- *Corresponding Author:
- Bo Han
Department of Cardiothoracic Vascular Surgery, The Third Affiliated Hospital of Inner Mongolia Medical University (Inner Mongolia Baotou Steel Hospital), Baotou, Inner Mongolia 010107, China
E-mail: hanbobaotou@aliyun.com
| Date of Received | 02 September 2021 |
| Date of Revision | 24 July 2022 |
| Date of Acceptance | 27 January 2023 |
| Indian J Pharm Sci 2023;85(1):256-260 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the function of kaempferol on the lung cancer cell A549 and the microRNA-199 variation is the objective of the study. The cell proliferation was detected by cell counting kit-8. The expression of mammalian target of rapamycin, phosphorylated mammalian target of rapamycin, light chain 3-?/?, beclin-1, B-cell lymphoma 2 and B-cell lymphoma 2-associated X protein was showed by Western blot. The microRNA-199 expression was determined by quantitative polymerase chain reaction. The adenocarcinomic human alveolar basal epithelial cells, A549 proliferation was gradually low with the concentration of kaempferol rising. Kaempferol could inhibit the microRNA-199, phosphorylated mammalian target of rapamycin, light chain 3-?/?, beclin-1 and B-cell lymphoma 2-associated X protein expression and promoted B-cell lymphoma 2 expressions. When microRNA-199 inhibited the expression of phosphorylated mammalian target of rapamycin, light chain 3-?/? and beclin-1 in the kaempferol+microRNA-199 inhibitor group was lower than blank group and kaempferol added only group. In the kaempferol+microRNA-199 inhibitor group, the B-cell lymphoma 2 was lower than the blank group and kaempferol added only group. When the mammalian target of rapamycin was inhibited the expression of light chain 3-?/? and beclin-1 was low. The B-cell lymphoma 2 was low and the B-cell lymphoma 2-associated X protein was high in the two groups. The kaempferol promoted the apoptosis and inhibited autophagy in A549 cells via microRNA/mammalian target of rapamycin axis.
Keywords
Kaempferol, micro ribonucleic acid-199, mammalian target of rapamycin, non-small cell lung cancer, apoptosis, autophagy
In worldwide, lung cancer is the most normal cancer which caused death. Non-Small Cell Lung Cancer (NSCLC), as one of the subtype of lung cancer constitutes 4 %-17 %, which has 5 y survival rate[1,2]. The common method used was chemotherapy for the treatment of NSCLC. However, the chemotherapy has side effects[3-6]. Hence, to find an effective and cheap drug is the goal for clinicians.
Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one) is a polyphenol which is mainly contained in fruits and vegetables[7-9]. It has many functions such as anti-inflammation, antioxidant, antimicrobial, anti-cancer properties etc.[10,11]. Kaempferol can inhibit the proliferation and increases apoptosis in cancer cells by triggering apoptosis, cell cycle and downregulation of signaling pathways[10,12-14].
Micro Ribonucleic Acid (RNA), as a small non-coding RNA, can regulate the RNA expression[15-17]. Previous study shows that microRNA (miR)-199 was low expressed in many cancers such as liver cancer, lung cancer etc.[18,19]. In present study, the function of kaempferol on the lung cancer cell (adenocarcinomic human alveolar basal epithelial cells-A549) was explored and the miR-199 variation was also determined.
Cell culture used in this study was explained here. The A549 cells (purchased from Science Cell No. CP3000) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Sigma-Aldrich) containing 10 % Fetal Bovine Serum (FBS, Gibco), 100 Units/ml penicillin and 0.1 mg/ml streptomycin, respectively. The cells were cultured in the 37° in 5 % Carbon dioxide (CO2).
Cell Counting Kit-8 (CCK-8) assays was used here. Cell proliferation after kaempferol treatment was assessed using the CCK-8 kit. Cells (1×104 cells per well) were plated in the 96-well plates (100 μl/well) and 10 μl of CCK-8 solution was added at each time point (24 h, 48 h and 72 h) for 2 h at 37°. The absorbance values at 450 nm were then measured using an enzyme marker.
