- *Corresponding Author:
- B. K. Manjunatha
Centre for Biotechnology, Pravara Institutes of Medical Sciences, Loni, Maharashtra 413736, India
E-mail: dr.manjunath.toce@gmail.com
| Date of Received | 17 July 2022 |
| Date of Revision | 09 July 2023 |
| Date of Acceptance | 08 November 2023 |
| Indian J Pharm Sci 2023;85(6):1702-1712 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Cancer is one of the leading causes of death worldwide. With the help of current scientific developments and traditional knowledge about medicinal plants, great progress could be made in the fight against cancer. The present study attempts to scientifically validate the ethnomedicinal and anticancer effects of Mesua ferrea Linn from the Western Ghats of Karnataka, India. Using preparative HPLC, two main fractions (C1 and C2) were isolated from the methanolic extract of Mesua ferrea and their bioactivity was investigated. Gas chromatography/Mass spectrometry analysis of Mesua ferrea methanol extract revealed the presence of 7 phytocomponents. Fraction C2 shows maximum potency against liver HepG2 cancer cell lines. Fraction C was further purified and subjected to spectroscopic analysis by 13C NMR, 1H NMR, FTIR and Liquid chromatography-mass spectrometry techniques. The structure is predicted and identified as 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one. This compound was found to have antiproliferative activity with an IC50 value of 352.007 µg/ml compared to the standard drug (camptothecin) with an IC50 value of 52.253 µg/ml and a crude drug of 177.006 µg/ml. Anticancer efficacy of the purified compound assessed by Annexin V apoptosis Study Using HepG2 cell lines. The compound exhibits significant early and late apoptosis of 13.6 % and 15.5 %, respectively, compared to the standard drug camptothecin with 28.7 % and 18.7 % early and late apoptosis and 7.02 % and 11.8 %. Caspase-3 expression analysis using HepG2 cell lines revealed that the purified compound inhibited early apoptosis in HepG2 cells at a concentration of 352 µg/ml, activating the caspase-3 cascade by 15.3 % compared with 17 % of the standard drug CTP. The result of the DNA fragmentation test supported the results of annexin V and caspase 3 expression and showed significant protection. Our results indicate that 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one isolated from Mesua ferrea may be a new lead molecule for the development of an antiproliferative, DNA-protective and effective anticancer drugs.
Keywords
Ethnomedicine, Mesua ferrea, anti-cancer, apoptosis, caspase, Annexin-V
Advances in molecular and genomic research have shed light on complexities and changed perceptions of cancer. Ongoing research has increased our understanding of how cancer develops and how drugs can be used to target specific types of cancer. However, cancer remains a major global health problem, with lifestyle, environmental and genetic factors increasing the risk of developing cancer. In chemotherapy approaches, researchers continually seek scientific validation of traditionally recommended medicinal plants for their anti-cancer properties.
Mesua ferrea (Linn.) (M. ferrea) is a well-known evergreen tropical tree (Clusiaceae), commonly known as Indian rose chestnut/and Naga sampige/Nagakesara (Kannada), used in folk medicine to treat fever and dyspepsia[1,2]. M. ferrea is known to possess anticancer, antioxidant, antiviral, immunosuppressive, antimicrobial and hepatoprotective activities[3-7]. It is also used to treat wounds, itching, other rashes, dandruff and rheumatism[8]. Traditionally, it has been used as a tonic, antiseptic, laxative, blood purifier and anthelmintic[9].
The plant is examined for its Ty1 copia retrotransposons[10]. Cytotoxicity, anticancer, antimycobacterial and anti-inflammatory effects of the bioactive flavonoid mesuaferrin-A from M. ferrea have been demonstrated in LPS-induced proinflammatory cytokines[11,12]. M. ferrea root is used as a bitter tonic to treat gastritis and bronchitis, while leaves and flowers are used as antidotes for snake and scorpion stings[7,9]. M. ferrea is chemically well characterized and contains phenylcoumarins, xanthones and triterpenoids[13-15]. The seeds of M. ferrea are reported to contain mesuol, mesuagin, mammeisin, and mesuon (all 4-phenylcoumarins), while the stamens are rich in sitosterol, A-and B-mesuaferrol, mesuanic acid and amyrin, a well-known component of nagakeshara-Remedy[16].
There are several references to anticancer properties of M. ferrea in the scientific literature. In vitro anticancer properties of M. ferrea stem bark extract against human colon carcinoma HCT116 cells have been previously reported[17]. It reduces chemotherapy toxicity in women with breast cancer[18]. Crude ethanolic extract of M. ferrea has been shown to be active against human cholangiocarcinoma (CL-6), human laryngeal carcinoma (HEP-2), and human hepatocarcinoma (HepG2), whereas methanolic extract has been reported to be active against Ehrlich ascites tumor in mice[19,20].
The present study attempts to scientifically validate the ethnomedical claim of M. ferrea Linn from the Western Ghats of Karnataka. In Unani medicinals, M. ferrea functions as an important component of Jawarish Shehryaran, a liver tonic[21]. In Ayurveda, Gulgulwasavam is a preparation made from Nagakesara (M. ferrea) used to treat liver and spleen diseases. It is used in India and various other parts of the world as a medicine to treat multisystem disorders and has held an important place in Ayurveda as a plant with anticancer properties[17,21].
In this context, this study provides the molecular characterization of phytocomponents of M. ferrea with anticancer activity. The phytocomponents were purified from crude methanol extract by column chromatography and preparative High-Performance Liquid Chromatography (HPLC). Molecular characterization of the purified compounds was performed using 13C NMR, 1H Nuclear Magnetic Resonance (NMR), Fourier Transform Infrared (FTIR), and Liquid Chromatography-Mass Spectrometry (LC-MS) techniques. The molecules were analyzed for their cytotoxic activity against liver HepG2 cancer cell lines using the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT), annexin V apoptosis and caspase-3 expression assays.
