- *Corresponding Author:
- R. Manikkam
Centre for Drug Discovery and Development, Col. Dr. Jeppiar Research Park, Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu 600119, India
E-mail: mrkactinos@gmail.com
| Date of Received | 10 June 2021 |
| Date of Revision | 18 November 2022 |
| Date of Acceptance | 15 September 2022 |
| Indian J Pharm Sci 2022;84(5):1150-1160 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The current study is aimed to isolate endophytic actinobacteria from various medicinal plants for exploring its antimicrobial activity against clinical pathogens. Out of 50 actinobacterial cultures isolated from eleven medicinal plants, 58 % of the cultures showed antimicrobial activity against at least two out of nine pathogens tested. Strain KCA1 isolated from Phyllanthus niruri leaves showed maximum zone of inhibition against all of the nine pathogens tested. Strain KCA1 exhibited maximum level of antimicrobial metabolite production in solid state fermentation during 9 d of incubation. Three well separated spots were observed when the ethyl acetate extract of strain KCA1 was analyzed through thin-layer chromatography. One spot with retardation factor value 0.82 was found to inhibit Staphylococcus aureus in thin-layer chromatography bioautographic assay. The active fraction eluted from thin-layer chromatography was further characterized by gas chromatography-mass spectral analysis. The mass spectral data revealed the presence of three compounds viz., 2,4-di-tert-butylphenol (C14H22O), 1-hexadecanol (C16H34O) and 1-nonadecene (C19H38) present in major proportions which are responsible for the antimicrobial activity of the strain KCA1. In fermentation experiment, variables such as glucose, yeast extract and sodium chloride were found to influence the antimicrobial compound production. The potential strain KCA1 was identified as Streptomyces sp. on the basis of microscopic, cultural, physiological and 16S ribosomal ribonucleic acid analysis.
Keywords
Endophytic actinobacteria, Streptomyces, antimicrobial, bioautography, gas chromatographymass spectroscopy
Antimicrobial Resistance (AMR) is one of the most concerning issues of human health[1,2]. It is reported that more than 700 000 deaths occur every year because of AMR in global level. It is statistically estimated that about 10 million people may possibly die every year due to AMR by 2050 without global intervention which would actually be more compared with cancer deaths by 2050. In India, it is estimated that there would be two million AMR-related deaths by 2050 without global intervention[3]. The management of AMR is difficult and requires a multinational approach. Indeed, no single initiative in the prevention and containment of AMR has proven entirely successful and hence development of a series of necessary mechanisms has been suggested[4]. The reduction in the number of novel antibiotics that have been found and produced in recent years is indeed a challenge to the researchers[5]. This has called for increased search for novel antibiotics to be discovered.
Microorganisms are producing a wide variety of nanoparticles, which can perform wide range of bioactivities[6,7]. This is especially notable for the phylum actinobacteria, which have been reported to produce more than 10 000 different bioactive molecules. Among them almost 45 % of the bioactive metabolites are currently known and most of which have been isolated from the Streptomyces[8]. Endophytic microorganisms live in healthy plant tissues and develop a symbiotic relationship with the host plant without causing any disease. The biodiversity of endophytic actinobacteria is known to be a valuable resource. A variety of studies have explored a wide spectrum of taxonomic status of medicinal plant-associated endophytic actinobacteria[7,9-11].
To our knowledge, to date, there are very limited reports on the diversity and antimicrobial potential of endophytic actinobacteria associated with Phyllanthus niruri (P. niruri), Ocimum tenuiflorum (O. tenuiflorum), Azadirachta indica (A. indica) and Mentha arvensis (M. arvensis) and also no reports were found with Cassia fistula (C. fistula), Eclipta prostrate (E. prostrate), Lawsonia inermis (L. inermis), Senna auriculata (S. auriculata), Rhizophora apiculata (R. apiculata), Murraya koenigii (M. koenigii) and Coriandrum sativum (C. sativum). Thus, the objectives of our study are as follows: To isolate and identify the endophytic actinobacteria from selected medicinal plants; to screen the antimicrobial potential of endophytic actinobacteria against various clinical pathogens; to separate and analyses the bioactive compounds from potential endophytic actinobacteria KCA1 using Thin Layer Chromatography (TLC) based bio autography and Gas Chromatography-Mass Spectroscopy (GC-MS) analysis.
Materials and Methods
Sample collection:
Medicinal plants were collected from regions of Trivandrum, Kerala and vicinity of Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu. A total of 33 samples including root, stem and leaf of 11 different medicinal plants such as P. niruri, C. fistula, A. indica, O. tenuiflorum, M. arvensis, E. prostrate, L. inermis, S. auriculata, R. apiculata, M. koenigii and C. sativum were collected for this study. The plant parts were rinsed thoroughly in running tap water to remove sand and dust particles and placed inside a zip-lock cover and were brought to the laboratory.
Isolation and characterization of endophytic actinobacteria:
The plant parts were cut into small pieces and were subjected to surface sterilization so as to ensure the removal of surface microbes. The most commonly used surface sterility includes ethanol (70 % for 1 min) and a strong oxidant or general disinfectant like household bleach (Sodium hypochlorite (NaOCl)) with 2 %-5 % (w/v), available chlorine (for 2-4 min). Then the surface sterilized samples were crushed in a mortar and pestle. 1 ml of crushed sample was serially diluted from 10-1 to 10-5 using sterile 9 ml water blank. 100 μl of aliquot from 103-105 dilutions were plated on Starch Casein Agar (SCA) supplemented with nalidixic acid (20 μg/ml) to inhibit the growth of fastgrowing eubacteria colonies and nystatin (50 μg/ml) to suppress the fungal growth. After incubating the plates for 20 d-28 d at 28°, all the endophytic actinobacterial colonies were recovered and sub-cultured in individual International Streptomyces Project-2 (ISP2) agar plates. After confirming the purity, morphologically dissimilar actinobacterial colonies were sub-cultured on ISP2 agar slants as well as in 20 % glycerol broth and stored at 4° and -20°, respectively. Cultural characteristics such as growth rate, consistency, aerial mass color, reverse side pigment and soluble pigment production and microscopic characteristics such as presence of aerial and substrate mycelium, mycelia fragmentation and spore chain morphology were recorded.
