhibiting the growth of MRSA at 200 µg/ml.
| Concentration (µg/ml) | Zone of inhibition (mm) | |
|---|---|---|
| SALE | STFE | |
| 50 | 11.66 ± 0.58 | 9.66 ± 0.58 |
| 100 | 12.66 ± 0.56 | 10.66 ± 0.56 |
| 150 | 13.66 ± 0.57 | 14.66 ± 0.57 |
| 200 | 17.33 ± 0.56 | 16.00 ± 0.58 |
Table 1: Antimicrobial Activity of Sale and Stfe Extracts Against MRSA
MIC values were calculated to evaluate the effect of SALE and STFE on MRSA growth. Here both SALE and STFE was found to have a MIC value of 5-10 mg/ ml (Table 2). Ethanolic extracts of S. androgynus and S. torvum at a concentration of 5 mg/ml was defined as the lowest concentration on inhibiting growth of MRSA after incubation when compared to control.
| Plant extracts | Concentrations (mg/ml) | ||||||
|---|---|---|---|---|---|---|---|
| 0.3125 | 0.625 | 1.25 | 2.5 | 5 | 10 | MIC | |
| SALE | + | + | + | + | - | - | 5 |
| STFE | + | + | + | + | - | - | 5 |
| Gentamycin | - | - | - | - | - | - | 0.3125 |
‘+’: Indicates growth; ‘-‘: Indicates no growth
Table 2: Mic of Plant Extracts Against MRSA
The effect of SALE and STFE on inhibition of biofilm formation was identified using microtitre plate method.
It was found that both SALE and STFE has got MBIC of 5 mg/ml on inhibiting MRSA biofilm (Table 3). No visible growth was observed on the wells containing extracts of 5-10 mg/ml, thus concluding the potential ability of SALE and STFE on MRSA biofilm inhibition and its growth.
| Plant extracts | Concentrations (mg/ml) | ||||||
|---|---|---|---|---|---|---|---|
| 0.3125 | 0.625 | 1.25 | 2.5 | 5 | 10 | MBIC | |
| SALE | + | + | + | + | - | - | 5 |
| STFE | + | + | + | + | - | - | 5 |
‘+’: Indicates growth; ‘-‘: Indicates no growth
Table 3: MBIC of Plant Extracts Against MRSA
CSH plays a major role in the adhesion property of bacterial biofilm formation. The obtained data showed that with the increase in concentration of plant extracts, cell surface hydrophobicity of MRSA was found to be increased (Table 4), The percentage of hydrophobicity obtained for MRSA isolate was 33.96 % and it was found to be increased up to 61.90 %, when the isolate was treated with SALE extract at 150 µg/ml. Likewise, ethanolic extract of S. androgynus (SALE) exhibited a maximum of 61.90 % hydrobhobicity (150 µg/ml) and minimum of 44.47 % hydrophobicity (100 µg/ml) whereas ethanolic extract of S. torvum (STFE) showed maximum of 46.8 % hydrophobicity at 150 µg/ml and minimum of 44.1 % at 100 µg/ml.
| Plant extracts | Concentration (µg/ml) | CSH % |
|---|---|---|
| SALE | 100 | 44.47±0.04 |
| 150 | 61.90±0.03 | |
| STFE | 100 | 44.10±0.05 |
| 150 | 46.80±0.03 | |
| CONTROL | - | 33.96±0.02 |
Table 4: Effect of Sale and STFE on the Cell Surface Hydrophobicity of MRSA
Anti-adherence effect of SALE and STFE on the adhering property of MRSA was carried out with two different concentrations (50 and 60 µg/ml) (Table 5). It was found that with increase in concentration of plant extracts, the adhering property of MRSA were reduced while compared to MRSA devoid of plant extracts. Antiadherence effect of S. androgynus ethanolic extract showed excellent activity compared to S. torvum ethanolic extract. SALE and STFE showed minimum adherence around 0.008 and 0.016 (50 µg/ ml) and maximum of 0.028 and 0.035 OD (40 µg/ml), respectively.
| Plant extracts | Concentration (µg/ml) | OD at 600 nm |
|---|---|---|
| SALE | 40 | 0.028±0.02 |
| 50 | 0.008±0.02 | |
| STFE | 40 | 0.035±0.04 |
| 50 | 0.016±0.02 | |
| CONTROL | - | 0.063±0.03 |
Table 5: Inhibitory Effects of Plant Extracts at Varying Concentration on Glass Dependant Adherence of MRSA
In order to control common health complications, medicinal plants are widely used as natural antimicrobial agents in foods which have been used traditionally for thousands of years. Natural plant product based antimicrobials achieved great importance as newly discovered drugs are likely to be effective against multi drug resistant microbes. Multidrug resistant pathogen MRSA plays significant role in nosocomial infections. Natural products from medicinal plants are a potential important source for natural drug discovery. We therefore studied the antimicrobial and anti-biofilm activities of S. androgynus and S. torvum extracts against MRSA.