Materials and Methods
Plant collection and extraction of crude drug:
The stem bark of M. ferrea (Linn.) was collected from the Shettyhalli reserve forest of the Western Ghats of Karnataka. The plant material has been identified, authenticated and the sample has been given the document no. OX/BT-240 and deposited at Department of Biotechnology, TOCE Bangalore for future reference. The crude phytocomponents of M. ferrea stem bark were extracted in methanol as previously described[22].
Chromatograhic purification of
phytoconstituents: Phytoconstituents dissolved in methanol were subjected to Thin Layer Chromatography (TLC) analysis using mobile methanol-water (9:1) and methanol-water (1:1) systems. This fraction was also applied to an activated silica column, 60-120 mesh. 2 g of the fraction was bound with 2 g of silica gel. The column used had a length of 450 mm and a bore of 30 mm. First, 40 fractions were collected at 5 ml intervals using methanol as the mobile phase. The mobile phase was then switched to methanol-water (9:1) and 20 fractions were collected at 5 ml intervals. The fraction collected with methanol-water (9:1) was thoroughly dried and used for maximum identification with Ultraviolet spectrometer (Labman). The solvent for the preparative HPLC was identified by TLC. The solvent system was methanol-water (1:1) for preparative HPLC purification. The percentage yield is determined using the following formula.
Percentage yield=(Weight of fraction obtained)/ (Weight of sample loaded)×100
LC–MS analysis:
LC-MS analysis of the compound was performed using SPD 2010-APCI-SHIMADZU Japan, a device with a C-18 column with the dimensions 25×2.5. Methanol:water (9:1) was used as the mobile phase, 5 µl of the compound was injected at a flow rate of 0.2 ml/min. The LC was recorded at 254 nm with a photodiode array detector.
FTIR analysis:
The main functional groups were identified by their characteristic vibrational frequencies using FTIR and the spectrum of the compound was recorded with a PerKln Elmer FTIR spectrometer.
NMR spectroscopy analysis
Two types of NMR analysis were performed; proton NMR (1H NMR) and carbon (13C NMR). An NMR spectrum was recorded by Bruker at 400 MHz. The sample whose spectra were to be recorded was first carefully dried in a high-vacuum pump so that no organic solution or water remained. Thereafter, the test sample was dissolved in about 1.5 ml of CDCl3 solution and transferred to a standard NMR sample tube for further determination.
MTT assay:
Cells were trypsinized and aspirated into a 50 ml centrifuge tube. The cell pellet was obtained by centrifugation at 300 xg. The cell number was adjusted using Dulbecco's modified eagle medium low glucose medium with 10 % Fetal Bovine Serum (FBS) such that 200 µl of cell suspension contained approximately 15 000 cells. About 200 µl of the cell suspension was added and the plate was incubated for 24 h at 37° and 5 % CO2 atmosphere. After 24 h, the spent medium was aspirated. 200 µl of selected plant extracts were added to the respective wells and then incubated for 24 h at 37° and 5 % CO2 atmosphere. 200 µl of medium containing 10 % MTT reagent was added to each well to give a final concentration of 0.5 mg/ ml and incubated again for 3 h at 37 and 5 % CO2 atmosphere. The culture medium was completely removed without disturbing the formed crystals. Then 100 µl of solubilization solution (dimethyl sulfoxide) was mixed and gently shaken on a gyratory shaker to solubilize the formed formazan. Optical Density (OD) was recorded at 570 nm and 630 nm respectively. Percent inhibition of growth was calculated after subtracting background/blank and generating the concentration of test sample required to inhibit growth by 50 % (IC50) from the dose-response curve for the cell line.
Annexin-V apoptosis assay:
Approximately 3×105 cells/2 ml are cultured and incubated overnight at 37° in a CO2 incubator. Aspirate the spent media and wash with 1X Phosphate Buffered Saline (PBS). Cells were treated with selected plant extract/test sample and control in 2 ml culture medium and incubated overnight at 37°. The media were treated with the required amount of toxic compound in 2 ml culture medium and incubated for 12-15 h. After treatment, the media was removed from the wells and the cells placed in 5 ml centrifuge tubes and washed with 0.5 ml PBS. 200 µl of Ethylenediaminetetraacetic Acid (EDTA)-trypsin were added to this and incubated at 37° for 3-4 min. Culture medium was poured into the appropriate wells and the cells were harvested. The cells were centrifuged at 300 xg for 5 min at 25°. The supernatant was decanted and the cells washed with PBS. Resuspend cells in 1X binding buffer at a concentration of 1×106 cells/ ml. About 100 µl of the solution (1×105 cells) and 5 µl of FITC Annexin-V were placed in a separate tube and incubated for 15 min at room temperature (25°) in the dark. Approximately 5 µl of propidium iodide solution and 400 µl of 1X binding buffer were prepared and immediately analyzed by flow cytometry.
Caspase-3 expression:
Approximately 3×105 cells/2 ml are cultured and incubated overnight at 37° in a CO2 incubator. Aspirate the spent media and wash with 1X Phosphate Buffered Saline (PBS). Cells were treated with selected plant extract/test sample and control in 2 ml culture medium and incubated overnight at 37°. The media were treated with the required amount of toxic compound in 2 ml culture medium and incubated for 12-15 h. After treatment, the media was removed from the wells and the cells placed in 5 ml centrifuge tubes and washed with 0.5 ml PBS. 200 µl of EDTA-trypsin were added to this and incubated at 37° for 3-4 min. The medium was washed with PBS and the PBS was completely decanted. Thereto was added 0.5 ml of 2 % P-formaldehyde solution and incubated for 20 min, then washed with 0.5 % Bovine Serum Albumin (BSA) in 1X PBS. Approximately 0.1 % Triton-X 100 in 0.5 % BSA solution was added and washed again with 0.5 % BSA in 1X PBS (2x). At the end, 20 µl of anti-caspase-3 antibody was added, mixed thoroughly and incubated for 30 min in the dark at room temperature (25°).
