- *Corresponding Author:
- Yingming Xie
Department of Respiratory Medicine, Harbin Institute of Technology Hospital, Harbin, Heilongjiang Province 150006, China
E-mail: 724928302@qq.com
| Date of Received | 30 January 2022 |
| Date of Revision | 24 February 2023 |
| Date of Acceptance | 28 September 2023 |
| Indian J Pharm Sci 2023;85(5):1444-1451 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This article investigated the endogenous mechanism of blueberry anthocyanins in regulating apoptosis through growth inhibition of human gastric adenocarcinoma BGC-823 and their xenograft tumors in nude mice. The Massachusetts Institute of Technology and terminal deoxynucleotidyl transferase dUTP nick end labeling methods were used to detect the proliferation and apoptosis of human gastric adenocarcinoma BGC-823. The nude mice BGC823 human gastric adenocarcinoma xenograft tumor model was established and treated with blueberry anthocyanins of 0 μg/ml, 50 μg/ml and 100 μg/ml, respectively. The expression of B-cell lymphoma 2, B-cell lymphoma 2 associated X-protein, and p-c-Jun N-terminal kinase protein in nude mice transplanted with human gastric adenocarcinoma cells was detected by Western blot. The proliferation inhibition rate and apoptosis rate of human gastric adenocarcinoma BGC-823 gradually increased with the increase of blueberry anthocyanins concentration; blueberry anthocyanins could reduce the growth of transplanted tumor in nude mice; blueberry anthocyanins decrease the B-cell lymphoma 2 protein and increase the Bcl-2 associated X-protein and p-c-Jun N-terminal kinase protein in the tumor tissue of nude mice with n human gastric adenocarcinoma transplanted tumor model (all p<0.05). Blueberry anthocyanins can inhibit the proliferation of BGC-823 and the growth of transplanted tumors, and may play a positive role in inhibiting human gastric adenocarcinoma by regulating the c-Jun N-terminal kinase signaling pathway.
Keywords
Blueberry anthocyanins, human gastric adenocarcinoma, apoptotic pathway, nude mice
Gastric Adenocarcinoma (GAC) is a malignant tumor originating from gastric mucosal epithelial cells, which is one of the common digestive system tumors. It usually originates from the gastric mucosa layer, and gradually invades other gastric parietal layer and surrounding tissues with the progression of the disease[1]. The current treatment methods include surgical resection, radiotherapy, and chemotherapy, etc. However, due to the complexity of GAC and the emergence of drug resistance, the therapeutic effect is still limited. Therefore, finding new therapeutic strategies and drugs has become an urgent need. In recent years, natural plant compounds have attracted much attention in cancer treatment. Blueberry Anthocyanins (BA) are important natural plant compounds that have been shown to have a variety of biological activities, including antioxidant, anti-inflammatory, and anti-tumor outcomes[2-4]. In particular, its role in inhibiting the proliferation and inducing apoptosis of various cancer cells has attracted much research attention[5]. Studies have shown that anthocyanins can weaken the activity of activating enzymes and inhibit the migration ability of cancer cells, and the induction dose and action time have a great impact on the proliferation and development of cancer cells[6,7]. As a kind of polyphenolic flavonoid organic compounds, anthocyanins can reduce the activities of Superoxide Dismutase (SOD) and Glutathione Peroxidase (GSH-PX) in tissues, effectively suppress cell oxidative damage[8], and even improve the immune function of the body by affecting the levels of cytokines Tumor Necrosis Factor-Alpha (TNF-α) and Interleukin-10 (IL-10) [9,10]. BA is the main active ingredient of blueberry, and there are more than ten kinds of BA. BA has many effects and is widely used in health care products, medicine and cosmetics. Its low toxic and side effects make it a relatively safe natural substance, and its toxic and side effects are low, so it has been paid much attention and respected.