Preliminary determination of antimicrobial activity:
Antimicrobial activity of all the endophytic actinobacterial cultures were tested by agar plug method against clinical pathogens such as Staphylococcus aureus (S. aureus)-ATCC 29213, Bacillus cereus, Micrococcus sp., Escherichia coli (E. coli)-ATCC 25922, Providencia sp., Vibrio sp., Aeromonas hydrophila (A. hydrophila), Klebsiella pneumonia (K. pneumoniae)-ATCC 13882 and Candida albicans (C. albicans). Inoculum with 0.5 McFarland’s standard was prepared using sterile nutrient broth and inoculated onto Muller Hinton Agar (MHA) plates using sterile cotton swabs. Agar plugs of 10 d old endophytic actinobacterial strains with 5 mm diameter were taken from ISP2 agar plates and placed over the MHA agar plates seeded with test pathogens. Zone of inhibition was measured after 24 h of incubation at 37° and the results were expressed in millimetre in diameter. Actinobacterial strain which showed promising antimicrobial activity was selected as potential strain for further studies.
Effect of fermentation method on bioactive metabolites production by the strain KCA1:
Effect of solid-state and submerged fermentation on bioactive metabolite production by the strain KCA1 was investigated. Spores of the endophytic actinobacterial strain KCA1 was inoculated into 5 ISP2 agar plates (90 mm) and 100 ml of ISP2 broth. ISP2 agar plates were kept in the incubator at 28º for 15 d whereas the ISP2 broth containing flasks were incubated in rotary shaker with 120 rpm at 28º for 15 d. After 3 d, for every 24 h, agar plug from ISP2 agar plates were taken and tested for antimicrobial activity against S. aureus by agar plug method. Similarly, each 2 ml of endophytic actinobacterial strain KCA1 inoculated in ISP2 broth was taken and centrifuged at 10 000 rpm for 10 min. Further, the cell free supernatant was collected and antimicrobial activity was tested against S. aureus by adopting agar well diffusion method.
Extraction of bioactive metabolites:
The spores of the endophytic actinobacteria KCA1 was inoculated into ISP2 agar plates for its growth and bioactive metabolite production. After incubating the plates for 7 d, the spores were scrapped out and the agar blocks were collected and mixed with hexane, ethyl acetate, chloroform and methanol (1:2 ratio) for the extraction of metabolites. After 24 h, the agar blocks were removed and the solvents were collected and evaporated using rotary evaporator so as to get the crude extract. The antimicrobial activity against S. aureus and E. coli was tested for four different extracts of the strain KCA1 by agar well diffusion method.
Secondary determination of antimicrobial activity:
The crude ethyl acetate extract was then checked for antimicrobial activity against various clinical pathogens using disc diffusion method. Sterile empty filter paper disc (HiMedia) with 5 mm diameter was impregnated with 20 μl of endophytic actinobacteria KCA1 ethyl acetate crude extract (100 μg/ml) and kept for drying. The panel of clinical pathogens, as mentioned above, was grown in nutrient broth. Inoculum equivalent to 0.5 McFarland standards was spread evenly over the surface of MHA plate using sterile cotton swab. Once the discs were dried, they were placed over the pathogens inoculated MHA plates and incubated at 37°. After 24 h of incubation the diameter of the zone of inhibition was measured and expressed in millimeter.
Purification of bioactive compounds:
The crude ethyl acetate extract of strain KCA1, which showed promising antimicrobial activity, was subjected to separation through TLC. 3 μl of crude extract was spotted onto silica gel plates (pore size 60Å, mesh size: 230-400, particle size 40-63 μm, Merck) using capillary tube and then developed with ethyl acetate:dichloromethane (8:2, v/v) gradient as an eluting solvent mixture. The separated components were visualized under Ultraviolet (UV) light at 254 and 365 nm. The antimicrobial active fraction was detected by bio autography approach. The developed TLC plates were overlaid on MHA agar medium supplemented with 0.1 % (w/v) 2, 3, 5-triphenyltetrazolium chloride (tetrazolium red) and S. aureus-ATCC 29213 used as the test pathogen at a final concentration of 107 CFU/ ml. The plate was incubated at 37° for 24 h. Clear zone of inhibition indicated the position of antimicrobial compounds on the TLC plates and the Retention factor (Rf) value was calculated. The active fraction was eluted by preparative TLC and purified using ethyl acetate.
Identification of bioactive metabolites by GC-MS analysis
The partially purified TLC fraction was further subjected to identify the bioactive metabolites by using GCMS analysis. Agilent technologies 6890–5973N with capillary column TG-5 MS Phenyl Methyl Siloxane (30 m×250 μm×0.25 μm) system was used. Mass detector used in split mode and helium gas with flow rate of 1.0 ml/min was used as a carrier. Injector was operated at 230° and oven temperature for initial setup was 60° for 2 min, ramp 10 min to 280° for 8 min.
Characterization, optimization and taxonomy of potential actinobacterial strain:
The potential endophytic actinobacterial strain KCA1 was characterized based on their phenotypic[12] 16S ribosomal Ribonucleic Acid (16S rRNA) analysis. Morphological characteristics were observed in different culture media such as tryptone agar (ISP1), yeast extract-malt extract agar (ISP2), oatmeal agar (ISP3), inorganic salts-starch agar (ISP4), glycerolasparagine agar (ISP5), peptone-yeast extract-iron agar (ISP6) and tyrosine agar (ISP7). The results were noted after incubation at 30° for 7 d-10 d. The strain KCA 1 was studied for the utilization of carbon, nitrogen, minerals sources and enzyme production. Also the effect of different pH levels and Sodium chloride (NaCl) concentration on its growth were studied[13].
Strain KCA1 grown at all the above conditions was tested for its antimicrobial activity against S. aureus and E. coli by agar plug method as described above.