DNA fragmentation studies:
Approximately 1 ml of each sample was transferred to 2 microcentrifuge tubes, centrifuged at 10 000 rpm for 10 min and the supernatant discarded. 500 µl of sodium chloride-tris-EDTA buffer, 20 µl of Sodium Dodecyl Sulfate (SDS), and 10 µl of mercaptoethanol were mixed with pellet and incubated at 55° in water bath for 1 h. To this, 300 µl of phenol:chloroform:isoamyl alcohol (25:24:1), were added and centrifuged at 10 000 rpm for 10 min. The resulting supernatant was transferred to a microcentrifuge tube. To this was added an equal amount of chloroform:isoamyl alcohol and again gently mixed and centrifuged at 10 000 rpm for 10 min. 10 µl of 3M sodium acetate and 500 µl of isopropyl alcohol were added to the supernatant and incubated at -20° for 1 h. The tubes were centrifuged at 10 000 rpm for 10 min. The pellets were washed with 500 l of 70 % ethanol and the supernatant discarded. The dry plates were dissolved in 20 µl of sterile distilled water and the sample stored at -20°.
Statistical analysis:
The statistical analysis was performed using column and one-way ANOVA analysis. The data are expressed as mean±standard error of mean. Graph pad Prism V 3.0 used for all the statistical analysis.
Results and Discussion
Bioactive compounds derived from medicinal plants are target candidates of the pharmaceutical industry for the development and manufacture of new medicines or therapeutics. Screening of plant extracts and identification of active ingredients is an innovative approach to therapeutic agent discovery[23,24]. The plant M. ferrea (Linn.) is known for its medicinal properties and is actively sought after for therapeutic principles. Here an attempt is made to identify novel lead molecules from the bark of M. ferrea with cytotoxic and anticancer effects.
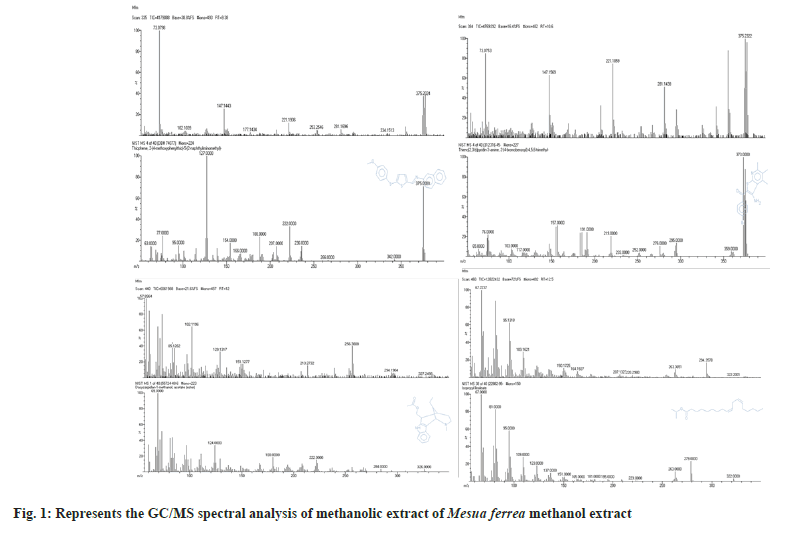
Gas Chromatography-Mass Spectrometry (GC-MS) spectral analysis is suitable for identifying phytoconstituents and has already been described for number plants[23-25]. Fig. 1 shows the GC-MS spectral analysis of the methanolic extract of M. ferrea with different retention times.
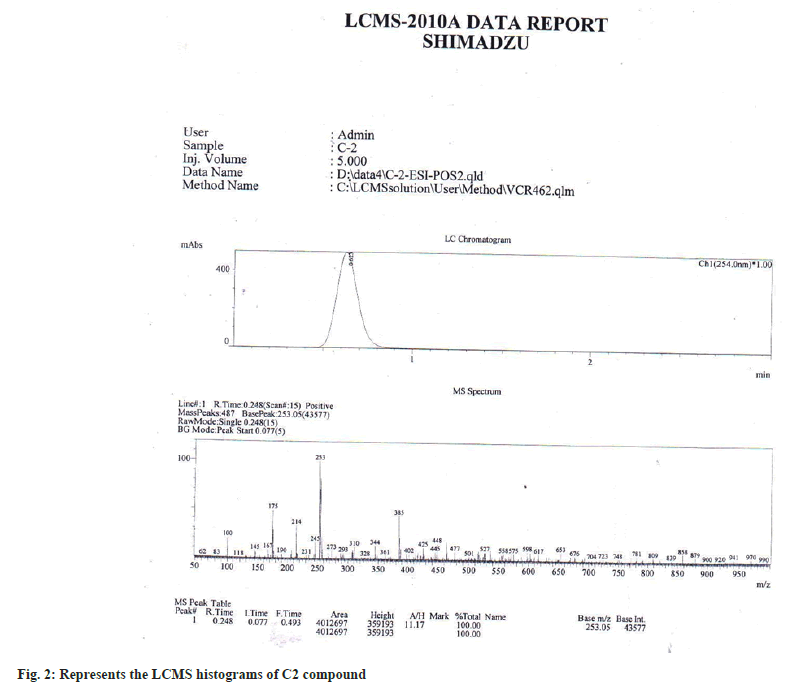
The results of GC-MS analysis of M. ferrea methanolic extract led to the identification of 7 compounds (Table 1) viz., Thiophene, 2-[4-methoxyphenylthio]-5-[2-naphthyliminomethyl], Thieno[2,3-b]pyridin-3amine, 2-[4-bromobenzoyl]-4,5,6,trimmethyl, Dasycarpidan-1-methanol, acetate[ester], Isopropyl linoleate 1H-Indane, 1-ethylideneoctahydro-7a-methyl-, cis. Aristolochic acid [2-Methoxy-1,3,2-thiozin][5,10,9]androstan-3,17-dione were present in the methanolic extracts of M. ferrea. After purification by Preparative HPLC, compound C2 was obtained as yellow crystals, mp 180-183°. LC-MS spectrum exhibited protonated molecular ion peak at base m/z 253.6 (fig. 2). The ultraviolet-absorption bands at λ max (MetOH) location 256 and 264 nm with peak value of 3.722 and 4.555 respectively. From the obtained isolated compound C-2 IR (in KBr) spectral peak at cm-1=3000-3500 clearly indicate the presence of –OH group, Sharp peak at 2000-1200 indicate the presence of aliphatic –CH and 1710 clearly indicate the presence of C=O group and aromatic system in the molecule.
