- *Corresponding Author:
- I. E. Shohin
Scientific Center for Expertise of Medical Products, Moscow-127 051
E-mail: sovdep2007@yandex.ru
| Date of Submission | 12 January 2011 |
| Date of Revision | 8 July 2011 |
| Date of Acceptance | 19 July 2011 |
| Indian J Pharm Sci, 2011, 73 (4): 443-446 |
Abstract
The WHO biowaiver procedure for BCS Class II weak acids was evaluated by running two multisource IR ibuprofen drug products (Ibuprofen, 200 mg tablets, Tatchempharmpreparaty, Russia and Ibuprofen, 200 mg tablets, Biosintez, Russia) with current Marketing Authorizations (i.e. in vivo bioequivalent) through that procedure. Risks associated with excipients interaction and therapeutic index were considered to be not critical. In vitro dissolution kinetic studies were carried out according WHO Guidance (WHO Technical Report Series, No. 937, Annexes 7 and 8) using USP Apparatus II (paddle method) at 75 rpm. Dissolution profiles of test and reference ibuprofen tablets were considered equivalent in pH 4.5 using factors f1 (13) and f2 (72) and not equivalent in pH 6.8 (factor f1 was 26 and f2 was 24). Drug release of ibuprofen at pH 1.2 was negligible due to its weak acid properties. Therefore, two in vivo bioequivalent tablets were declared bioinequivalent by this procedure, indicating that procedure seems to be over-discriminatory.
Keywords
Biowaiver, dissolution test, ibuprofen, interchangeability
Currently interchangeability of multisource drugs is established by clinical trials, comparative pharmacodynamic studies in humans, comparative pharmacokinetic studies in humans (bioequivalence studies) and comparative in vitro tests. A comparative in vitro dissolution profile similarity can be used to document equivalence of a multisource with a comparator product for solid oral dosage forms containing APIs with suitable properties according to Biopharmaceutical Classification System (BCS). Such studies, used to approve equivalence other than through in vivo equivalence testing, are called “biowaivers” [1]. Biowaiver procedure involves aqueous solubility and intestinal permeability studies of API (performed by literature search, or experimentally, is necessary data is absent), evaluation of risks associated with therapeutic indications and therapeutic index, evaluation of risks associated with excipient interaction, dissolution rate and dissolution kinetics studies called “in vitro equivalence test”. This test includes comparison of the dissolution profile between test product and reference product in three media: pH 1.0–1.2 (or SGF without enzymes), pH 4.5 and pH 6.8 (or SIF without enzymes) [1,2]. Biowaiver procedure is already accepted by United States Food and Drug Administration (FDA) in 2001 and European Medical Agency (EMA) in 2010 and recommended by World Health Organization WHO in 2006 for some classes of drug substances [1-3]. Evaluation of biowaiver possibility for WHO Essential Medicines is one of the important activities of International Pharmaceutical Federation [4].
Ibuprofen is a nonsteroidal antiinflammatory drug (NSAID) with analgesic and antipyretic properties. Ibuprofen has pharmacologic actions similar to those of other prototypical NSAIDs, that is thought to be associated with the inhibition of prostaglandin synthesis. Ibuprofen is used to treat rheumatoid arthritis, osteoarthritis, dysmenorrhea, and to alleviate moderate pain. The exact mechanism of action of ibuprofen is unknown. Its antiinflammatory effects are believed to be due to inhibition of both cylooxygenase-1 and cylooxygenase-2 which leads to the inhibition of prostaglandin synthesis, and results in the inhibition of prostaglandin synthesis. Antipyretic effects may be due to action on the hypothalamus, resulting in an increased peripheral blood flow, vasodilation, and subsequent heat dissipation [5]. Ibuprofens chemical name is (RS)-2- (4-Isobutylphenyl)propionic acid [6].
Biopharmaceutical characteristics of ibuprofen were evaluated by literature search. The most reliable data are presented in biowaiver monograph series, published online at website of International Pharmaceutical Federation (www.fip.org/bcs). These monographs are essentially literature reviews, gathering and organizing relevant data to be taken into consideration whether a biowaiver can be recommended for a new formulation of that API. Discussed are: Solubility, pharmacokinetics and permeability, dissolution of dosage forms, the therapeutic use and therapeutic window of the API, data on excipient interactions and problems with bioavailability and/or bioequivalence. The monographs have no formal regulatory status, but represent the best scientific opinions currently available [4]. Ibuprofen biowaiver monograph describes it as BCS Class II API (“low” solubility, “high” permeability) [7]. Current FDA and EMA BCS and Biowaiver Guidances do not recommend biowaiver procedure for drug products containing Class II compounds [2,3]. But WHO Guidance states that biowaiver can apply to Class II weak acids, highly soluble at pH 6.8 but not at pH 1.2 and pH 4.5 [1]. Such substances are readily ionized in small intestine (pH about 6.8) and their behavior is similar with that of Class I APIs. Ibuprofen fulfills these criteria as described aboveand its in vitro equivalence may be evaluated under biowaiver conditions for BCS Class II.