Molecular characterization:
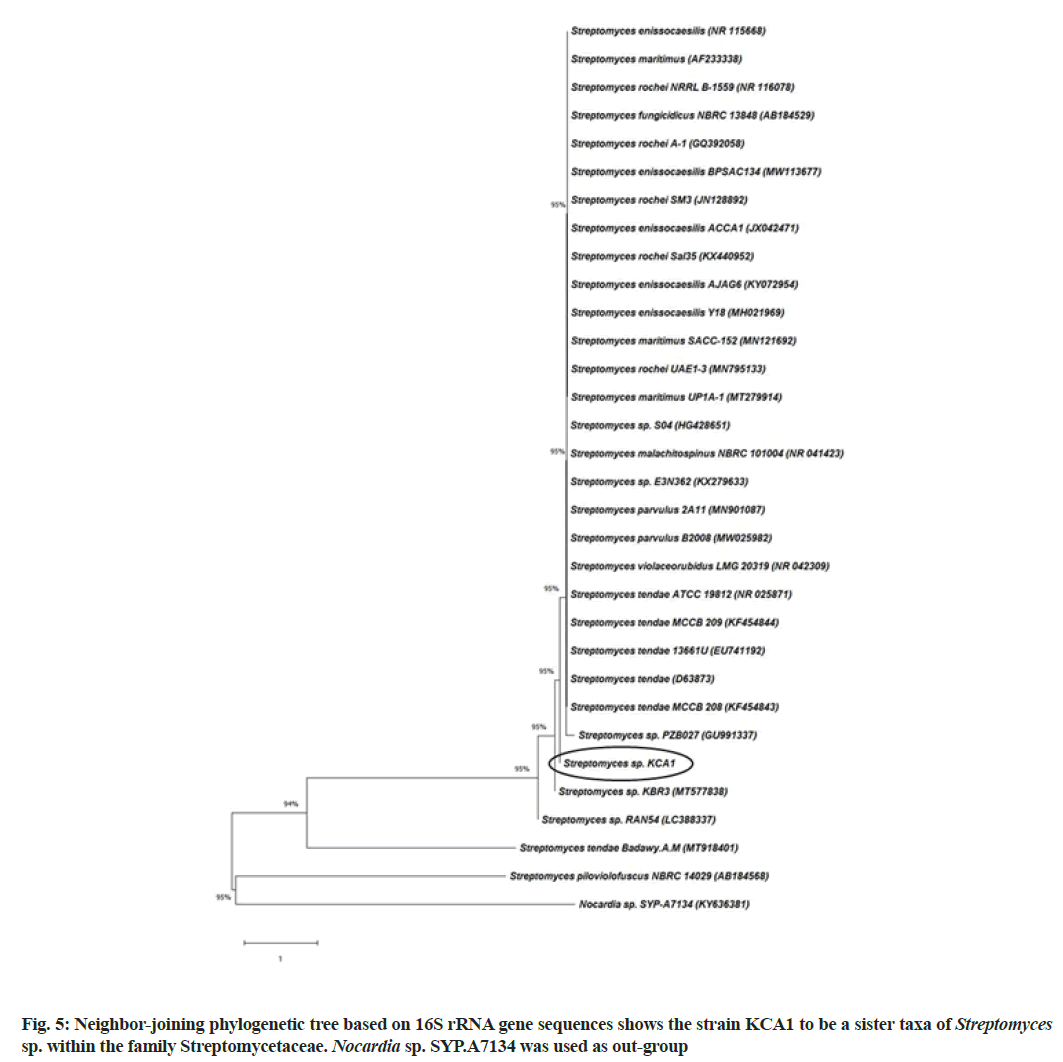
The genomic Deoxyribonucleic acid (DNA) of potential endophytic actinobacteria KCA1 was isolated using solute ready genomic DNA kit (HiMedia- HiPurA Streptomyces DNA purification kit), according to the manufacturer’s guidelines. The polymerase chain reaction amplification of 16S rRNA gene of strain KCA1 was performed by using the primers 27F 5'-AGAGTTTGATCMTGGCTCAG-3' (forward) and 1492R 5'-TACGGYTACCTTGTTACGACTT-3' (reverse)[14]. Amplified product was carried out for sequencing at Euro fins Genomics India pvt. Ltd., Bangalore. The identification of phylogenetic 9 h and calculation of pairwise 16S rRNA gene sequence similarities were achieved using the Molecular Evolutionary Genetics Analysis (MEGA) version 7 and basic local alignment search tool analysis (http:// blast.ncbi.nlm. nihgov/Blast. cgi). The phylogenetic tree was constructed using the aligned sequences by the neighbor-joining method[15] using Kimura-2-parameter distances in the MEGA 7 software. To determine the support of each clade, bootstrap analysis was performed with 1000 replications. The obtained 16S rRNA sequence was submitted to GenBank to get the accession number.
Results and Discussion
Totally 50 endophytic actinobacterial cultures were isolated from eleven medicinal plant species based on different colony morphology. All the cultures showed good growth on ISP2 agar medium while most of them have the typical morphology of Streptomyces (Table 1). Among the 50 cultures, 37 of them appear to be powdery in nature while 10 others showed a leathery consistency and 3 strains appeared to be smooth. The aerial mass colour of the actinobacterial cultures were found to be grey (28), white (9), cream (5), brown (3), orange (2), pink (2) and yellow (1) with a diverse range of reverse side pigments like pale yellow (31), brown (10), pale brown (5), pink (2) and orange (2). Some of the strains had soluble pigments like pale yellow (9), pink (1) and brown (3) which were dispersed all over the plates. All the endophytic actinobacteria showed the presence of aerial and a branched substrate mycelium which differentiate into short, straight to flexuous chains of smooth-surfaced spores. Passari et al.[16] isolated 42 endophytic actinobacteria from seven medicinal plants and most of them are identified as Streptomyces. Similar to this present study surface sterilization is one of the important processes to study endophytic actinobacterial isolation, whereas Qin et al.[17] conducted similar work and isolated 2174 actinobacteria from 90 plant samples.