| S. No. | RT (min) | Name of the compound | Peak area % |
|---|---|---|---|
| 1. | 9.38 | Thiophene, 2-[4-methoxyphenylthio]-5-[2-naphthyliminomethyl]- | 2.06 |
| 2. | 10.6 | Thieno[2,3-b]pyridin-3amine, 2-[4-bromobenzoyl]-4,5,6,trimmethyl | 2.57 |
| 3. | 12 | Dasycarpidan-1-methanol, acetate[ester] | 5.15 |
| 4. | 12.5 | Isopropyl linoleate | 24.74 |
| 5. | 13.32 | 1H-Indane, 1-ethylideneoctahydro-7a-methyl-, cis. | 56.7 |
| 6. | 14.73 | Aristolochic acid | 4.63 |
| 7. | 16.25 | [2-Methoxy-1,3,2-thiozin][5,10,9]androstan-3,17-dione | 4.12 |
Table 1: GC/MS analysis of methanolic extract of M. Ferrea methanol extract
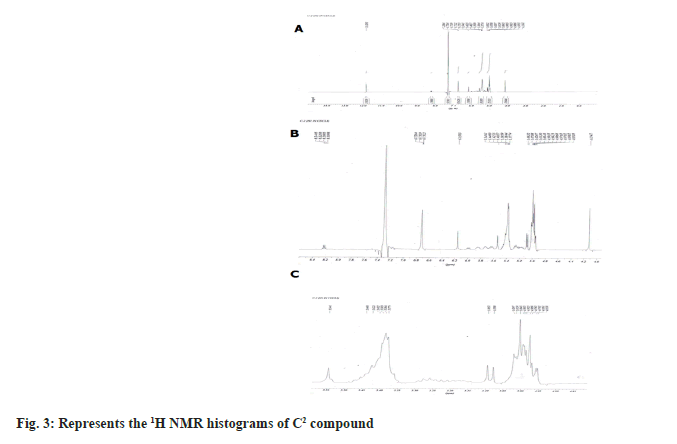
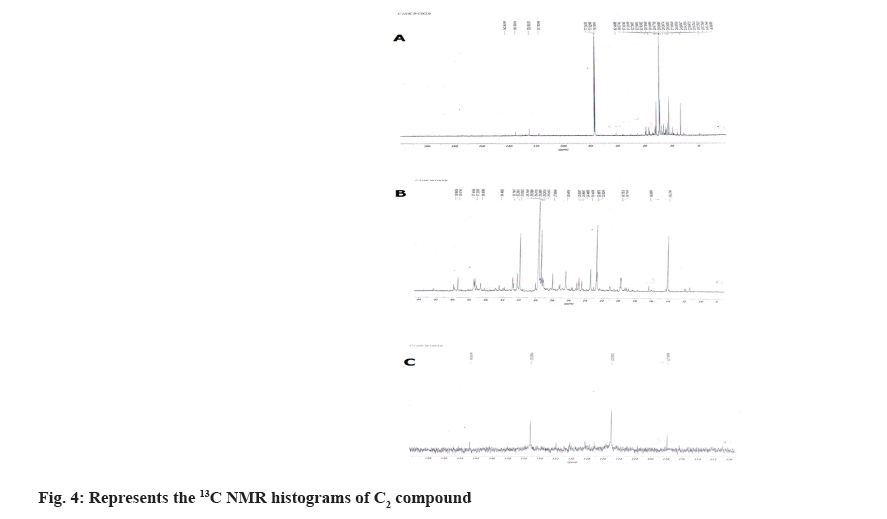
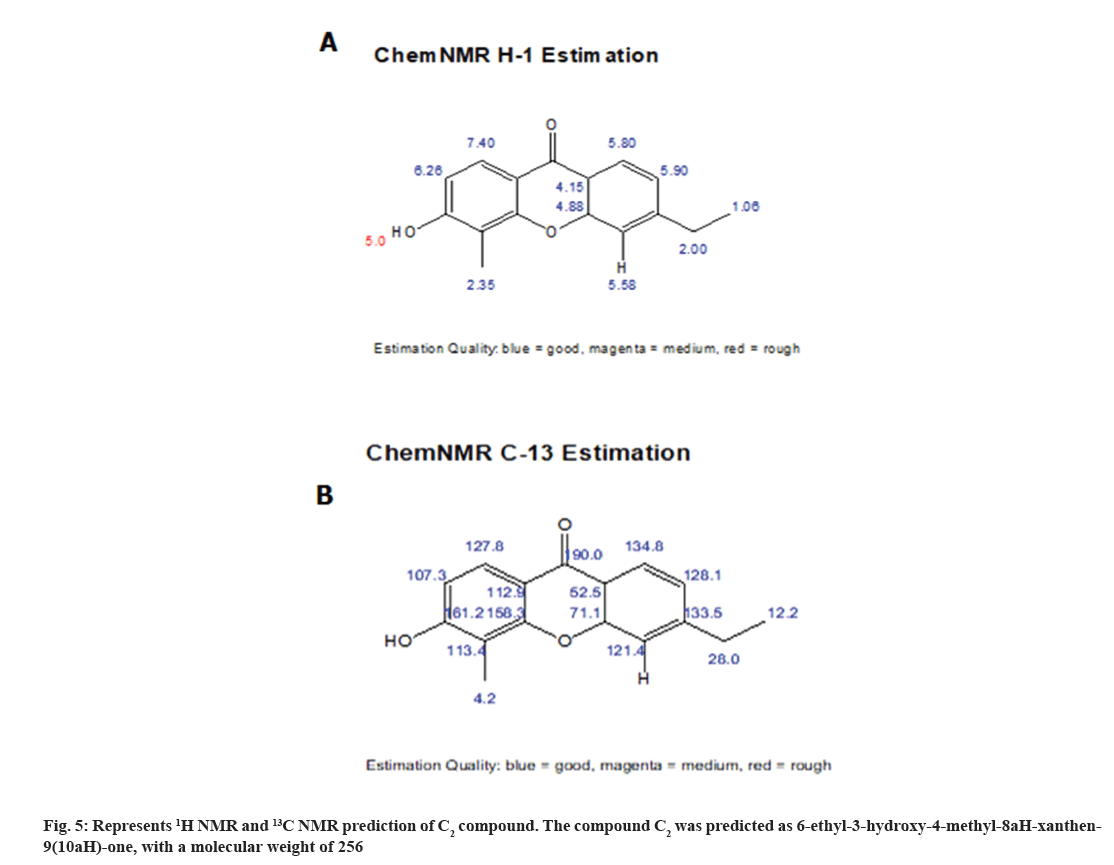
1H NMR spectrum showed the four aromatic protons at δH 6.26-7.26, 7.40-7.26, 4.88-2.15, 4.15-2.15, 5.90-5.90, 5.80-5.80 (m) (fig. 3; Table 2) and in 13C NMR spectrum showed five aromatic methines at δC 17-40 (2C, 77 2C) and quaternary aromatic carbons at δC 107.3-128.5, 127.8-128.5, 71.1-49.0, 52.5- 29.3, 121.4-123.3, 134.8-123.3 (2C) (fig. 4). From the above data compound C2 was characterized 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one (fig. 5). It was further confirmed by comparison of its physicochemical data with that of the reported and comparison of TLC with authentic sample, TLC system Ethyl acetate:Methanol:Acetic acid (7:3 v/v).