Apoptosis is an important cell death process, also known as programmed cell death, which is a key link in normal cell development, tissue maintenance, immune regulation and other biological processes. Apoptosis is usually achieved through a series of precisely regulated molecular signaling pathways[11]. The endogenous apoptotic pathway is an important signal transduction network involved in the regulation of apoptosis. The key molecules in this pathway include apoptosis regulatory factors and apoptosis effector factors. Among the regulatory factors of apoptosis, B-Cell Lymphoma 2 (Bcl-2) family proteins are important members[12]. This family includes members that inhibit apoptosis (such as Bcl-2, Bcl-xL) and members that promote apoptosis (such as Bcl-2 Associated X-Protein (BAX), Bad). Bcl-2 family proteins control cell life and death decisions by regulating mitochondrial membrane permeability[13,14]. In addition, the key factors in the apoptotic signaling pathway also include caspases family proteins. These proteins play a major role in the execution stage of apoptosis, including caspase-8 and caspase-9, etc. When activated, they can initiate a series of signaling cascades, eventually leading to cell apoptosis[15,16]. C-Jun N-Terminal Kinase (JNK) is an important serine/threonine protein kinase that belongs to the Mitogen-Activated Protein Kinase (MAPK) family and plays a key regulatory role in the transmission of apoptotic signals during cellular stress response[17]. The activation of JNK is mainly achieved by phosphorylation. When cells are subjected to a series of stress stimuli, such as oxidative stress, extracellular signal stimulation, Deoxyribonucleic Acid (DNA) damage, the activity of JNK is stimulated, which triggers a series of cell signal transduction[18]. It can evaluate the degree of apoptosis and the activity of JNK signaling pathway by detecting the phosphorylation state of JNK and the level of related proteins in its downstream signaling pathways, and further explore its regulatory relationship with BA on apoptosis.
It aimed to investigate the inhibitory effect of BA on human BGC-823 GAC cells and their xenograft tumors in nude mice, and to explore the regulatory outcome of BA on the endogenous apoptotic pathway. The results of this article are expected to provide new ideas and strategies for the treatment of GAC, experimental basis for the application of BA, and reference for the development of new anticancer drugs.
Materials and Methods
Experimental reagents and instruments:
BA (Qinghai Jinmaiqi Biotechnology Co., Ltd.), Dulbecco's Modified Eagle Medium (DMEM) (Gibco Company), 3-[4,5-Dimethylthiazol-2- yl]-2,5 Diphenyl Tetrazolium Bromide (MTT) dry powder (Sigma Company), Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) kit (Promega Company), Phosphate Buffer Saline (PBS) buffer, Fetal Bovine Serum (FBS) (Gibco Company), protein lysate, Bicinchoninic Acid (BCA) protein quantification kit, and Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel preparation kit (Beijing Solarbio Technology Co., Ltd.,), Bcl-2 mouse anti-human monoclonal antibody, BAX rabbit anti-human polyclonal antibody (Biodragon Company), p-JNK mouse anti-human monoclonal antibody (BOSTER Company); immunocytochemical kit (Beijing Zhongshan golden bridge Biotechnology Co., Ltd.,).
KSD-1211 Carbon dioxide (CO2) incubator (Thermo scientific Company), ultra-clean table (Jiangsu Tongjing Purification Equipment Co., Ltd.,), microplate reader (Multiskan Company), inverted fluorescence microscope (NIKON Company) and flow cytometer (Beckman Coulter Company).
Cell lines and animal models:
Culture and grouping of human GAC cells: The cryopreserved human GAC strain BGC- 823 (Shanghai Cell Bank, Chinese Academy of Sciences) was resuscitated and sub cultured. 10 % FBS and 100 U/ml penicillium streptomycin were added into DMEM medium. When about 70 % to 80 % of BGC-823 grew and fused, the medium was discarded. It was washed with PBS and treated with trypsin-Ethylenediaminetetraacetic Acid (EDTA) digestion solution for 5 min. The suspension was collected and transferred to a centrifuge tube. Following counting, the cells were seeded into 24- well plates with DMEM medium at 1×106 cells/ well, and 500 μl of cell diluent was put to each well. The cells were divided into three groups, and anthocyanin mother liquor at 0 μg/ml, 50 μg/ ml and 100 μg/ml was put. It was adjusted to the final volume of 2 ml. After that, the samples of the three groups were routinely cultured in a cell incubator at 37° with 5 % CO2 for 24 h. The cells were collected and centrifuged at 1000 rpm for 3 min at 4°. The supernatant was discarded, and 10 % FBS was put and mixed. Dimethylsulfoxide (DMSO) reagent was put at FBS:DMSO=9:1. It was stored in the refrigerator at -80°.