| Compound name | RT | MM | Area (%) | MF | Activity | References |
|---|---|---|---|---|---|---|
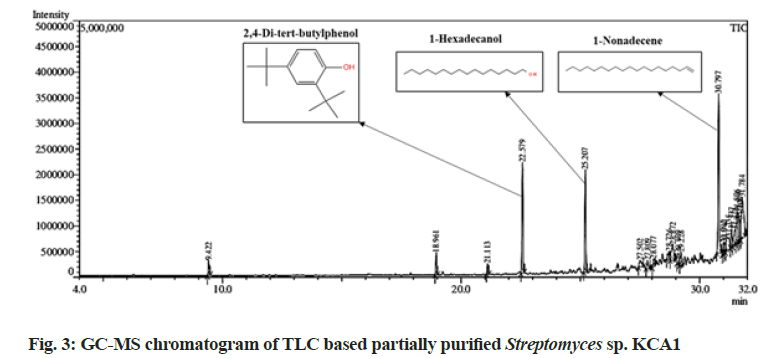
| 2,4-Di-tert-butylphenol | 22.579 | 206 | 12.58 | C14H22O | Antibacterial; antiviral; antifungal; antioxidant; anti-inflammatory; cytotoxicities; phytotoxicity: allelopathy and autotoxicity; insecticidal and nematicidal activities | [30-33] |
| 1-Hexadecanol | 25.207 | 242 | 11.8 | C16H34O | Antimicrobial; antifungal activity | [34,35] |
| 1-Nonadecene | 30.797 | 266 | 19.45 | C19H38 | antituberculosis, anticancer, antioxidant, antimicrobial activity | [36-39] |
Table 1: Bioactive Metabolites Identified from The from Streptomyces Strain KCA1 Through by GC-MS Analysis
They reported most of the strains as Streptomyces (87 %) according to the preliminary morphology identification. Bhatia et al.[18] isolated most of the actinobacteria in SCA media from fresh and dried root and shoot materials of P. niruri. Gangwar et al.[19] isolated forty endophytic actinobacteria from root, leaves and stem of medicinal plants including O. sanctum and most of them are identified as Streptomyces. According to literature survey, this study might be the first report of endophytic actinobacterial isolation from medicinal plants such as C. fistula, E. prostrata, L. inermis, S. auriculata, R. apiculata, M. koenigii and C. sativum.
Out of 50 endophytic actinobacterial isolates, 29 isolates showed activity against minimum of 2 out of 9 pathogens tested whereas two isolates namely KCA1 and KCA2 showed positive activity against all of the 9 pathogens tested (Table 2). The isolate KCA1, isolated from the leaves of P. niruri showed maximum inhibition against all the pathogens with the zone of inhibition ranging 13.2±0.6 mm to 22.8±0.4 mm in diameter. Endophytic actinobacteria isolated from medicinal plants P. niruri, O. sanctum, A. indica and M. arvensis showed strong antimicrobial activity against various pathogens[18-20]. Bhatia et al.[18] reported that endophytic actinobacteria isolated from P. niruri showed maximum zone of inhibition against gram negative pathogen P. fluorescens, which is correlated with our present study, KCA1 isolated from P. niruri also showing highest zone inhibition against the gram negative pathogens E. coli (14.8±0.4 mm), Vibrio sp. (13.2±0.6 mm), Providencia sp. (13.2±0.2), A. hydrophila (20.5±0.28) and K. pneumoniae (22.5±0.3). Similar to our study, endophytic actinobacteria isolated from A. indica showed antimicrobial activity against P. fluorescens, E. coli and Bacillus subtilis[21].
| Characteristics | Variables | Growth | Antimicrobial activity S. aureus (mm) |
|---|---|---|---|
| Cultural Characteristics | ISP1 (Tryptone agar) | +++ | 15.2±0.2 |
| ISP2 (Yeast extract malt extract) | +++ | 22.2±0.2 | |
| ISP3 (Oatmeal agar) | +++ | 0.0 | |
| ISP4 (Inorganic salts- starch) | ++ | 19.8±0.4 | |
| ISP5 (Glycerol aspargine) | + | 0.0 | |
| ISP6 (Peptone Yeast extract iron ) | ++ | 0.0 | |
| ISP7 (Tyrosine agar) | +++ | 16.2±0.2 | |
| Utilization of Carbon source | Glucose | ++ | 20.2±0.2 |
| Arabinose | + | 0.0 | |
| Sucrose | ++ | 14.5±0.3 | |
| Xylose | + | 13.2±0.2 | |
| Inositol | + | 0.0 | |
| Mannitol | + | 17.8±0.2 | |
| Fructose | ++ | 17.5±0.3 | |
| Rhamnose | - | 0.0 | |
| Raffinose | - | 0.0 | |
| Cellulose | - | 0.0 | |
| Utilization of nitrogen source | Peptone | ++ | 14.8±0.2 |
| Yeast Exract | +++ | 20.5±0.3 | |
| Malt Extract | +++ | 0.0 | |
| KNO3 | - | 0.0 | |
| Soybean (NaNO3) | +++ | 12.5±0.3 | |
| Enzyme production | Asparginase | +++ | 0.0 |
| Glutaminase | +++ | 0.0 | |
| Amylase | ++ | 0.0 | |
| Protease | ++ | 0.0 | |
| Lipase | - | 0.0 | |
| pH | 5 | - | 0.0 |
| 7 | +++ | 22.2±0.2 | |
| 9 | ++ | 0.0 | |
| 11 | - | 0.0 | |
| NaCl tolerance (%) | 0 | ++ | 11.8±0.2 |
| 1 | +++ | 21.5±0.3 | |
| 2.5 | + | 0.0 | |
| 5 | - | 0.0 | |
| 7.5 | - | 0.0 | |
| 10 | - | 0.0 |
Table 2: Effect of Critical Medium Components on Antimicrobial Compounds Production by Streptomyces Strain KCA1
The strain KCA1 showed a good bioactive metabolite production in both solid as well as in submerged state fermentation. From 3 d to 15 d, it was noted that the metabolites produced by the strain KCA1 showed maximum activity against S. aureus in solid state fermentation compared to that of submerged fermentation (fig. 1). The metabolite production was thus confirmed to be faster and stable in solid state having an inhibition zone of 15.2±0.2 mm in diameter on 3 d to 21.5±0.9 mm, which was the maximum, on 9 d when compared to that of submerged fermentation where the zone of inhibition (12.5±0.8 mm) showed on 7 d and it reaches the maximum level on 9 d with the inhibition zone of 14.8±0.7 mm. However, a gradual reduction in the inhibition zone was observed from 8th and 10th d of its growth in solid and submerged state fermentation respectively. Our present study correlated with Radhika et al.[22] who reported that the bioactive metabolite production from Streptomyces showed antimicrobial activity against S. aureus in both solid and submerged state fermentation. But most of the Streptomyces strains were reported to show bioactive metabolite production in solid state and failed to show activity when produced in liquid culture. Gebreyohannes et al.[23] studied using eleven potential actinobacteria for solid and submerged state fermentation and bioactivity was also checked against various pathogens. Similar to our study, they reported that both the fermentation methods were suitable for bioactive metabolite production and showed provident antimicrobial activity against various pathogens.