| Position | δH (J in Hz) | δC |
|---|---|---|
| CH | 6.26-7.26 | 107.3-128.5 |
| 7.40-7.26 | 127.8-128.5 | |
| 4.88-2.15 | 71.1-49.0 | |
| 4.15-2.15 | 52.5-29.3 | |
| 5.90-5.90 | 121.4-123.3 | |
| 5.80-5.80 | 134.8-123.3 | |
| CH3 | 2.35-0.86 | 4.2-24.3 |
| CH2 | 2.00-1.37 | 28.0-19.5 |
| OH | 5.00-5.00 | |
| CH3 | 1.06-0.86 | 12.2-9.1 |
| H | 5.58-5.80 |
Table 2: 1H AND 13C NMR (300 and 75 MHZ) data of C2 in CDCl3
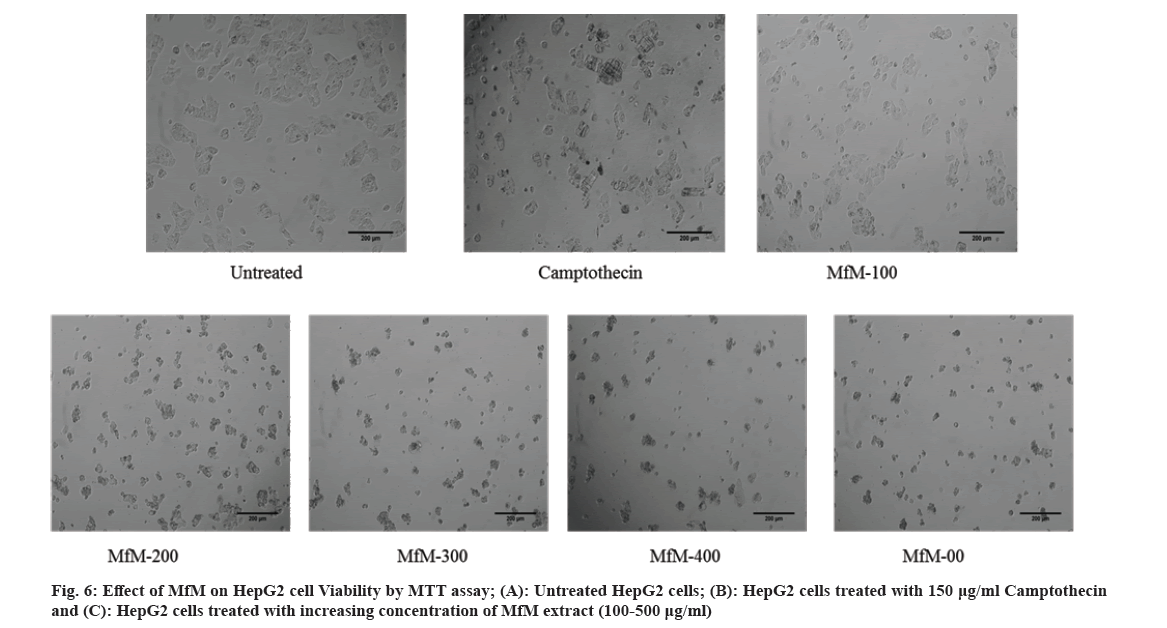
The MTT assay was reliable and widely used to study cell proliferation[26,27]. It reduces tetrazolium salts. The formation of solubilized violet formazan was quantified with a spectrophotometer. In our present study, proliferation in HepG2 cells was assessed by treatment with C1, 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one and the standard drug camptothecin (fig. 6). The IC50 value of the methanol extract of M. ferrea for the HepG2 cell line was 177.006 g/ml, whereas the IC50 values of the test compounds were C1 and 6-ethyl-hydroxy-4-methyl-8H-xanthan-9-one for the HepG2 cell lines were found to be 615.535 µg/ml and 352.007 µg/ml (fig. 2 and fig. 7).
Fig. 7: Effect of C1 and 6-ethyl-hydroxy-4-methyl-8H-xanthan-9-one compounds on HepG2 cell Viability by MTT assay; (A): Untreated HepG2 cells; (B): HepG2 cells treated with 150 μg/ml Camptothecin; (C): HepG2 cells treated with increasing concentration of C1 (100-500 μg/ml) and (D): HepG2 cells treated with increasing concentration of 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one (100-500 μg/ml)
FITC-annexin V staining precedes the failure of membrane integrity that accompanies the final phase of cell death due to apoptosis or necrotic[28]. Annexin V is used in conjunction with PI, which helps differentiate early apoptotic cells (PI Ve and Annexin-V +Ve).