Modeling and grouping of transplanted tumors in nude mice:
Thirty Specific Pathogen Free (SPF) female Bagg Albino (BALB/C) nude mice (6 w old, 15±2 g) were fed under the ambient temperature of 24°±2° and humidity of 60 %±5 % for 12 h. The mice were fed with standard mouse diet every day for a fixed time, and they were free to drink water. After 5 d of adaptive feeding, the nude mice were transplanted with tumor to establish the model. All female BALB/C nude mice were subcutaneously injected with 1×106/0.2 ml human GAC BGC-823 through the right axillary region under sterile conditions. BALB/C nude mice inoculated with human GAC cells were randomly divided into 3 groups with 10 mice in each group. BA was administered at 0, 50, and 100 μg/ml, respectively 2 w after inoculation, once a day at 2 p.m., for 20 d. After the end of administration, the nude mice in each group were sacrificed under anesthesia with 2.5 % sodium pentobarbital. The subcutaneous tumors of the nude mice were dissected and weighed. The experiment was approved by the Ethics Committee of our Hospital (approval number: X2022051794).
Proliferation and apoptosis analysis of human GAC BGC-823:
MTT assay to detect the proliferation inhibition rate of human GAC BGC-823: Human GAC BGC- 823 in log phase was seeded into the plate with 96 wells, about 9000 (about 200 μl) to each well. 24 h later, the plate was subjected to centrifugation to discard the supernatant. The wells were grouped, and 0 μg/ml BA was used as the controls, and 50 and 100 μg/ml BA were used as the administration groups for intervention, respectively. 24 h and 48 h later, 20 μl of 5 mg/ml MTT buffer was put to all wells, and the supernatant was taken after 4 h of incubation at 37°. The experiment was repeated three times. The Optical Density (OD) value of each well at 570 nm was measured by ELX-800 microplate reader. The proliferation inhibition rate of human GAC BGC-823 treated with various concentrations of BA was calculated.
Cell proliferation inhibition rate=(OD 570 nm of controls-OD 570 nm of treatment group)/OD 570 nm of controls×100 %
Apoptosis rate of human GAC BGC-823:
In the plate with 24 wells, intervention with DMEM medium, the cells cultured at 37° in 5 CO2 for 24 h and 48 h according to the instructions of TUNEL kit. The cells were fixed with 4 % cold buffered acetaldehyde, washed twice with PBS buffer, and the cell membrane was permeabilize with 0.1 % Triton X-100 solution for 30 min. The cells were added with TUNEL reaction solution, incubation at 37° for 1 h. The cells were washed twice with PBS, stained with 4′,6-Diamidino-2-Phenylindole (DAPI) nuclear staining solution, and pictures were taken under fluorescence microscope following sealing slides. The number of apoptotic cells in human GAC BGC-823 was counted and computed.
Apoptosis rate (%)=apoptotic number/total number×100 %
Bcl-2, BAX, and p-JNK protein in nude mice transplanted with human GAC cells detected by Western blot: GAC cells transplanted in nude mice were sampled, and protein lysates were used for cell extraction. The supernatant was obtained by centrifugation, and proteins were separated by SDS-PAGE gel electrophoresis until protein bands were formed in the gel. The target bands were transferred to the Polyvinylidene Difluoride (PVDF) membrane using a wet electrophoresis device for 2 h at 200 mA. The PVDF membrane was placed in 5 % skim milk to block non-specific binding sites on the transfer membrane, incubation for 1.5 h. It was then placed in the primary antibody and incubated overnight at 4°. The membrane was rinsed twice with PBS buffer, transferred in the secondary antibody, and subjected to incubation for 1 h at ambient temperature. It was rinsed twice with PBS buffer, and ECL reagent was adopted for color development. The fluorescence or Chemiluminescence signals of the transfer membrane were recorded. ImageJ software was adopted for quantitative analysis of protein bands, and the relative protein levels in human GAC cells in nude mice were computed.
Statistical methods:
Statistical Package for the Social Sciences (SPSS) 22.0 software was adopted for statistical analysis. Measurement data were expressed as mean±standard deviation (x? ±s), and one-way analysis of variance was adopted for multiple contrast. When the data conformed to normal distribution, Dunnett’s t test was adopted for contrast. When the data did not conform to the normal distribution, the rank sum test was adopted for analysis. p<0.05 was considered statistically meaningful.