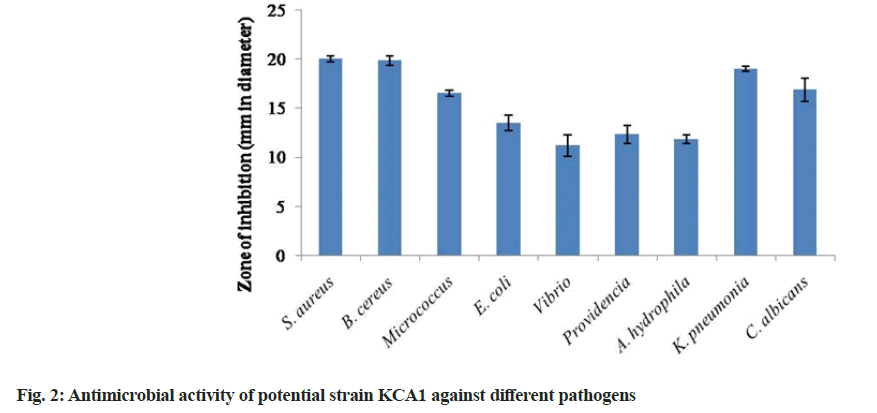
The ethyl acetate extract of KCA1 showed activity against all the test pathogens the maximum activity was observed against S. aureus (20.0±0.3 mm) followed by Bacillus cereus (19.83±0.4 mm), K. pneumoniae (19.0±0.3 mm) and C. albicans (16.83±1.2 mm). Also, the crude extract of KCA1 showed least antimicrobial activity against Vibrio sp. (11.17±1.1 mm) followed by A. hydrophila (11.83±0.4 mm), Providencia (12.33±0.9 mm) and E. coli (13.5±0.8 mm) (fig. 2). Our present finding of secondary screening also correlated with that of other reports. This evidenced that the crude extract of actinobacterial strains produced by solid state fermentation showed antimicrobial activity against wide range of pathogens such as S. aureus, E. coli, P. aeruginosa, K. pneumoniae and S. typhi in secondary screening using disc diffusion method[23]. They also reported that the promising antimicrobial inhibition against various pathogens suggested that endophytic actinobacteria may be the crucial candidates for the production of bioactive metabolites[17].
Among the different solvents used for extraction of secondary metabolites from the potential strain KCA 1, ethyl acetate extract showed maximum zone of inhibition against S. aureus (20.5±0.7 mm), while no results were obtained for hexane and methanol extracts. However, chloroform extract showed 9.5±0.3 mm zone of inhibition against S. aureus. Similarly, Das et al.[24] also reported that ethyl acetate extract of Streptomyces showed maximum activity against S. aureus. The ethyl acetate extract of Streptomyces sp. VITPSA showed highest range zones of inhibition against P. aeruginosa and S. aureus, which was also higher than the standard chloramphenicol[25]. Our present study exactly correlates with other reports showing the antimicrobial activity of different solvent extracts of endophytic actinobacteria against E. coli, P. mirabilis, P. aeruginosa, K. pnumoniae and Enterobacter and proving that ethyl acetate extract was the most predominate than other solvents[26].
A total of six bioactive compounds were detected by TLC using the solvent system of dichloromethane and chloroform with the ratio of 8:2. In which, one bioactive compound showed a clear zone of inhibition against S. aureus and had an Rf value of 0.82. The antimicrobial activity of the bioactive compound was found to be 21.8±0.3 mm zone of inhibition against S. aureus which was collected by preparative TLC. Our research finding exactly correlated with Vaishnavi et al.[27] reported that partially purified ethyl acetate extract from Streptomyces showed inhibition against S. aureus, which was purified and detected by TLC and bio autography techniques. TLC based bioautography is a high throughput method which can be used for screening of bioactive molecules and to study their activity[28]. Our present study also correlated with other report stated that antibacterial compound Actinomycin D produced by Streptomyces TBRC 8912 was purified and detected by TLC based bioautography[29].
In this present study GC-MS analysis of partially purified active TLC fraction of Streptomyces sp. KCA1 confirmed the presence of major three compounds with different retention times as illustrated in fig. 3. The identified compounds and their retention time, molecular mass, peak area (%), molecular formula and their potential activities are shown in Table 1. The most prevailing major compounds such as 2, 4-di-tert-butylphenol (C14H22O), 1-Hexadecanol (C16H34O) and 1-Nonadecene (C19H38) were found to be the derivatives of aromatic compounds. Similarly, C14H22O by TLC based approach and showed strong antimicrobial activity against Aspergillus[30,31]. Also, Streptomyces mutabilis G61 isolated from Sahara soil sample which produced C14H22O having strong antimicrobial activity[32]. The endophytic Streptomyces globosus JQ926176 produced C14H22O which has strong antioxidant activity[33]. Another remaining two bioactive compounds C16H34O and C19H38 were also isolated from Streptomyces strains showed antimicrobial, anticancer and anti-tubercular activity[34-39]. Thus, the present study is clearly revealed that the three major compounds identified through GC-MS could be the key contributing factors to the antagonistic potential of Streptomyces strain KCA1.