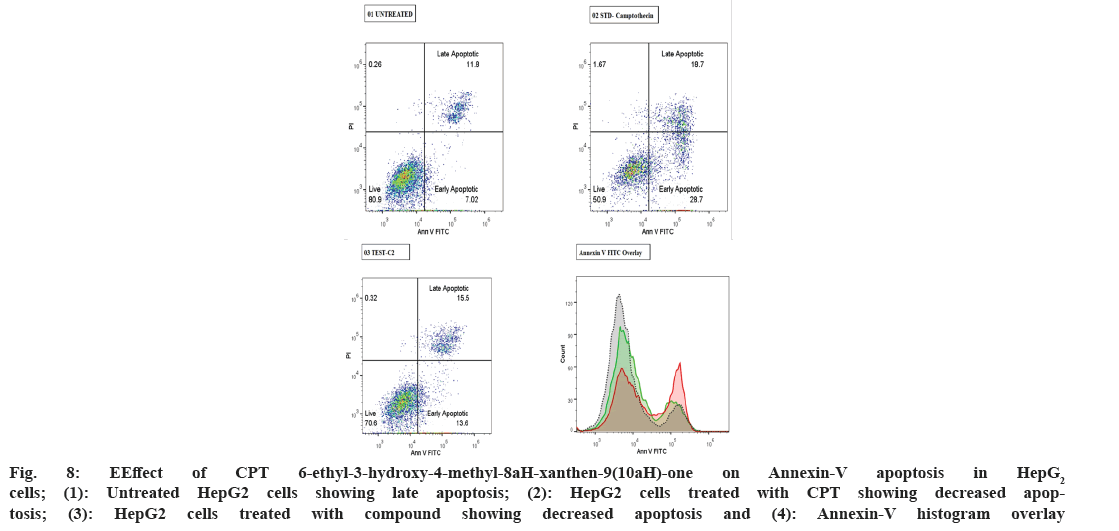
In our current study, HepG2 cells treated with 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one showed 13.6 % and 15.5 % early and late apoptosis, respectively, compared to the standard drug camptothecin 28.7 % and 18.7 % early and late apoptosis and 7.02 % and 11.8 %, respectively (Table 3). The study results indicated that cells in early apoptosis were viable and were annexin V and PI negative. This shows the effectiveness of the test compound and the standard drug (fig. 8) HepG2 cells undergoing apoptosis have phosphatidylserine occurring at the outer membrane of the plasma membrane, identified by fluorochrome-linked annexin-V. Previous studies reported that Annexin V-mediated apoptosis in the mitocurcumin-treated A549 cell line significantly correlated with our results[29]. A similar study was also previously reported with SiHa cells[30]. Furthermore, from the above results it can be concluded that the test compound is a potent anti-cancer molecule.
| S No. | Sample Name | % of Cells | Geometric mean fluorescence intensity (MFI) of FITC Annexin V (FL1-A parameter) | ||
|---|---|---|---|---|---|
| Live | Early Apoptotic | Late Apoptotic | |||
| 1 | Untreated | 80.9 | 7.02 | 11.8 | 7636 |
| 2 | Standard drug Camptothecin (CPT) -100 µg/mL | 50.9 | 28.7 | 18.7 | 20768 |
| 3 | 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one | 70.6 | 13.6 | 15.5 | 11351 |
Table 3: Effect of CPT and 6-ethyl-3-hydroxy-4-methyl-8ah-xanthen-9(10ah)-one -on annexin-v apoptosis in HepG2 cells
Fig. 8: EEffect of CPT 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one on Annexin-V apoptosis in HepG2 cells; (1): Untreated HepG2 cells showing late apoptosis; (2): HepG2 cells treated with CPT showing decreased apoptosis; (3): HepG2 cells treated with compound showing decreased apoptosis and (4): Annexin-V histogram overlay
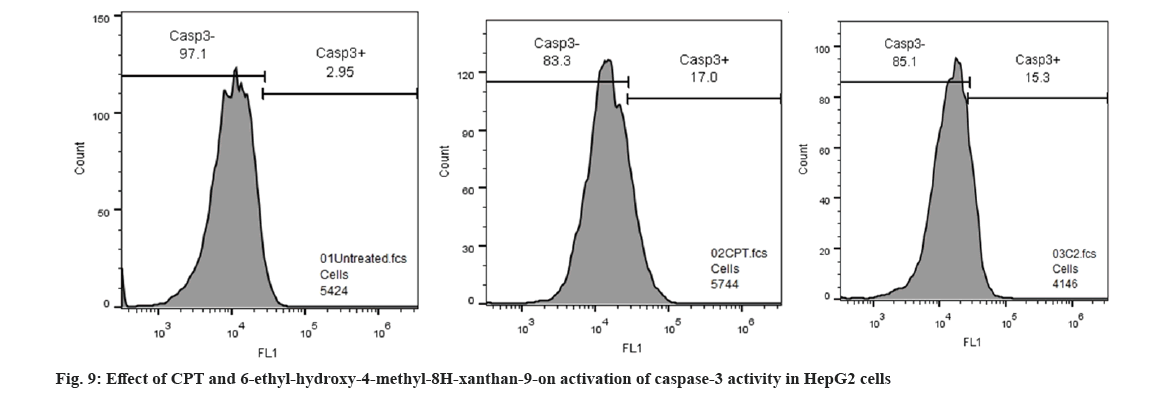
Caspase-3 is a key protease mediator activated during the early stages of apoptosis, by self-proteolysis of several cellular proteases[31]. In our current investigation, caspase-3 activity was measured in 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one compound treated HepG2 cells undergoing apoptosis; 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one compound inhibits early apoptosis in HepG2 cells at 352 µg/ ml concentration, which activates caspase-3 cascade 15.3 % compared with standard drug CTP 17 % this indicates anticancer potency of the compound (Table 4, fig. 9). From the study it is concluded that 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one compound significantly inhibits apoptosis by activating caspase-3 activity and it can be accounted as potent anticancer agent. Similar activity was previously reported in Hopea odorata, which significantly correlates with our study results[32].