FT-IR measurements were carried out to identify the functional groups of active constituents in S. torvum and S. androgynus powder. In FT-IR spectrum of S. androgynus, the presence of alkyl halides and alkynes were identified. Similarly, the presence of aromatic compounds, alkanes, nitro compounds, primary amines, phenols, alcohols and essential oil from S. androgynus are found to be similar with previous reports [37,38]. In FT-IR spectrum of S. torvum, various peaks indicated the presence of alkyl halides, aliphatic amines, nitro compounds, alkynes groups, alcohols and phenols. The shoulder at 2929.87 cm-1 is a characteristic vibration of carboxylic acid group. Likewise, the FT-IR spectrum obtained in S. torvum and the presence of flavonoidal groups and the active constituents of its essential oils also match with the findings of previous works [39-44]. Upon comparison, ethanolic extract of S. androgynus and S. torvum were found to be highly active against MRSA (more or less similar to conventional antibioticslinezolid, rifampicin and vancomycin). Also, there was a correlation between the number of polyphenols and flavanoid content in S. torvum and their effectiveness against bacteria [42-44].
The antibacterial activity of SALE is due to the presence of multi vitamins, peptides, glycosides, alkaloids, saponins, terpenoids and flavonoids [45]. The antimicrobial activities of the SALE and STFE at 200 µg/ml matches with the findings of previous studies [46,37]. Also, the MIC and MBIC values of plant SALE and STFE obtained against MRSA at a concentration of 5 mg/ml were considered as a promising result.
Biofilm formations by microorganisms are the reason for the diverse problems in medical, food industry and everyday life [47]. For the detection of biofilm formation, the classical 96-well microtitre plate assay was used [29]. The most important stage in biofilm formation is its attachment to the solid substratum after which the bacterial growth occurs. The adhesion is reversible for initial few hours of growth on the surface [48]. In this study, both SALE and STFE prevented the bacterial adhesion at varying concentrations and inhibited biofilm formation. This is due to the presence of phytochemicals present in the plant extracts which efficiently inhibited the biofilm. Anti-biofilm activity against MRSA has not been reported with the extracts of S. torvum. We report the first study on the anti-biofilm activity of STFE, hydrophobicity and anti- adhesive activities of SALE and STFE against the test organism MRSA.
Bacterial adhesion is one of the most important characteristic in biofilm formation. The cell surface hydrophobicity needs to be increased in order to decrease bacterial adhesion which can suppress biofilm formation [49]. In this study, both SALE and STFE extracts increased the cell surface hydrophobicity at 150 µg/ml concentration. The physicochemical theory for bacterial adhesion implies that a decrease in bacterial cell surface hydrophobicity may result in increased adhesion to hydrophobic surfaces [50]. The results of the current study corroborate the findings of earlier reports [15,51]. The growth of MRSA occurs soon after its attachment to the solid surface which is one of the most significant phases in the sequence of biofilm formation [50]. The bacterial adhesion is directly proportional to the formation of biofilm and degree of virulence. All the plant extracts were subjected to anti-adhesion test, which showed a proficient result in decreasing the bacterial adhesion, in turn preventing biofilm formation. Among the two plant extracts, SALE proved to be more beneficial. Increased hydrophobicity of SALE extract was found to decrease the ability for biofilm formation by MRSA. Plant extracts which show promising activity in biofilm reduction can be explored for future studies. Similar studies on effect of plant extracts against MRSA have been reported previously [52,53].
The current study demonstrates that the ethanolic extract of S. androgynus and S. torvum could be considered as a natural source for treating MRSA infection and preventing biofilm due to their anti-biofilm and anti- adhesive activity. Based on the demonstrated activity of S. androgynus and S. torvum against MRSA, it can be suggested that both these plant parts might be helpful for use as an antiseptic in the prophylaxis and treatment of MRSA related skin infections. In conclusion, although the antimicrobial activity of S. androgynus and S. torvum has been previously reported, this is the first study to demonstrate the reasonable efficiency of plant extracts on the reduction of bacterial biofilm with increased hydrophobicity and anti-adhesive activity against MRSA. Accordingly, these findings justify the consideration of these plant extracts as potential natural therapeutic sources.
Conflict of interests:
The authors declare that they have no conflicts of interest.
Acknowledgements:
Authors thank the Department of Biotechnology, Karunya Institute of Technology and Sciences, Coimbatore, India for providing facilities to carry out this study.
References
- Garau J, Bouza E, Chastre J, Gudiol F, Harbarth S. Management of methicillin-resistant Staphylococcus aureus infections. Clin Microbiol Infect 2009;15(2):125-36.