The cultural characteristics of the strain KCA 1 is shown in Table 1. It showed good growth on ISP1, ISP2, ISP3 and ISP7 and moderate growth on ISP4, ISP5 and ISP6. The potential strain KCA1 was found to utilize wide range of carbon (Glucose, Arabinose, Sucrose, Xylose, Inositol, Fructose, Mannitol, Raffinose, Rhamnose, and cellulose), nitrogen (Peptone, Yeast extract, Malt extract, Potassium nitrate (KNO3) and Soybean) and mineral sources (NaCl, Magnesium chloride, Manganese chloride, Potassium chloride, Calcium carbonate) for their growth and secondary metabolite production. The strain KCA1 was also found to have asparaginase, glutaminase, amylase and protease production. Other factors like pH and NaCl were also found to regulate the growth and secondary metabolite production of the strain KCA1 (Table 1). Among all the parameters tested, the potential strain KCA1 was found to show maximum growth and antimicrobial activity against S. aureus and E. coli on utilization of glucose as carbon source, yeast extract as nitrogen source, NaCl as mineral source and pH 7 (Table 2). Similarly, Manikkam et al.[40] also studied the influence of different carbon sources for the growth and metabolite production against S. aureus. It showed glucose as the most predominant carbon source followed by fructose, xylose and sucrose which were also correlated with our present study which showed highest growth of inhibition of S. aureus (20.2±0.2 mm) followed by mannitol (17.8±0.2 mm), fructose (17.5±0.3 mm), sucrose (14.5±0.3) and xylose (13.2±0.2). Al-ghazali et al.[41] reported that Streptomyces sp. LH9 used dextrose as a carbon source and produced maximum activity against E. coli,P. aeruginosa,S. aureus and S. agalagtiae. They also studied different nitrogen sources for metabolite production and reported peptone as the excellent nitrogen source against E. coli,P. aeruginosa,S. aureus and S. agalagtiae, whereas our present study showed yeast extract having maximum activity followed by peptone and soybean against S. aureus and E. coli[41]. Manikkam et al.[40] reported that malt extract used as nitrogen source showed maximum activity against S. aureus. Our results correlates with Abdelwahed et al.[42] who reported soybean meal and yeast extract as the most favorable nitrogen source for antimicrobial compound production for Streptomyces cyaneus DN.37 and Streptomyces lavendulae DN.7, respectively. Al-Farraj et al.[43] also reported that glucose and yeast extract used as the carbon and nitrogen sources influenced maximum antibiotic productions than others. Actinobacteria can tolerate wide range of pH and are able to grow in higher saline condition[44]. Our present study reported that the strain KCA1 showed good growth in pH 7 and was able to tolerate NaCl up to 1 %. This investigation also coincides with other’s reports[40,45].
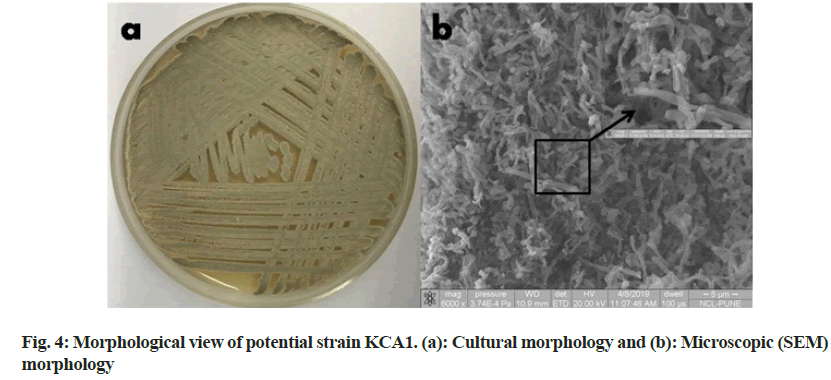
The strain KCA1 showed powdery type of colony with gray aerial mass color in ISP2 agar medium (fig. 4a). Scanning electron microscope images of this strain showed substrate mycelia and extensively branched aerial hyphae that are further differentiated into smoothly surfaced spores (fig. 4b). The 16S rRNA gene sequence of the strain KCA1 showed 99.45 % similarity with Streptomyces sp. and had a query length of 1452 bp. The accession number MW470667 was received from Genbank for the strain KCA1. A phylogenetic tree was constructed based on 16S rRNA gene sequences to show the comparative relationship between strain KCA1 and other related Streptomyces species (fig. 5). The comparative analysis of 16S rRNA gene sequence and phylogenetic relationship showed that strain KCA1 lies in clade close with Streptomyces sp. strain KBR3 and Streptomyces sp., RAN54. This microscopic and molecular identification is also agreed with other reports[27,46].
Acknowledgements:
Authors thank the authorities of Sathyabama Institute of Science and Technology for their research support and encouragements. Authors also thank the Science Engineering Research Board (SERB), Department of Science and Technology, New Delhi for their support in the form of research grant (YSS/2015/001887).
Conflict of interests:
The authors declared no conflict of interests.
References
- Licata F, Quirino A, Pepe D, Matera G, Bianco A. Antimicrobial resistance in pathogens isolated from blood cultures: A two-year multicenter hospital surveillance study in Italy. Antibiotics 2020;10(1):10.
[Crossref] [Google Scholar] [PubMed]
- Paun VI, Lavin P, Chifiriuc MC, Purcarea C. First report on antibiotic resistance and antimicrobial activity of bacterial isolates from 13,000-year old cave ice core. Sci Rep 2021;11(1):514.
- AMR Industry Alliance; 2020 Progress Report. 2020.