Real-time quantitative Polymerase Chain Reaction (qPCR) analysis was used in the study. The total RNA was extracted from cells by using the commercial TRIzol reagent (Invitrogen, United States of America (USA)) following the manufacturer’s instruction. The quality of the total RNA was determined by agarose electrophoresis and the RNA was reversely transcribed into complementary Deoxyribonucleic Acid (cDNA) by using the TIANScript Reverse Transcription (RT) kit (Tiangen Biotech, China). Then, the SYBR Premix Ex Taq™ II Kit (Takara, Japan) was used to determine relative gene expression levels and enrichment.
Western blot technique was used in this study. Total cellular proteins were extracted using high performance radio immunoprecipitation analysis lysis buffer (C0481, Sigma-Aldrich) containing 1 % protease inhibitor and 1 % phosphatase inhibitor (Beyotime). The protein concentration of each sample was determined by a bicinchoninic acid kit (23227, Thermo Fisher Scientific). The proteins were separated by polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, Massachusetts (MA), USA) which were blocked with 5 % Bovine Serum Albumin (BSA) at room temperature for 1 h. Primary antibodies were incubated with membranes overnight at 4°. The membranes were incubated with Horseradish Peroxide (HRP)-labeled goat anti-rabbit immunoglobulin G (ab205718, Abcam) dilutions at room temperature for 1.5 h. After incubation, the membranes were developed by using developing liquid (NCI4106, Pierce, Rockford, Illinois, USA). Protein quantification was performed using Clinx Chemi Analysis software (ChemiScope 6000, Shanghai, China).
The miR-199 inhibitor was purchased from GenePharma (Shanghai, China). Cell transfection was conducted using Lipofectamine™ 2000 (Thermo Fisher Scientific, Waltham, MA, USA). After 48 h, cells were harvested for following experiments.
Statistical analyses of data were performed using Graphpad Prism 9.0 (GraphPad Software, San Diego, California, USA). The measurement data were expressed as mean±Standard Deviation (SD). Independent sample t-test was used in comparisons between groups, and one-way Analysis of Variance (ANOVA) was used for comparison among multiple groups, followed by Tukey’s post hoc tests. Comparison of data between groups at different time points was performed using two-way ANOVA. A p<0.05 indicated statistically significant.
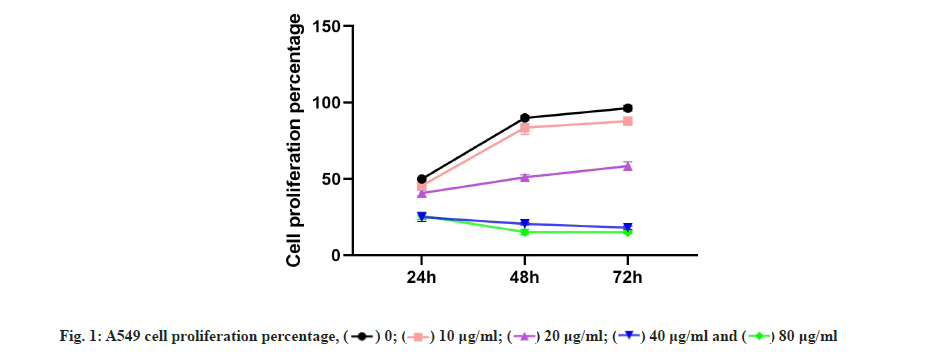
Kaempferol inhibited the A549 proliferation which was shown in fig. 1. To detect the effect of kaempferol on the A549 proliferation, the CCK-8 was used. The A549 proliferation was gradually low with the concentration of kaempferol raising (fig. 1) and the optimum concentration of kaempferol on the A549 proliferation was 40 μg/ml.