- VandenBergh MF, Kluytmans JA, van Hout BA, Maat AP, Seerden RJ, McDonnel J, et al. Cost-effectiveness of perioperative mupirocin nasal ointment in cardiothoracic surgery. Infect Control Hosp Epidemiol 1996;17(12):786-92.
- Zafar KS, Singh PS, Raj P. Staphylococcus aureus septicemia presenting as disseminated intravascular coagulation-thrombotic thrombocytopenic purpura overlap and thrombus in inferior vena cava, right atrium and right ventricle: A case report. Int J Res Med Sci 2015;3(1):368-72.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis 2001;7(2):178-82.
- Otto M. Staphylococcalbiofilms. Curr Top Microbiol Immunol 2008;322:207-28.
- Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute. 25th Information Supplement, Wayne, PA, USA, CLSI Publication M100-S25; 2015.
- Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Sci Am 1978;238(1):86-95.
- Van Oss CJ, Good RJ, Chaudhury MK. The role of van der Waals forces and hydrogen bonds in hydrophobic interactions between biopolymers and low energy surfaces. J Colloid Interface Sci 1986;111(2):378-90.
- Colombo ML, Bosisio E. Pharmacological activities of Chelidonium majus L.(Papaveraceae). Pharmacol Res 1996;33(2):127-34.
- Rojas R, Bustamante B, Bauer J, Fernández I, Albán J, Lock O. Antimicrobial activity of selected Peruvian medicinal plants. J Ethnopharmacol 2003;88(2-3):199-204.
- Scazzocchio F, Cometa MF, Tomassini L, Palmery M. Antibacterial activity of Hydrastis canadensis extract and its major isolated alkaloids. Planta Med 2001;67(6):561-4.
- Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Toxicol 2008;46(12):3774-9.
- Essawi T, Srour M. Screening of some Palestinian medicinal plants for antibacterial activity. J Ethnopharmacol 2000;70(3):343-9.
- Quave CL, Plano LR, Pantuso T, Bennett BC. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J Ethnopharmacol 2008;118(3):418-28.
- Sandasi M, Leonard CM, Viljoen AM. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett Appl Microbiol 2010;50(1):30-5.
- Nahak G, Sahu RK. Free radical scavenging activity of multi-vitamin plant (Sauropus androgynus L. Merr). Researcher 2010;2(11):6-14.
- Christi VE. Study of pharmacognostical, anti-inflammatory and antioxidant activity of Saropus androgynus plant. Int J Adv Pharm Res 2014;5(3):198-207.
- Ong HC. Vegetables: Food and medicine properties (Khasiat Makan & Ubatan). Utusan Publications, Kuala Lumpur, Malaysia; 2003.
- Singh S, Singh DR, Salim KM, Srivastava A, Singh LB, Srivastava RC. Estimation of proximate composition, micronutrients and phytochemical compounds in traditional vegetables from Andaman and Nicobar Islands. Int J Food Sci Nutr 2011;62(7):765-73.
- Arthan D, Svasti J, Kittakoop P, Pittayakhachonwut D, Tanticharoen M, Thebtaranonth Y. Antiviral isoflavonoid sulfate and steroidal glycosides from the fruits of Solanum torvum. Phytochemistry 2002;59(4):459-63.
- Nguelefack TB, Feumebo CB, Ateufack G, Watcho P, Tatsimo S, Atsamo AD, et al. Anti-ulcerogenic properties of the aqueous and methanol extracts from the leaves of Solanum torvum Swartz (Solanaceae) in rats. J Ethnopharmacol 2008;119(1):135-40.
- Ndebia EJ, Kamgang R, Nkeh-Chungag Anye BN. Analgesic and anti-inflammatory properties of aqueous extract from leaves of Solanum torvum (Solanaceae). Afr J Tradit Complement Altern Med 2007;4(2):240-4.
- Sivapriya M, Leela S. Isolation and purification of a novel antioxidant protein from the water extract of Sundakai (Solanum torvum) seeds. Food Chem 2007;104(2):510-7.
- Kala CP. Ethnomedicinal botany of the Apatani in the Eastern Himalayan region of India. J Ethnobiol Ethnomed 2005;1(1):1-8.
- Akoto O, Borquaye LS, Howard AS, Konwuruk N. Nutritional and mineral composition of the fruits of Solanum torvum from Ghana. Int J Chem Biomol Sci 2015;1(4):222-6.
- Tonk S, Bartarya R, Kumari KM, Bhatnagar VP, Srivastava SS. Effective method for extraction of larvicidal component from leaves of Azadirachta indica and Artemisia annua Linn. J Environ Biol 2006;27(1):103-5.