- Nofiani R, Weisberg AJ, Tsunoda T, Panjaitan RG, Brilliantoro R, Chang JH, et al. Antibacterial potential of secondary metabolites from Indonesian marine bacterial symbionts. Int J Microbiol 2020;2020:8898631.
- Sharma P, Thakur D. Antimicrobial biosynthetic potential and diversity of culturable soil actinobacteria from forest ecosystems of Northeast India. Sci Rep 2020;10(1):4104.
[Crossref] [Google Scholar] [PubMed]
- Abdel-Razek AS, El-Naggar ME, Allam A, Morsy OM, Othman SI. Microbial natural products in drug discovery. Processes 2020;8(4):470.
- Girão M, Ribeiro I, Ribeiro T, Azevedo IC, Pereira F, Urbatzka R, et al. Actinobacteria isolated from Laminaria ochroleuca: A source of new bioactive compounds. Front Microbiol 2019;10:683.
[Crossref] [Google Scholar] [PubMed]
- Amin DH, Abdallah NA, Abolmaaty A, Tolba S, Wellington EM. Microbiological and molecular insights on rare Actinobacteria harboring bioactive prospective. Bull Nat Res Centre 2020;44(1):1-2.
- Musa Z, Ma J, Egamberdieva D, Abdelshafy Mohamad OA, Abaydulla G, Liu Y, et al. Diversity and antimicrobial potential of cultivable endophytic actinobacteria associated with the medicinal plant Thymus roseus. Front Microbiol 2020;11:191.
- Singh R, Dubey AK. Isolation and characterization of a new endophytic actinobacterium Streptomyces californicus strain ADR1 as a promising source of anti-bacterial, anti-biofilm and antioxidant metabolites. Microorganisms 2020;8(6):929.
[Crossref] [Google Scholar] [PubMed]
- Rante H, Alam G, Pakki E, Umar U, Ali A. Identification and antibacterial activity of actinomycetes isolated from medicinal plant Andrographis paniculata rhizosphere soil. Crescent J Med Biol Sci 2020;7(4):467-73.
- Shirling ET, Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Bacteriol 1966;16(3):313-40.
- Kumar PS, Raj JP, Duraipandiyan V, Ignacimuthu S. Antibacterial activity of some actinomycetes from Tamil Nadu, India. Asian Pac J Trop Biomed 2012;2(12):936-43.
- Kumar Gothwal R, Kumar Nigam V, Mohan MK, Sasmal D, Ghosh P. Extraction of bulk DNA from Thar desert soils for optimization of PCR-DGGE based microbial community analysis. Electron J Biotechnol 2007;10(3):400-8.
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 1987;4(4):406-25.
[Crossref] [Google Scholar] [PubMed]
- Passari AK, Mishra VK, Saikia R, Gupta VK, Singh BP. Isolation, abundance and phylogenetic affiliation of endophytic actinomycetes associated with medicinal plants and screening for their in vitro antimicrobial biosynthetic potential. Front Microbiol 2015;6:273.
[Crossref] [Google Scholar] [PubMed]
- Qin S, Li J, Chen HH, Zhao GZ, Zhu WY, Jiang CL, et al. Isolation, diversity, and antimicrobial activity of rare actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna, China. Appl Environ Microbiol 2009;75(19):6176-86.
[Crossref] [Google Scholar] [PubMed]
- Bhatia N, Gupta T, Sharma B, Sarethy IP. Endophytes from Phyllanthus niruri: Selection, characterization and metabolite production. J Mater Sci Surf Eng 2019;6(6):888-94.
- Gangwar M, Dogra S, Sharma N. Antagonistic bioactivity of endophytic actinomycetes isolated from medicinal plants. J Adv Lab Res Biol 2011;2(4):154-7.
- Singh MJ, Padmavathy S. Nocardiopsis sp. 5 endophytic to tulsi leaves-isolation and antimicrobial activity. Br Microbiol Res J 2015;5(3):194-202.
- Verma VC, Gond SK, Kumar A, Mishra A, Kharwar RN, Gange AC. Endophytic actinomycetes from Azadirachta indica A. Juss.: Isolation, diversity, and anti-microbial activity. Microb Ecol 2009;57(4):749-56.
[Crossref] [Google Scholar] [PubMed]
- Radhika, Selvaraj B, Radhakrishnan M, Rama B. Bioprospecting of fresh water actinobacteria: Isolation, characterization and antagonistic potential of selected actinobacteria. J Pharma Res 2011;44:2584-6.
- Gebreyohannes G, Moges F, Sahile S, Raja N. Isolation and characterization of potential antibiotic producing actinomycetes from water and sediments of Lake Tana, Ethiopia. Asian Pac J Trop Biomed 2013;3(6):426-35.
[Crossref] [Google Scholar] [PubMed]
- Das R, Romi W, Das R, Sharma HK, Thakur D. Antimicrobial potentiality of actinobacteria isolated from two microbiologically unexplored forest ecosystems of Northeast India. BMC Microbiol 2018;18(1):71.
- Pooja S, Aditi T, Naine SJ, Subathra Devi C. Bioactive compounds from marine Streptomyces sp. VITPSA as therapeutics. Front Biol 2017;12(4):280-9.
- Ramachandran G, Rajivgandhi G, Maruthupandy M, Manoharan N. Isolation and identification of antibacterial compound from marine endophytic actinomycetes against multi drug resistant bacteria. Ann Microbiol Immunol 2018;1(1);1003.
- Vaishnavi M, Manigundan K, Smalia T, Nandhini SU, Gopikrishnan V, Kumar A, et al. Antibacterial and anti-HIV activity of extracellular pigment from Streptomyces sp. S45 isolated from Sabarimala forest soil, India. Indian J Exp Biol 2020;58(12):861-8.
- Choma IM, Jesionek W. TLC-direct bioautography as a high throughput method for detection of antimicrobials in plants. Chromatography 2015;2(2):225-38.