Results and Discussion
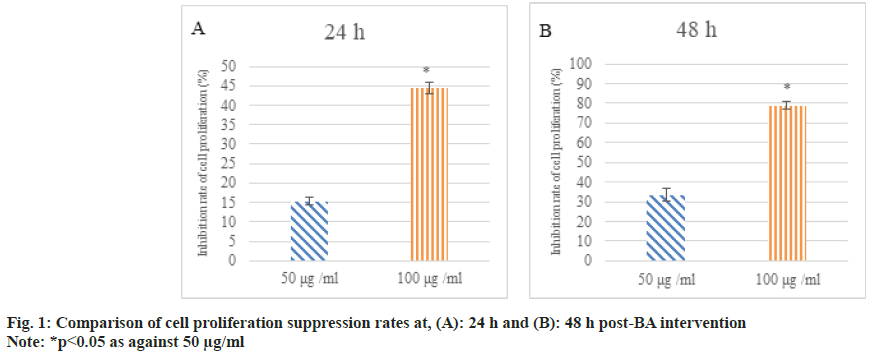
After 24 h and 48 h of BA intervention, the proliferation of human GAC BGC-823 was analyzed. As against 0 μg/ml BA, the inhibition rate of cell proliferation was increased at 50 μg/ ml and 100 μg/ml BA and as against 50 μg/ml, 100 μg/ml intervention markedly increased the suppression rate of cell proliferation (p<0.05) as shown in fig. 1.
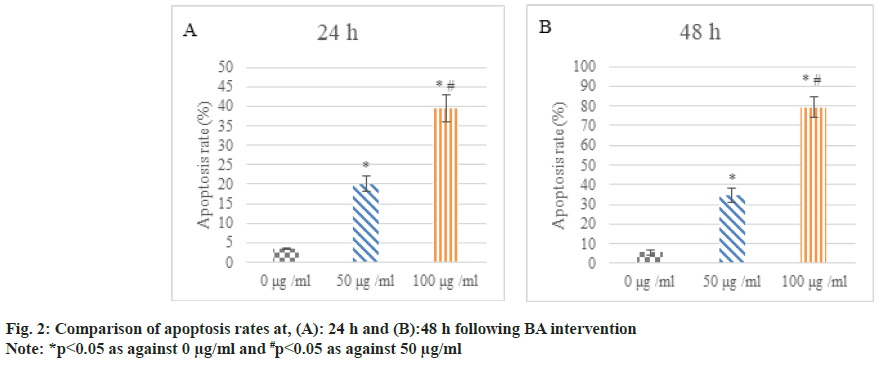
Following 24 h and 48 h of BA intervention, the apoptosis of human GAC BGC-823 was analyzed. The apoptosis rate of the cells treated with 50 μg/ ml and 100 μg/ml was higher relative to 0 μg/ml; the increase in apoptosis rate was clearer in 100 μg/ml intervention than in 50 μg/ml (p<0.05) as shown in fig. 2.
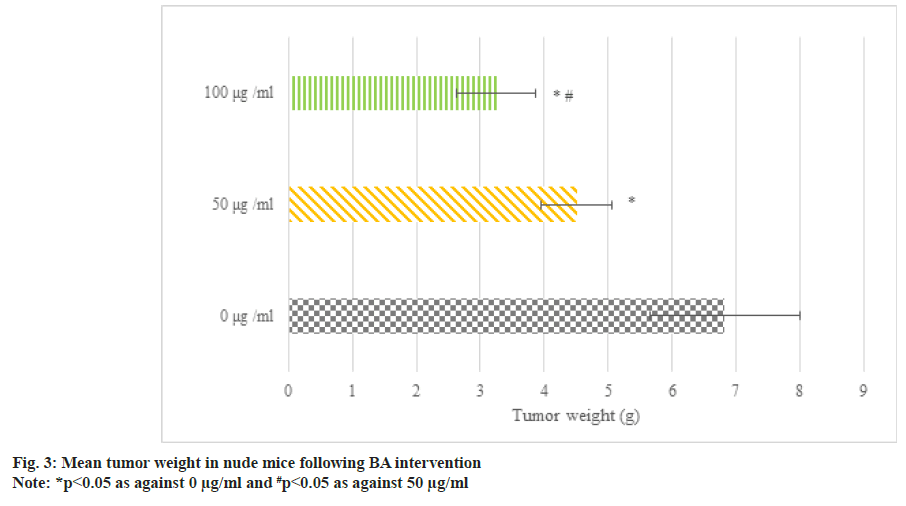
Fig. 3 illustrates the transplanted tumors in BGC823 human GAC nude mice following BA intervention. The average tumor weight following 50 μg/ml and 100 μg/ml BA intervention was less, i.e. as against 0 μg/ml BA, the tumor weight of nude mice following 50 μg/ml and 100 μg/ml BA intervention was smaller; as against 50 μg/ml, the tumor shrinkage following 100 μg/ml intervention was clearer (p<0.05).