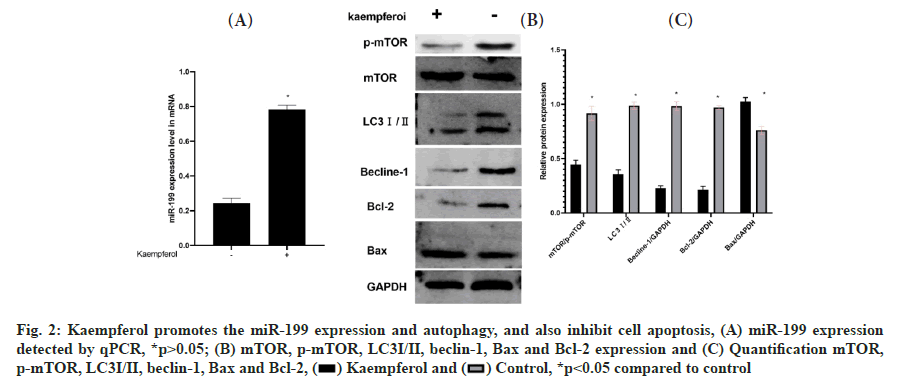
Kaempferol promotes the miR-199 expression and autophagy and also inhibit apoptosis in A549 cells was shown in fig. 2A-fig. 2C. To detect the miR-199 expression, the qPCR was used and the miR-199 expression was higher in the kaempferol (40 μg/ml for 48 h) group than blank group. Kaempferol inhibited the autophagy in A549 cells. Hence, the expression of phosphorylated mammalian Target of Rapamycin (p-mTOR), Light Chain 3-?/? (LC3?/?) and beclin-1 was lower in the kaempferol group than blank group. However, kaempferol promoted the A549 apoptosis and the B-cell lymphoma 2 (Bcl-2) expressions was low and Bcl-2-associated X protein (Bax) was high in the kaempferol group.
Fig. 2: Kaempferol promotes the miR-199 expression and autophagy, and also inhibit cell apoptosis, (A) miR-199 expression detected by qPCR, *p>0.05; (B) mTOR, p-mTOR, LC3?/?, beclin-1, Bax and Bcl-2 expression and (C) Quantification mTOR, p-mTOR, LC3?/?, beclin-1, Bax and Bcl-2, *p<0.05 compared to control.
*p<0.05 compared to control.
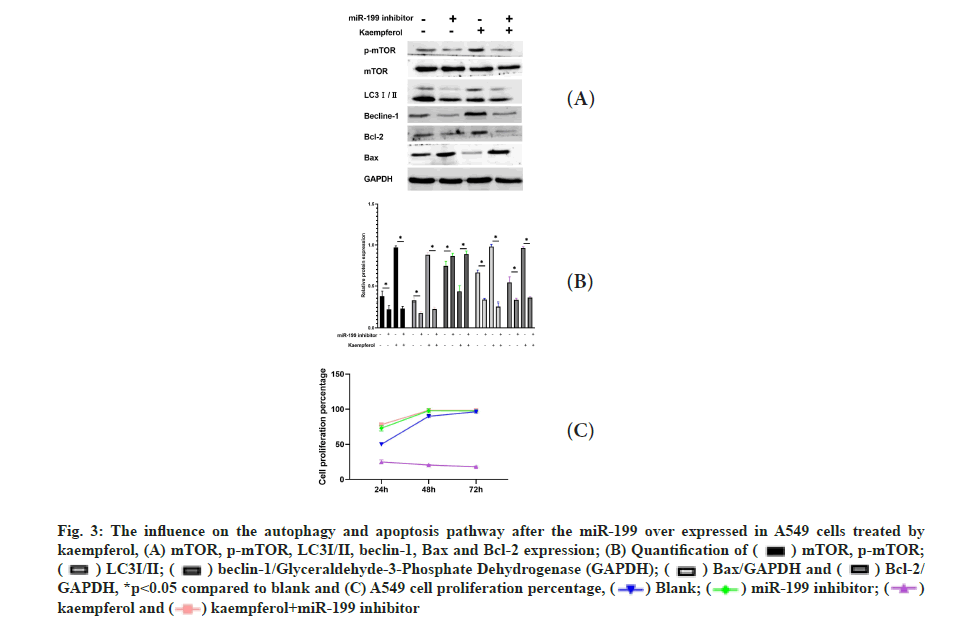
The influence on the autophagy and apoptosis pathway after the miR-199 silencing in A549 cells treated by kaempferol was shown in fig. 3A-fig. 3C. To explore the function of miR-199 in the kaempferol promoting autophagy and inhibiting apoptosis in A549 cells, the miR-199 was inhibited. The expression of p-mTOR, LC3?/? and beclin-1 in the kaempferol+miR-199 inhibitor group was lower than blank group and kaempferol added only group. In the kaempferol+miR-199 inhibitor group, the Bcl-2 was lower than the blank group and kaempferol added only group. However, the Bax was higher. The cell proliferation in the kaempferol+miR-199 inhibitor group was higher than the blank group and kaempferol added only group.