- Jumpathong J, Nuengchamnong N, Masin K, Nakaew N, Suphrom N. Thin layer chromatography-bioautography assay for antibacterial compounds from Streptomyces sp. TBRC 8912, a newly isolated actinomycin D producer. Chiang Mai J Sci 2019;46(5):839-49.
- Zhao F, Wang P, Lucardi RD, Su Z, Li S. Natural sources and bioactivities of 2, 4-di-tert-butylphenol and its analogs. Toxins 2020;12(1):35.
[Crossref] [Google Scholar] [PubMed]
- Teresa RC, Rosaura VG, Elda CM, Ernesto GP. The avocado defense compound phenol-2, 4-bis (1, 1-dimethylethyl) is induced by arachidonic acid and acts via the inhibition of hydrogen peroxide production by pathogens. Physiol Mol Plant Pathol 2014;87:32-41.
- Belghit S, Driche EH, Bijani C, Zitouni A, Sabaou N, Badji B, Mathieu F. Activity of 2, 4-Di-tert-butylphenol produced by a strain of Streptomyces mutabilis isolated from a Saharan soil against Candida albicans and other pathogenic fungi. J Mycol Med 2016;26(2):160-9.
[Crossref] [Google Scholar] [PubMed]
- Akshatha JV, Prakash HS, Nalini MS. Actinomycete endophytes from the ethno medicinal plants of southern India: Antioxidant activity and characterization studies. J Biol Active Prod Nat 2016;6(2):166-72.
- Baskaran R, Mohan PM, Madanan MG, Kumar A, Palaniswami M. Characterization and antimicrobial activity of Streptomyces sp. DOSMB-A107 isolated from mangrove sediments of Andaman Island, India. Indian J Mari Sci 2015; 44:714-723.
- Marimuthu S, Karthic C, Mostafa AA, Al-Enazi NM, Abdel-Raouf N, Sholkamy EN. Antifungal activity of Streptomyces sp. SLR03 against tea fungal plant pathogen Pestalotiopsis theae. J King Saud Univ Sci 2020;32(8):3258-64.
- Rukachaisirikul T, Siriwattanakit P, Sukcharoenphol K, Wongvein C, Ruttanaweang P, Wongwattanavuch P, et al. Chemical constituents and bioactivity of Piper sarmentosum. J Ethnopharmacol 2004;93(2-3):173-6.
[Crossref] [Google Scholar] [PubMed]
- Lee YS, Kang MH, Cho SY, Jeong CS. Effects of constituents of Amomum xanthioides on gastritis in rats and on growth of gastric cancer cells. Arch Pharm Res 2007;30(4):436-43.
[Crossref] [Google Scholar] [PubMed]
- Gherraf N, Zellagui A, Kabouche A, Lahouel M, Salhi R, Rhouati S. Chemical constituents and antimicrobial activity of essential oils of Ammodaucus leucotricus. Arabian J Chem 2017;10:S2476-8.
- Kumari N, Menghani E, Mithal R. GCMS analysis of compounds extracted from actinomycetes AIA6 isolates and study of its antimicrobial efficacy. Indian J Chem Technol 2019;26:362-370.
- Manikkam R, Venugopal G, Ramasamy B, Kumar V. Effect of critical medium components and culture conditions on antitubercular pigment production from novel Streptomyces sp D25 isolated from Thar desert, Rajasthan. J Appl Pharm Sci 2015;5(6):015-9.
- Al-ghazali LH, Omran R. Optimization of medium composition for antibacterial metabolite production from Streptomyces sp. Asian J Pharm Clin Res 2017;10(9):381-5.
- Abdelwahed NA, Abdallah NA, El-Ghawas DE, EL-Din SM, El-Diwany AI. Isolation, identification and optimization of antimicrobial metabolites produced by soil derived actinomycetes. Egypt J Exp Biol (Bot) 2012;8(2):205-17.
- Al-Farraj DA, Varghese R, Vágvölgyi C, Elshikh MS, Alokda AM, Mahmoud AH. Antibiotics production in optimized culture condition using low cost substrates from Streptomyces sp. AS4 isolated from mangrove soil sediment. J King Saud Univ Sci 2020;32(2):1528-35.
- Norovsuren ZH, Oborotov GV, Zenova GM, Aliev RA, Zvyagintsev DG. Haloalkaliphilic actinomycetes in soils of Mongolian desert steppes. Biol Bull 2007;34(4):417-22.
[Crossref] [Google Scholar] [PubMed]
- Sengupta S, Pramanik A, Ghosh A, Bhattacharyya M. Antimicrobial activities of actinomycetes isolated from unexplored regions of Sundarbans mangrove ecosystem. BMC Microbiol 2015;15(1):170.
[Crossref] [Google Scholar] [PubMed]
- Soundarya G, Manigundan K, Meganathan PR, Gomathi S, Balaji S, Jerrine J, et al. Antimicrobial and anti-tubercular potential of Streptomyces lomondensis SACC 63 isolated from soil samples in Himachal Pradesh, India. J Environ Biol 2020;41(1):131-8.
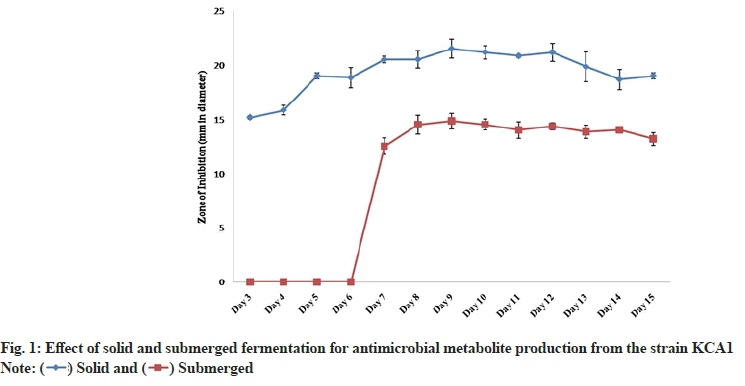
 ) Solid and (
) Solid and ( ) Submerged
) Submerged