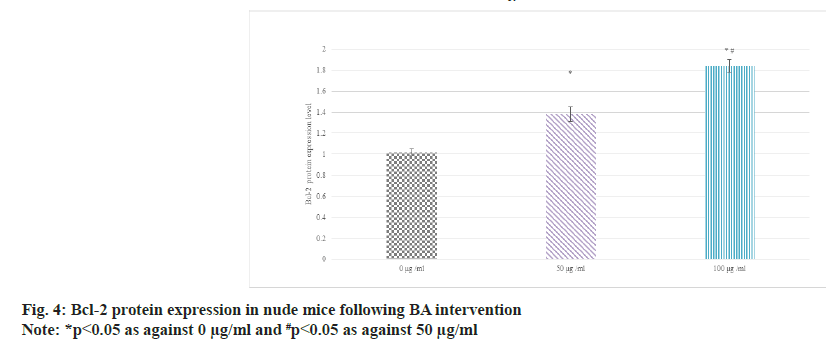
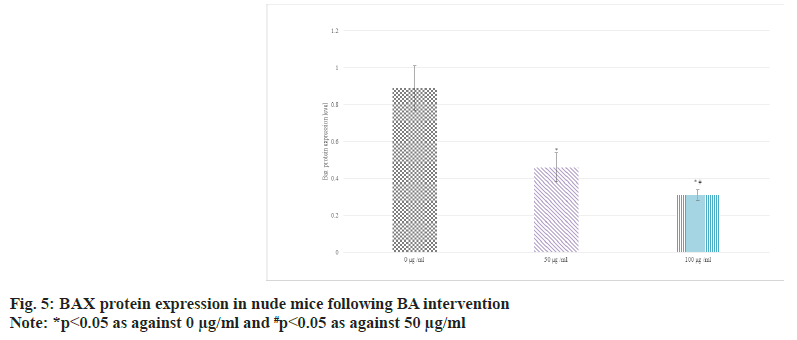
Western blot was adopted to detect the relative expression in the serum of BGC-823 human GAC nude mice. The results suggested that as against 0 μg/ml BA, the protein expression of Bcl-2 was raised and that of BAX was decreased in 50 μg/ ml and 100 μg/ml BA intervention; the distinction between 100 μg/ml and 50 μg/ml intervention was more obvious (p<0.05) as shown in fig. 4 and fig. 5.
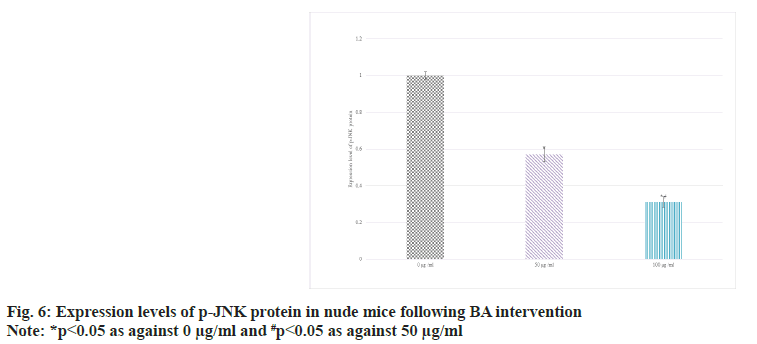
The expression in the serum of BGC-823 human GAC nude mice was detected by Western blot. The results suggested that as against 0 BA, the p-JNK protein in 50 and 100 μg/ml groups decreased; the distinction between 100 μg/ml and 50 μg/ml intervention was more obvious (p<0.05) as shown in fig. 6.