Fig. 3: The influence on the autophagy and apoptosis pathway after the miR-199 over expressed in A549 cells treated by kaempferol, (A) mTOR, p-mTOR, LC3?/?, beclin-1, Bax and Bcl-2 expression; 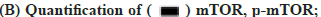
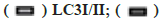 beclin-1/Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH);
beclin-1/Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH); 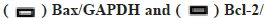 *p<0.05 compared to blank and (C) A549 cell proliferation percentage,
*p<0.05 compared to blank and (C) A549 cell proliferation percentage,  kaempferol and
kaempferol and 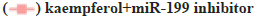 .
.
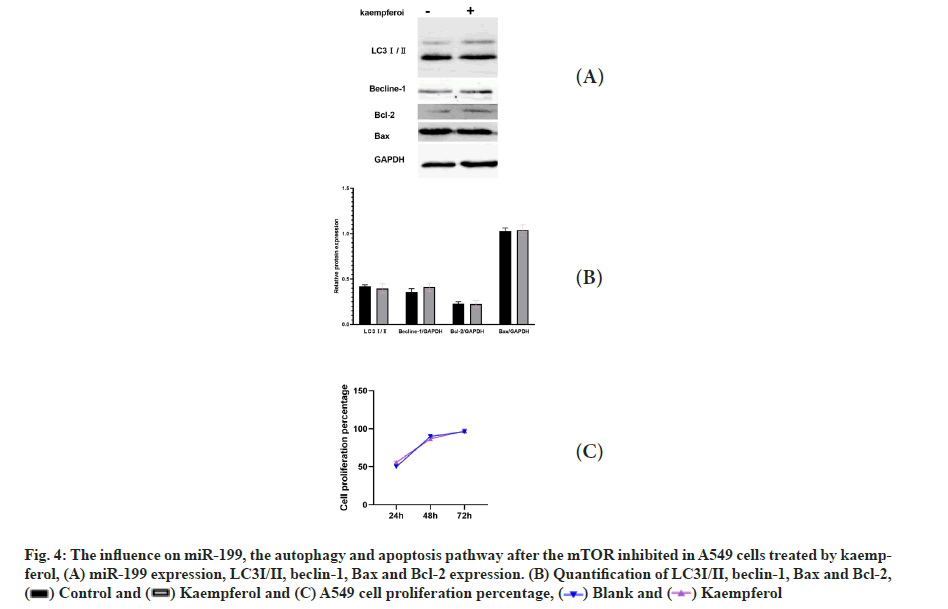
The influence on miR-199, the autophagy and apoptosis pathway after the mTOR inhibition in A549 cells treated by kaempferol was shown in fig. 4A-fig. 4C. When the mTOR was inhibited, the expression of LC3?/? and beclin-1 was low and there was no difference between the kaempferol group and blank group. The Bcl-2 was low, the Bax was high in the two groups and the cell proliferation has no difference.
Fig. 4: The influence on miR-199, the autophagy and apoptosis pathway after the mTOR inhibited in A549 cells treated by kaempferol, (A) miR-199 expression, LC3?/?, beclin-1, Bax and Bcl-2 expression. (B) Quantification of LC3?/?, beclin-1, Bax and Bcl-2, and (C) A549 cell proliferation percentage,
and (C) A549 cell proliferation percentage,  .
.
NSCLC is an alarming incidence, accounting for 85 % of lung cancers. NSCLC in the majority of patients already at an advanced stage during diagnosis, in which usually less than 5 % of patients survive only 5 y[20-23]. Kaempferol, as a main portion of fruits and vegetables, can act as antitumor[8]. In this study, we found kaempferol can inhibit the A549 cell proliferation.
MiR-199 was expressed low in many cancers[18,24-26] and miR-199 can regulate Mitogen-Activated Protein Kinase (MAPK) pathway[27]. In this study, kaempferol can promote the miR-199 expression and kaempferol also promoted the apoptosis and inhibited autophagy in A549 cells. When the miR-199 silenced, the function of kaempferol on A549 cells disappeared. This means miR-199 may join in the processes of kaempferol promoting the apoptosis and inhibiting autophagy in A549 cells. Kaempferol promoted the apoptosis in vitro. This may give us a new way for the treatment of NSCLC. mTOR is the key protein in the Phosphoinositide-3-Kinase (PI3K)/Protein Kinase B (AKT)/mTOR pathway[28-30]. This pathway can influence the cell autophagy pathway and apoptosis pathway[30].