Ibubrofen CRS (purity of 99%) was purchased from Scientific Center for Expertise of Medical Products (Moscow, Russia). Analytical grade hydrochloric acid, glacial acetic acid, potassium dihydrophosphate, disodium hydrophosphate dodecahydrate, and ethanol (96%) were purchased from Sigma-Aldrich (Moscow, Russia). Evaluated ibuprofen tablets (both are generic drug products, one of them was used as reference preparation), in strengths of 200 mg, were purchased ibuprofen in Moscow pharmacies. Test product was ibuprofen, Biosintez, Russia (series 110808, expire date 11.08.11), and reference product was Ibuprofen, Tatchempharmpreparaty, Russia (series 18112009, expire date 18.11.12). Both preparations have current Marketing Authorisation in Russia, i.e. they had to successfully pass in vivo bioequivalence studies against the innovator according to Russian guidances (90% CI of the AUC and Cmax for the multisource product within the 80–125% range of those of the comparator). Excipients containing in studied drug products are shown in Table 1.
| Excipient | Evaluated drug |
|---|---|
| Potato starch | 1, 2 |
| Magnesium stearate | 1, 2 |
| Silica | 2 |
| Vanillin | 1, 2 |
| Beeswax | 1, 2 |
| Gelatin | 1, 2 |
| Magnesium carbonate | 2 |
| Sucrose | 1, 2 |
| Povidone | 1, 2 |
| Titanium dioxide | 2 |
| Dextrin | 1 |
| Iron oxide red | 1, 2 |
Table 1: Excipients contained in reference (1) and test (2) drugs
All dissolution studies were performed using USP Apparatus 2 (Erweka DT 600, Frankfurt, Germany) at 75 rpm. Dissolution media were USP buffers pH 1.2, acetate buffer pH 4.5, and phosphate buffer pH 6.8 at 37±0.5º. Dissolution media volume was 900 ml. pH of dissolution media was evaluated using pH-meter MetroHM 744 (Herisau, Switzerland) and was corrected, if necessary, using 0.1M sodium hydroxide or 0.1M hydrochloric acid. Twelve capsules of each preparation were studied to evaluate statistical significance of the results. Time point schedule was 5, 10, 15, 20, 30, and 45 min for acetate buffer pH 4.5 and 10, 15, 20, 30, 45 and 60 min for phosphate buffer pH 6.8. Sample aliquots were taken using micropipettes and immediately replaced with equal volumes of fresh medium at the same temperature to maintain constant total volume during the test. All samples were filtered through 0.45-μm membrane filters (Millipore, Billerica, MA, USA). Drug release was assayed spectrophotometrically using a UV/ Vis spectrophotometer (Agilent 8453, Santa Clara, California, USA) at 264 nm using corresponding dissolution media as compensation liquid. 0.2 mg/ml ibuprofen CRS solution (in corresponding dissolution media) was used as reference solution.
Dissolution profile comparisons were made by calculation of factors f1 and f2 using formulas: f1 = {[Σ>sub>t=1 n(Rt – Tt)] / [Σt=1nRt]} × 100…(1) and f2 = 50 × log {[1 + (1/n)Σt=1n(Rt–Tt)2] – 0,5 × 100}… (2), where, n – the number of time points; Rt – mean drug release of reference drug product at time point t, %; Tt – mean drug release of test drug product at time point t, %; Statistical treatment was carried out using Microsoft Excel software. Relative standard deviation (RSD, %) was calculated for each time point to confirm the validity of results.
Biowaiver criteria for drugs containing BCS Class II active pharmaceutical ingredients with weak acid properties and high solubility at pH 6.8 but not at pH 1.2 and pH 4.5 are [1]: (1) The dosage form is rapidly dissolving (85% in 30 min or less) in pH 6.8 buffer (only). (2) The test product exhibits similar dissolution profiles, as determined by the f2 value or equivalent statistical evaluation, to those of the reference product at the three pH values (pH 1.2, 4.5 and 6.8).
In addition for drug products containing Class II APIs the excipients should additionally be critically evaluated in terms of type and amounts, e.g. of surfactants, in the formulation. Evaluated tablets contain only excipients presented in ibuprofen biowaiver monograph [7], (excipients containing in IR solid oral dosage forms approved in ICH or associated countries) therefore the risk of bioinequivalence due to an excipient interaction is low. No surfactants was containing in test and reference preparations.