Anthocyanins are a class of natural compounds found in a wide variety of fruits and vegetables, and blueberries have the highest amount of anthocyanins. Studies have shown that BA has a variety of biological activities, including antiinflammatory, antioxidant, anti-cancer, and immunomodulatory outcomes[19,20]. BA has shown clear suppressing outcomes against different types of cancer cells[21]. For example, in LS174T colon cancer cells, BA was found to suppress cell proliferation, induce apoptosis, and reduce invasiveness. Its mechanism of action may involve the up-regulation of P53 protein and the downregulation of caspase-8 protein[22,23]. Similarly, BA has also shown an suppressing outcome on A549 non-small cell lung cancer cells and HepG2 liver cancer cells by affecting cell proliferation and cell cycle, increasing histone acetylation modification, and promoting apoptosis[24,25]. In this article, the cell proliferation suppression rate and apoptosis rate of human GAC BGC-823 were calculated by interfering with the proliferation of human GAC BGC-823 with different concentrations of BA. The results suggested that BA intervention markedly suppressed the proliferation and promoted the apoptosis of human GAC BGC-823 in a concentration-dependent manner. Following 24 h and 48 h of BA intervention, the cell proliferation suppression rate and apoptosis rate of 50 μg/ml and 100 μg/ml intervention were higher than 0 μg/ml; the apoptosis rate of the cells treated with 100 μg/ml was markedly higher relative to 50 μg/ ml; in addition, as against the 0 μg/ml BA group, the nude mice treated with 50 μg/ml and 100 μg/ ml had smaller tumors; the tumor size of 100 μg/ ml group was markedly smaller relative to 50 μg/ ml group (all p<0.05). These results indicate that BA can play a positive role in the suppression of human GAC BGC-823.
Bcl-2 is an anti-apoptotic protein. It mainly exists on the outer membrane of mitochondria in cells, and can suppress mitochondrial permeability transition and apoptosis. High level of Bcl-2 can counteract the effect of apoptotic signals and maintain the survival state of cells[26-28]. In some tumors, the abnormally highly expressed Bcl- 2 is closely related to the evasion of apoptosis, proliferation, and development of treatment resistance of tumor cells[29]. The main role of BAX is to promote apoptosis. When cells are exposed to apoptotic signals, BAX is activated and translocalizes to the mitochondrial membrane, leading to increased mitochondrial membrane permeability, release of cytochrome c and other apoptosis-related molecules, and ultimately trigger the apoptotic process[30]. Thus, highly expressed BAX is generally associated with induction of apoptosis and increased cell death. Similarly, JNK pathway plays a central role in the regulation of apoptosis, which can effectively regulate apoptosis, etc.,[31]. The activation and suppression of JNK pathway play an important role in cell fate determination. In this article, as against 0 μg/ml, the protein levels of Bcl-2 were raised and those of BAX and p-JNK were decreased in 50 μg/ml and 100 μg/ml intervention; the distinction between 100 μg/ml and 50 μg/ml intervention was clearer (all p<0.05). It revealed BA can suppress the apoptosis of human GAC BGC-823.
Although this article provides a preliminary exploration of the suppressing outcome of BA on the regulation of endogenous apoptotic pathways, there are still some shortcomings. This article mainly adopted the human GAC cell line BGC-823 for experiments, and the nude mouse xenograft tumor model was adopted as the animal experimental model, but the analysis of its mechanism of action is relatively insufficient. In addition, GAC is a highly heterogeneous tumor, and different subtypes of GAC may have different biological characteristics and reactivity. Therefore, further studies should explore the outcomes of BA on key proteins in the endogenous apoptosis pathway and the interactions between BA and other signaling pathways, covering more GAC subtypes and clinical samples. It should provide direction for further research in the future, and present challenges and opportunities for improvement in the clinical application of BA.
BA reduces the proliferation suppression rate and apoptosis rate of human GAC BGC-823, and reduces the size of human GAC cells transplanted in nude mice. It increases the protein expression of Bcl-2, and reduces that of BAX and p-JNK. It indicates that BA can play a positive role in suppressing GAC by regulating JNK signaling pathway.