In this study, we found mTOR could inhibit the cell autophagy via inhibiting the expression of LC3?/? and beclin-1. mTOR could promote cell apoptosis via inhibiting the Bcl-2 expression and promoting Bax expression. Hence, in this study, we found that the kaempferol promoted the apoptosis and inhibited autophagy in A549 cells via miR-199/mTOR axis.
Conflict of interests:
The authors declared no conflict of interest.
References
- Bradley SH, Bhartia BS, Kennedy MP, Frank LK, Watson J. Investigating suspected lung cancer. BMJ 2022;378:e068384.
[Crossref] [Google scholar] [PubMed]
- Hamilton G. Comparative characteristics of small cell lung cancer and Ewing’s sarcoma: A narrative review. Transl Lung Cancer Res 2022;11(6):1185-98.
[Crossref] [Google scholar] [PubMed]
- El-Kishky AH, Moussa N, Helmy MW, Haroun M. GANT61/BI-847325 combination: A new hope in lung cancer treatment. Med Oncol 2022;39(10):1-11.
[Crossref] [Google scholar] [PubMed]
- Fairley JA, Cheetham MH, Patton SJ, Rouleau E, Denis M, Dequeker E, et al. Results of a worldwide external quality assessment of cfDNA testing in lung cancer. BMC Cancer 2022;22(1):1-2.
[Crossref] [Google scholar] [PubMed]
- Gao G, Ni J, Wang Y, Ren S, Liu Z, Chen G, et al. Efficacy and safety of camrelizumab plus apatinib in previously treated patients with advanced non-small cell lung cancer harboring EGFR or ALK genetic aberration. Transl Lung Cancer Res 2022;11(6):964-74.
[Crossref] [Google scholar] [PubMed]
- Gupta S, Vundavilli H, Osorio RS, Itoh MN, Mohsen A, Datta A, et al. Integrative network modeling highlights the crucial roles of Rho-GDI signaling pathway in the progression of non-small cell lung cancer. IEEE J Biomed Health Inform 2022;26(9):4785-93.
[Crossref] [Google scholar] [PubMed]
- Ashrafizadeh M, Tavakol S, Ahmadi Z, Roomiani S, Mohammadinejad R, Samarghandian S. Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother Res 2020;34(5):911-23.
[Crossref] [Google scholar] [PubMed]
- Calderon-Montano JM, Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem 2011;11(4):298-344.
[Crossref] [Google scholar] [PubMed]
- Devi KP, Malar DS, Nabavi SF, Sureda A, Xiao J, Nabavi SM, et al. Kaempferol and inflammation: From chemistry to medicine. Pharmacol Res 2015;99:1-10.
[Crossref] [Google scholar] [PubMed]
- Imran M, Salehi B, Sharifi-Rad J, Aslam Gondal T, Saeed F, Imran A, et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019;24(12):2277.
[Crossref] [Google scholar] [PubMed]
- Sharma N, Biswas S, Al-Dayan N, Alhegaili AS, Sarwat M. Antioxidant role of kaempferol in prevention of hepatocellular carcinoma. Antioxidants 2021;10(9):1419.
[Crossref] [Google scholar] [PubMed]
- Rajendran P, Rengarajan T, Nandakumar N, Palaniswami R, Nishigaki Y, Nishigaki I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur J Med Chem 2014;86:103-12.
[Crossref] [Google scholar] [PubMed]
- Taylor LP, Miller KD. The use of a photoactivatable kaempferol analogue to probe the role of flavonol 3-O-galactosyltransferase in pollen germination. Flavonoids Cell Funct 2002;505:41-50.
[Crossref] [Google scholar] [PubMed]
- Wong SK, Chin KY, Ima-Nirwana S. The osteoprotective effects of kaempferol: The evidence from in vivo and in vitro studies. Drug Des Devel Ther 2019;13:3497-514.
[Crossref] [Google scholar] [PubMed]
- Cui X, Guo Y, Wang Q, Li X. MiR?199?3p-Dnmt3a-STAT3 signalling pathway in ovalbumin?induced allergic rhinitis. Exp Physiol 2019;104(8):1286-95.
[Crossref] [Google scholar] [PubMed]
- Li S, Wang Q, Huang L, Fan S, Li T, Shu Y, et al. miR-199-5p regulates spermiogenesis at the posttranscriptional level via targeting Tekt1 in allotriploid crucian carp. J Anim Sci Biotechnol 2022;13(1):1-3.