- Sasidharan S, Chen Y, Saravanan D, Sundram KM, Latha LY. Extraction, isolation and characterization of bioactive compounds from plants extracts. Afr J Tradit Complementary Altern Med 2011;8(1):1-10.
- Hameed IH, Ibraheam IA, Kadhim HJ. Gas chromatography mass spectrum and fourier-transform infrared spectroscopy analysis of methanolic extract of Rosmarinus oficinalis leaves. J Pharmacogn Phytother 2015;7(6):90-106.
- Stepanovic S, Vukovi? D, Hola V, Bonaventura GD, Djuki? S, ?irkovi? I, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007;115(8):891-9.
- Performance Standards for Antimicrobial Susceptibility Tests. National Committee for Clinical Laboratory Standards Institute. Villanova, PA, USA, M2-A5; 1993.
- Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 1998;64(8):711-3.
- Performance Standards for Antimicrobial Disc Susceptibility Tests. Clinical and Laboratory Standards Institute M100 S26, 27th ed; 2017.
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharma Anal 2016;6(2):71-9.
- Martin MA, Pfaller MA, Massanarl RM, Wenzel RP. Use of cellular hydrophobisity, slime production and species identification markers for the clinical significance of congulase-negative staphylococcal isolates. Am J Infect Control 1989;17(3):130-5.
- Hamada S, Torii M. Effect of sucrose in culture media on the location of glucosyltransferase of Streptococcus mutans and cell adherence to glass surfaces. Infect Immun 1978;20(3):592-9.
- Shih SL, Tsai WS, Lee LM, Kenyon L. Molecular characterization of begomoviruses infecting Sauropus androgynus in Thailand. J Phytopathol 2013;161(2):78-85.
- Wei LS, Wendy WE, Siong JY, Syamsumir DF. Characterization of antimicrobial, antioxidant, anticancer properties and chemical composition of Sauropus androgynus stem extract. Acta Med Litu 2011;18(1):12-6.
- Barbosa QP, da Camara CA, Silva TM, Ramos CS. Chemical constituents of essential oils from Solanum torvum leaves, stems, fruits and roots. Chem Nat Compd 2012;48(4):698-9.
- Agrawal AD, Bajpei PS, Patil AA, Bavaskar SR. Solanum torvum Sw.—A phytopharmacological review. Der Pharmacia Lett 2010;2(4):403-7.
- González M, Zamilpa A, Marquina S, Navarro V, Alvarez L. Antimycotic spirostanol saponins from Solanum hispidum leaves and their structure activity relationships. J Nat Prod 2004;67(6):938-41.
- Shan B, Cai YZ, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol 2007;117(1):112-9.
- Wu C, Chen F, Wang X, Kim HJ, He GQ, Haley-Zitlin V, et al. Antioxidant constituents in feverfew (Tanacetum parthenium) extract and their chromatographic quantification. Food Chem 2006;96(2):220-7.
- Hara-Kudo Y, Kobayashi L, Sugita-Konishi Y, Kondo K. Antibacterial activity of plants used in cooking for aroma and taste. J Food Protect 2004;67(12):2820-4.
- Jaabir MM, Vigneshwaran R, Hassan TM. Study on the antimicrobial activity of ethanolic extract of the fruits of Solanum torvum and its phytochemical analysis by GC-MS. Biomed Pharmacol J 2015;3(1):117-21.
- Ariharan VN, Devi VM, Prasad PN. Antibacterial activity of sauropus and rogynous leaf extracts against some pathogenic bacteria. Rasayan J Chem 2013;6(2):134-7.
- Hood SK, Zottola EA. Biofilms in food processing. Food Control 1995;6(1):9-18.
- Frank JF, Chmielewski R. Influence of surface finish on the cleanability of stainless steel. J Food Protect 2001;64(8):1178-82.
- Høiby N, Johansen HK, Moser C, Song Z, Ciofu O, Kharazmi A. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect 2001;3(1):23-35.
- Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science 1999;286(5445):1732-5.
- Bos R, Van der Mei HC, Busscher HJ. Physico-chemistry of initial microbial adhesive interactions–its mechanisms and methods for study. FEMS Microbiol Rev 1999;23(2):179-230.
- Dorota W, Marta K, Dorota TG. Effect of asiatic and ursolic acids on morphology, hydrophobicity and adhesion of UPECs to uroepithelial cells. Folia Microbiol 2013;58(3):245-52.
- Al-Bakri AG, Othman G, Afifi FU. Determination of the antibiofilm, antiadhesive and anti-MRSA activities of seven Salvia species. Pharmacogn Mag 2010;6(24):264-70.
- Bozic DD, Milenkovic M, Ivkovic B, Cirkovic I. Newly-synthesized chalcones-inhibition of adherence and biofilm formation of methicillin-resistant Staphylococcus aureus. Braz J Microbiol 2014;45:263-70.