Conflict of interests:
The authors declared no conflict of interests.
References
- Jiang N, Liu A, Cao C, Chen G. miRNA-497 inhibits proliferation, migration and induces apoptosis of gastric cancer cells by negatively regulating pbx3 expression. Acta Med Mediter 2021;37(1):575-80.
- Alnadari F, Abdin M, Ennab W, Mohedein A, Nasiru MM. Metabolism of anthocyanins and modulation of gut microbiome in inflammatory bowel disease. J Food Chem Nanotechnol 2020;6:207-17.
- Amjadi S, Armanpour V, Ghorbani M, Tabibiazar M, Soofi M, Roufegarinejad L. Determination of phenolic composition, antioxidant activity and cytotoxicity characteristics of kombucha beverage containing Echium amoenum. J Food Meas Charact 2023;17:1.
- Li J, Wu T, Li N, Wang X, Chen G, Lyu X. Bilberry anthocyanin extract promotes intestinal barrier function and inhibits digestive enzyme activity by regulating the gut microbiota in aging rats. Food Funct 2019;10(1):333-43.
[Crossref] [Google Scholar] [PubMed]
- Banach M, Wiloch M, Zawada K, Cyplik W, Kujawski W. Evaluation of antioxidant and anti-inflammatory activity of anthocyanin-rich water-soluble aronia dry extracts. Molecules 2020;25(18):4055.
[Crossref] [Google Scholar] [PubMed]
- Bhatt U, Shah G, Soni V. Therapeutic, protective and industrial significances of anthocyanins: A review. Avicenna J Med Biochem 2022;10:82-93.
- Chirumbolo S, Bjorklund G, Lysiuk R, Vella A, Lenchyk L, Upyr T. Targeting cancer with phytochemicals via their fine tuning of the cell survival signaling pathways. Int J Mol Sci 2018;19(11):35-68.
[Crossref] [Google Scholar] [PubMed]
- Eskra JN, Schlicht MJ, Bosland MC. Effects of black raspberries and their ellagic acid and anthocyanin constituents on taxane chemotherapy of castration-resistant prostate cancer cells. Sci Rep 2019;9(1):4367.
[Crossref] [Google Scholar] [PubMed]
- Fagundes FL, Pereira QC, Zarricueta ML, Dos Santos RD. Malvidin protects against and repairs peptic ulcers in mice by alleviating oxidative stress and inflammation. Nutrients 2021;13(10):3312.
[Crossref] [Google Scholar] [PubMed]
- Fernandez J, Garcia L, Monte J, Villar CJ, Lombo F. Functional anthocyanin-rich sausages diminish colorectal cancer in an animal model and reduce pro-inflammatory bacteria in the intestinal microbiota. Genes 2018;9(3):133.
[Crossref] [Google Scholar] [PubMed]
- Ribera-Fonseca A, Jiménez D, Leal P, Riquelme I, Roa JC, Alberdi M, et al. The anti-proliferative and anti-invasive effect of leaf extracts of blueberry plants treated with methyl jasmonate on human gastric cancer in vitro is related to their antioxidant properties. Antioxidants 2020;9(1):45.
[Crossref] [Google Scholar] [PubMed]
- Layosa MA, Lage NN, Chew BP, Atienza L, Mertens-Talcott S, Talcott S, et al. Dark sweet cherry (Prunus avium) phenolics enriched in anthocyanins induced apoptosis in Mda-Mb-453 breast cancer cells through mapk-dependent signaling and reduced invasion via Akt and Plcγ-1 downregulation. Nutr Cancer 2021;73(10):1985-97.
[Crossref] [Google Scholar] [PubMed]
- Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients 2020;12(2):457.
[Crossref] [Google Scholar] [PubMed]
- Wei L, Chen Q, Guo A, Fan J, Wang R, Zhang H. Asiatic acid attenuates CCl4-induced liver fibrosis in rats by regulating the PI3K/AKT/mTOR and Bcl-2/Bax signaling pathways. Int Immunopharmacol 2018;60:1-8.
[Crossref] [Google Scholar] [PubMed]
- Ju X, Yang Z, Zhang H, Wang Q. Role of pyroptosis in cancer cells and clinical applications. Biochimie 2021;185:78-86.