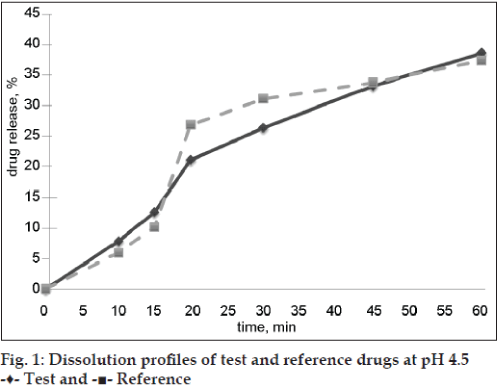
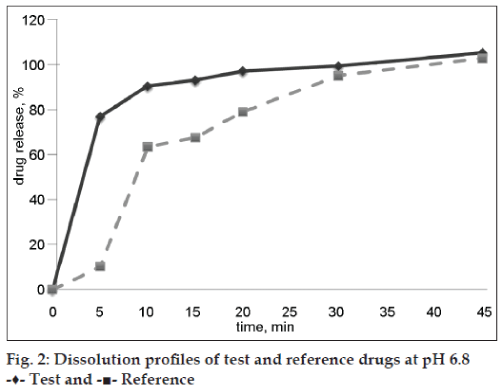
Both evaluated drug products were “rapidly dissolving” (Table 2) at pH 6.8 because the drug release at time point 30 min was more than 85 %, and only test drug product was “very rapidly dissolving” at pH 6.8 (more than 85 % released in 15 min). Dissolution profiles of test and reference preparations are shown in figs. 1 and 2. Dissolution profiles in pH 6.8 were considered not similar because calculated factors f2 (24) and f1 (26) did not meet acceptance criteria (50 ≤ f2 ≤ 100; 0 ≤ f1 ≤ 15) and similar in pH 4.5: factor f2 was 80 and factor f1 was 3 (Table 3). Drug release of ibuprofen at pH 1.2 was negligible due to its weak acid properties and low (about 0.04 mg/ml in pH 1.2 at 37°) solubility [7]. The percent relative standard deviation (% RSD) for all time points fulfills all requirements (≤20% for first time point, ≤10 % for other time points), so obtained results were valid.
| Medium | Test product | Reference product | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % dissolved,( X ),15 min | % dissolved,(X), 30 min | % dissolved,(X), 15 min | % dissolved,(X), 30 min | ||||||||
| pH 6.8 | 92,99 | 99,30 | 67,55 | 94,90 | |||||||
| “rapidly dissolving”, “very rapidly dissolving”, or “not rapidly dissolving” | |||||||||||
‘‘rapidly dissolving’’, ‘‘very rapidly dissolving’’, or ‘‘not rapidly dissolving’’
Table 2: Dissolution rate for evaluated drug products
| Medium | f1 | f2 |
|---|---|---|
| pH 4.5 | 3 | 80 |
| pH 6.8 | 26 | 24 |
Table 3: f1 and f2 values for evaluated drug products
Therefore evaluated ibuprofen preparations do not meet biowaiver criteria for drug products containing BCS Class II substances. Both drug products are “rapidly dissolving”, but they do not fulfill the criteria for dissolution profile similarity, f1 and f2. These products are considered to be in vitro inequivalent. The results show that these biowaiver criteria seem to be over-discriminatory for drug products containing BCS Class II substances, because this procedure declares the drug products to be bioinequivalent, whereas they have to be bioequivalent because of their Marketing Authorisations. From the other side recent publications have shown that biowaiver procedure for IR ibuprofen preparation could not detect bioinequivalence in terms of Cmax [8]. Therefore, biowaiver possibility for BCS Class II weak acids like ibuprofen has to be still discussed.
Acknowledgements
The authors wish to acknowledge Prof. V. G. Kukes, acad. RAMS for providing laboratory base.
References
- World Health Organization (WHO). 2006. Multisource (generic) pharmaceutical products: Guidelines on registration requirements to establish interchangeability. Technical Report Series, No 937, 40th Report, Annexes 7 and 8 of WHO Expert Committee on Specifications for Pharmaceutical Preparations. Available from: http://whqlibdoc.who. int/trs/WHO_TRS_937_eng.pdf, [Last accessed on 2011 Jan 11].
- European Medicines Evaluation Agency (EMEA), Committee for Proprietary Medicinal Products (CPMP). 2001. Note for Guidance on the Investigation of Bioavailability and Bioequivalence. Available from: http://www.emea.eu.int/pdfs/human/ewp/140198en.pdf, [Last accessed on 2011 Jan 11].
- U.S., Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). 2000. Guidances for industry: Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a Biopharmaceutics Classification System. Available from: http://www.fda.gov/CDER/GUIDANCE/3618fnl.pdf, [Last accessed on 2011 Jan 11].
- International Pharmaceutical Federation (FIP). 2009. Biopharmaceutics Classification System (BCS). Available from: http://www.fip.org/bcs, [Last accessed on 2011 Jan 11].
- Drug Bank database. Drug card for Ibuprofen. Available from: http:// www.drugbank.ca/drugs/DB01050, [Last accessed on 2011 Jan 11].
- The Merck Index. 13th ed. Rahway, New Jersey: Merck Research Laboratories; 2005.
- Potthast H, Dressman JB, Junginger HE, Midha KK, Oeser H, Shah VP, et al. Biowaiver monographs for immediate release solid oral dosageforms: Ibuprofen. J Pharm Sci 2005:94;2121-31.
- Alvarez C, Núñez I, Torrado JJ, Gordon J, Potthast H, García- Arieta. Investigation on the possibility of biowaivers for ibuprofen. J Pharm Sci 2011;100:2343-9.