| S No. | Sample Name | Geometric mean fluorescence intensity (MFI) of FITC Caspase-3 (FL1-A parameter) | % of Cells | |
|---|---|---|---|---|
| Caspase-3 -ve | Caspase-3 +ve | |||
| 1 | Untreated | 9541 | 97.1 | 2.9 |
| 2 | Std drug (CPT) -100 µg/ml | 14695 | 83.3 | 17 |
| 3 | 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one | 14483 | 85.1 | 15.3 |
Table 4: Effect of CPT and 6-ethyl-3-hydroxy-4-methyl-8ah-xanthen-9(10ah)-one -on activation of caspase-3 activity in HepG2 cells
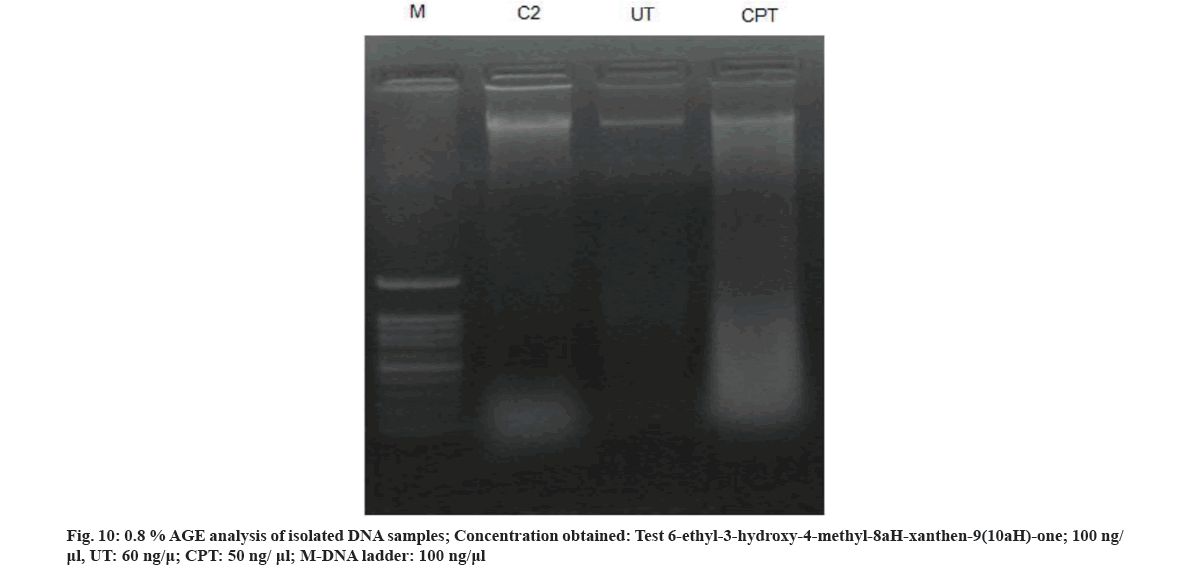
DNA fragmentation is an indicative and hallmark of apoptosis[33]. This assay was used to confirm the anticancer efficacy of 6-ethyl-3-hydroxy-4-methyl-8aH-xanthen-9(10aH)-one compound that significantly inhibits apoptosis (fig. 10). Based on the results of the present study it is evident that the active constituent isolated from M. ferrea could be a novel lead molecule for developing potent anticancer, antiproliferative and DNA protecting drug. Further study on its toxicity profile, SAR, dosage and bioavailability will help in developing natural radioprotector and anticancer drug candidate.
Acknowledgements:
The authors are greatly thankful to Vision group of Science and Technology, Karnataka (VGST-KFIST (L1) GRD-388), Government of Karnataka. We greatly acknowledge the constant support of RB and HSD, Bhabha Atomic Research Center, Mumbai, and Board of Research in Nuclear Science, Government of India (Sanction No. 2012/34/21/BRNS) and Director of Minorities, Govt. of Karnataka.
Conflict of interests:
The authors express that no conflict of interest exists.
References
- Ali MA, Sayeed MA, Bhuiyan MS, Sohel FI, Yeasmin MS. Antimicrobial screening of Cassia fistula and Mesua ferrea. J Med Sci 2004;4(1):24-9.
- Kumar VP, Chauhan NS, Padh H, Rajani M. Search for antibacterial and antifungal agents from selected Indian medicinal plants. J Ethnopharmacol 2006;107(2):182-8.
[Crossref] [Google Scholar] [PubMed]
- Gopalakrishnan C, Shankaranarayanan D, Nazimudeen SK, Viswanathan S, Kameswaran L. Anti-inflammatory and CNS depressant activities of xanthones from Calophyllum inophyllum and Mesua ferrea. Indian J Pharmacol 1980;12(3):181-91.
- Choudhury S, Ahmed R, Barthel A, Leclercq PA. Volatile oils of Mesua ferrea (L.) from Assam, India. J Essential Oil Res 1998;10(5):497-501.
- Nordin K, Ahmad FB, Taufiq-Yap YH, Ali AM. Volatile components of methanol extract from the flower of malaysian Musea ferrea Linn. Orient J Chem 2004;20:69-72.
- Verotta L, Lovaglio E, Vidari G, Finzi PV, Neri MG, Raimondi A, et al. 4-Alkyl-and 4-phenylcoumarins from Mesua ferrea as promising multidrug resistant antibacterials. Phytochemistry 2004;65(21):2867-79.
[Crossref] [Google Scholar] [PubMed]
- Garg S, Kameshwar S, Rajeev R, Pankaj A, Parshuram M. In vivo antioxidant activity and hepatoprotective effects of methanolic extract of Mesua ferrea Linn. Int J Pharmatechnol Res 2009;1:1692-6.
- Sander JW, Shorvon SD. Epidemiology of the epilepsies. J Neurol Neurosurg Psychiatry 1996;61(5):433.
[Crossref] [Google Scholar] [PubMed]
- Parukutty B, Chandra GS. Studies on the medicinal uses of plants by the Boro tribals of Assam-II. J Econ Taxon Bot 1984;5:599-604.