[Crossref] [Google Scholar] [PubMed]
- Wang M, Sun X, Jiang Y, Tan Z. NET-1 promotes invasion, adhesion and growth of hepatocellular carcinoma by regulating the expression of BAX, caspase 3, caspase 8 and BCL2. Cell Mol Biol 2018;64(12):37-41.
[Crossref] [Google Scholar] [PubMed]
- Somade OT, Ajayi BO, Olushola MO, Omoseebi EO. Methyl cellosolve-induced renal oxidative stress and time-dependent up-regulation of pro-inflammatory cytokines, apoptotic and oncogenic markers in rats. Toxicol Rep 2020;7:779-87.
[Crossref] [Google Scholar] [PubMed]
- Gao Y, Wang Y, Wang X, Ma J, Wei M, Li N, et al. FABP4 regulates cell proliferation, stem ness, apoptosis and glycolysis in colorectal cancer via modulating ROS/ERK/mTOR pathway. Discov Med 2023;35(176):361-71.
[Crossref] [Google Scholar] [PubMed]
- Xue H, Sang Y, Gao Y, Zeng Y, Liao J, Tan J. Research progress on absorption, metabolism and biological activities of anthocyanins in berries: A review. Antioxidants 2022;12(1):3.
[Crossref] [Google Scholar] [PubMed]
- Wani MY, Ganie NA, Wani DM, Wani AW, Dar SQ, Khan AH, et al. The phenolic components extracted from mulberry fruits as bioactive compounds against cancer: A review. Phytother Res 2023;37(3):1136-52.
[Crossref] [Google Scholar] [PubMed]
- Sindhu RK, Verma R, Salgotra T, Rahman MH, Shah M, Akter R, et al. Impacting the remedial potential of nano delivery-based flavonoids for breast cancer treatment. Molecules 2021;26(17):51-63.
[Crossref] [Google Scholar] [PubMed]
- Ruan J, Wang S, Wang J. Mechanism and regulation of pyroptosis-mediated in cancer cell death. Chem Biol Interact 2020;323:109052-1104.
- Fonseca LR, Silva GR, Luís A, Cardoso HJ, Correia S, Vaz CV, et al. Sweet cherries as anti-cancer agents: From bioactive compounds to function. Molecules 2021;26(10):2941.
[Crossref] [Google Scholar] [PubMed]
- Safdar MA, Aslam RM, Shakeel A, Shiza WM, Jamil A, Mehmood MH, et al. Cyanidin as potential anticancer agent targeting various proliferative pathways. Chem Biol Drug Design 2023;101(2):438-52.
[Crossref] [Google Scholar] [PubMed]
- Venskutonis PR. Phytochemical composition and bioactivities of hawthorn (Crataegus spp.): Review of recent research advances. J Food Bioactives 2018;4:69-87.
- Tarantini S, Balasubramanian P, Delfavero J, Csipo T, Yabluchanskiy A, Kiss T, et al. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience 2021;43(5):2427-40.
[Crossref] [Google Scholar] [PubMed]
- Edlich F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem Biophys Res Commun 2018;500(1):26-34.
[Crossref] [Google Scholar] [PubMed]
- Kumar A, Sinha N, Bhardwaj A, Goel S. Clinical risk assessment of chronic kidney disease patients using genetic programming. Comput Methods Biomech Biomed Eng 2022;25(8):887-95.
[Crossref] [Google Scholar] [PubMed]
- Sulistiyowati I, Yunus J, Sari DC, Arfian N. Upregulation of p16, Bax and Bcl-2 mRNA expression associated with epithelial apoptosis and myofibroblast proliferation in kidney fibrosis model in mice. Malays J Med Sci 2020;27(2):37.
[Google Scholar] [PubMed]
- Moldoveanu T, Czabotar PE. BAX, BAK and BOK: A coming of age for the BCL-2 family effector proteins. Cold Spring Harbor Perspect Biol 2020;12(4):a036319.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Ruan Z, Song G, Wang R, Hu C, Zhu L. The effect and mechanism of action of danhong injection on lipid peroxidation and endothelial dysfunction coronary heart disease: An animal study. J Biol Regul Homeost Agents 2022;36(5):1519-26.