[Crossref] [Google scholar] [PubMed]
- Murakami Y, Toyoda H, Tanaka M, Kuroda M, Harada Y, Matsuda F, et al. The progression of liver fibrosis is related with overexpression of the miR-199 and 200 families. PLoS One 2011;6(1):e16081.
[Crossref] [Google scholar] [PubMed]
- Callegari E, Elamin BK, D’Abundo L, Falzoni S, Donvito G, Moshiri F, et al. Anti-tumor activity of a miR-199-dependent oncolytic adenovirus. PLoS One 2013;8(9):e73964.
[Crossref] [Google scholar] [PubMed]
- Lou Z, Gong YQ, Zhou X, Hu GH. Low expression of miR?199 in hepatocellular carcinoma contributes to tumor cell hyper?proliferation by negatively suppressing XBP1. Oncol Lett 2018;16(5):6531-9.
[Crossref] [Google scholar] [PubMed]
- Criner GJ, Agusti A, Borghaei H, Friedberg J, Martinez FJ, Miyamoto C, et al. Chronic obstructive pulmonary disease and lung cancer: A review for clinicians. Chronic Obstr Pulm Dis 2022;9:454-76.
[Crossref] [Google scholar] [PubMed]
- Ding Y, Lu Y, Xie X, Cao L, Zheng S. Ring finger protein 180 suppresses cell proliferation and energy metabolism of non-small cell lung cancer through downregulating C-myc. World J Surg Oncol 2022;20(1):1-10.
[Crossref] [Google scholar] [PubMed]
- Fang X, Zhu Y, Zhang T, Li Q, Fan L, Li X, et al. Fucoxanthin inactivates the PI3K/Akt signaling pathway to mediate malignant biological behaviors of non-small cell lung cancer. Nutr Cancer 2022;74(10):3747-60.
[Crossref] [Google scholar] [PubMed]
- Guberina N, Pöttgen C, Schuler M, Guberina M, Stamatis G, Plönes T, et al. Long-term survival of patients with central or >7 cm T4 N0/1 M0 non-small-cell lung cancer treated with definitive concurrent radiochemotherapy in comparison to trimodality treatment. Radiat Oncol 2022;17(1):1-4.
[Crossref] [Google scholar] [PubMed]
- Koshizuka K, Hanazawa T, Kikkawa N, Arai T, Okato A, Kurozumi A, et al. Regulation of ITGA 3 by the anti?tumor miR?199 family inhibits cancer cell migration and invasion in head and neck cancer. Cancer Sci 2017;108(8):1681-92.
[Crossref] [Google scholar] [PubMed]
- Ritter RA, Ulrich CH, Brzezinska BN, Shah VV, Zamora MJ, Kelly LE, et al. miR?199 plays both positive and negative regulatory roles in Xenopus eye development. Genesis 2020;58(3-4):e23354.
[Crossref] [Google scholar] [PubMed]
- Wei W, Wang L, Xu L, Liang J, Teng L. MiR-199 reverses the resistance to gemcitabine in pancreatic cancer by suppressing stemness through regulating the epithelial-mesenchymal transition. ACS Omega 2021;6(47):31435-46.
[Crossref] [Google scholar] [PubMed]
- Li X, Xiao J, Li K, Zhou Y. MiR-199-3p modulates the onset of puberty in rodents probably by regulating the expression of Kiss1 via the p38 MAPK pathway. Mol Cell Endocrinol 2020;518:110994.
[Crossref] [Google scholar] [PubMed]
- Battaglioni S, Benjamin D, Wälchli M, Maier T, Hall MN. mTOR substrate phosphorylation in growth control. Cell 2022;185(11):1814-36.
[Crossref] [Google scholar] [PubMed]
- Stefani C, Miricescu D, Stanescu-Spinu II, Nica RI, Greabu M, Totan AR, et al. Growth factors, PI3K/AKT/mTOR and MAPK signaling pathways in colorectal cancer pathogenesis: Where are we now? Int J Mol Sci 2021;22(19):10260.
[Crossref] [Google scholar] [PubMed]
- Akbarzadeh M, Mihanfar A, Akbarzadeh S, Yousefi B, Majidinia M. Crosstalk between miRNA and PI3K/AKT/mTOR signaling pathway in cancer. Life Sci 2021;285:119984.
[Crossref] [Google scholar] [PubMed]
 .
.