- Das R, Shelke RG, Rangan L, Mitra S. Estimation of nuclear genome size and characterization of Ty1-copia like LTR retrotransposon in Mesua ferrea L. J Plant Biochem Biotechnol 2018;27:478-87.
- Asif M, Jafari SF, Iqbal Z, Revadigar V, Oon CE, Majid AS, et al. Ethnobotanical and Phytopharmacological attributes of Mesua ferrea: a mini review. J Appl Pharm Sci 2017;7:242-51.
- Chaithanya KK, Gopalakrishnan VK, Hagos Z, Nagaraju B, Kamalakararao K, Kebede H, et al. In vitro and in vivo anti-inflammatory activities of Mesua ferrea Linn. Int J Pharmacogn Phytochem Res 2018;10:103-1.
- Bandaranayake WM, Selliah SS, Sultanbawa MU, Games DE. Xanthones and 4-phenylcoumarins of Mesua thwaitesii. Phytochemistry 1975;14(1):265-9.
- Raju MS, Srimannarayana G, Rao NS, Bala KR, Seshadri TR. Structure of mesuaferrone-b a new biflavanone from the stamens of Mesua ferrea Linn. Tetrahedron Lett 1976;17(49):4509-12.
- Joy PP, Thomas J, Samuel M, Baby PS. Kerala Agricultural University. Arom Med Plant Res. 1998:106-7.
- Govindachari TR, Pai BR, Subramaniam PS, Rao UR, Muthukumaraswamy N. Constituents of Mesua ferrea L.-I. Tetrahedron 1967;23:243-8.
- Asif M, Al-Mansoub MA, Khan MS, Yehya AH, Ezzat MO, Oon CE, et al. Molecular mechanisms responsible for programmed cell death-inducing attributes of terpenes from Mesua ferrea stem bark towards human colorectal carcinoma HCT 116 cells. J Appl Biomed 2017;15(1):71-80.
- Saxena A, Dixit S, Aggarwal S, Seenu V, Prashad R, Bhushan S, et al. An ayurvedic herbal compound to reduce toxicity to cancer chemotherapy: A randomized controlled trial. Indian J Med Paediatr Oncol 2008;29(2):11-8.
- Rana AY, Khanam JA, Asad-Ud-Daula M. Antineoplastic screening of some medicinal plants against Ehrlich ascites carcinoma in mice. J Med Sci 2004;4(2):142-5.
- Mahavorasirikul W, Viyanant V, Chaijaroenkul W, Itharat A, Na-Bangchang K. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complement Altern Med 2010;10(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Sharma A, Sharma S, Parashar B. Mesua ferrea Linn: A review of the Indian medical herb. Sys Rev Pharm 2017;8(1):19-23.
- Murthuza S, Manjunatha BK. In vitro and in vivo evaluation of anti-inflammatory potency of Mesua ferrea, Saraca asoca, Viscum album and Anthocephalus cadamba in murine macrophages raw 264.7 cell lines and Wistar albino rats. Beni-Suef Univ J Basic Appl Sci 2018;7(4):719-23.
- Starlin T, Prabha PS, Thayakumar BK, Gopalakrishnan VK. Screening and GC-MS profiling of ethanolic extract of Tylophora pauciflora. Bioinformation 2019;15(6):425-9.
[Crossref] [Google Scholar] [PubMed]
- Gopalakrishnan K, Udayakumar R. GC-MS Analysis of phytocompounds of leaf and stem of Marsilea quadrifolia(L.). Int J Biochem Res 2010;4:517-26.
- Kanthal LK, Dey A, Satyavathi K, Bhojaraju P. GC-MS analysis of bio-active compounds in methanolic extract of Lactuca runcinata DC. Pharmacogn Res 2014;6(1):58.
[Crossref] [Google Scholar] [PubMed]
- Xu JH, Zheng X, Li HZ, Cao JG. Induction of apoptosis of human ovarian cancer CoCl cells by 5-allyl-7-gemdifluoromethylenechrysin. Zhongguo Bijiao Yixue Zazhi 2008;18:5-9.
- Tan XW, Xia H, Xu JH, Cao JG. Induction of apoptosis in human liver carcinoma HepG2 cell line by 5-allyl-7-gen-difluoromethylenechrysin. World J Gastroenterol 2009;15(18):2234.
[Crossref] [Google Scholar] [PubMed]
- Plackal Adimuriyil George B, Tynga IM, Abrahamse H. In vitro antiproliferative effect of the acetone extract of Rubus fairholmianus gard. root on human colorectal cancer cells. BioMed Res Int 2015;2015:165037.
[Crossref] [Google Scholar] [PubMed]
- Jayakumar T, Liu CH, Wu GY, Lee TY, Manubolu M, Hsieh CY, et al. Hinokitiol inhibits migration of A549 lung cancer cells via suppression of MMPs and induction of antioxidant enzymes and apoptosis. Int J Mol Sci 2018;19(4):939.
[Crossref] [Google Scholar] [PubMed]
- Goswami P, Paul S, Banerjee R, Kundu R, Mukherjee A. Betulinic acid induces DNA damage and apoptosis in SiHa cells. Mutat Res Genet Toxicol Environ Mutagen 2018;828:1-9.
[Crossref] [Google Scholar] [PubMed]
- D’Amelio M, Sheng M, Cecconi F. Caspase-3 in the central nervous system: Beyond apoptosis. Trends Neurosci 2012;35(11):700-9.
[Crossref] [Google Scholar] [PubMed]
- Nguyen ST, Huynh KL, Nguyen HL, Nguyen Thi Thanh M, Nguyen Trung N, Nguyen Xuan H, et al. Hopea odorata extract inhibits hepatocellular carcinoma via induction of caspase-dependent apoptosis. Onco Targets Ther 2017:5765-74.
[Crossref] [Google Scholar] [PubMed]
- Bakshi H, Sam S, Rozati R, Sultan P, Islam T, Rathore B, et al. DNA fragmentation and cell cycle arrest: A hallmark of apoptosis induced by crocin from Kashmiri saffron in a human pancreatic cancer cell line. Asian Pac J Cancer Prev 2010;11(3):675-9.
[Google Scholar] [PubMed]