- *Corresponding Author:
- M. Ahmad
Department of Pharmacy, Faculty of Pharmacy and Alternative Medicine, The Islamia University of Bahawalpur, Bahawalpur‑63100, Pakistan
E‑mail: ma786_786@yahoo.com
| Date of Submission | 18 February 2013 |
| Date of Revision | 11 July 2013 |
| Date of Acceptance | 14 July 2013 |
| Indian J Pharm Sci 2013;75(5):569-577 |
Abstract
The present work was performed to develop and evaluate transdermal patches of combined antiasthmatic drugs (salbutamol sulphate and ketotifen fumarate). Polyvinyl alcohol membrane was used as backing membrane and eudragit RL-100 was used as matrix material to suspend the drugs in the continuous thickness of the patch. Methanol was solvent and propylene glycol was used as plasticizer. Tween 20, isopropyl myristate, eucalyptus oil, castor oil and span-20 were used as permeability enhancers. Thickness, weight variation and drug uniformity were investigated. The patch formulations were also subjected to drug release in dissolution media and permeation through rabbit skin. Effects of different enhancers were evaluated on release and permeation of drugs. F3 formulations having isopropyl myristate as permeation enhancer, showed maximum amounts of drugs release (88.11% of salbutamol sulphate and 88.33% of ketotifen fumarate) at the end of 24 h dissolution study. F3 also showed maximum permeation of both drugs (4.235 mg salbutamol sulphate and 1.057 mg ketotifen fumarate) after 24 h permeation study through rabbit skin mounted in Franz cell. The patches having no enhancer in the formulation also showed some drug release and permeation due to the presence of plasticizer. The results of the study suggested that new controlled release transdermal formulations of combined antiasthmatic drugs can be suitably developed as an alternate to conventional dosage forms.
Keywords
Ketotifen, permeability enhancers, transdermal patches, salbutamol
Transdermal patches are developed to deliver a drug to blood stream across a multilayer system of skin and keep therapeutically effective amount of drug in the body. The drug from patch first passes the stratum corneum (SC), then epidermis and finally enters into the systemic circulation. Transdermal delivery provides a leading edge over injectable and oral routes [1]. Transdermal patches can deliver the drug from skin to systemic circulation at controlled rate. It provides sustained effect of drug for desired time period [2,3]. Avoidance of first pass metabolism and gastrointestinal incompatibility increase the bioavailability and reduce side effects. Transdermal patch systems are suitable alternate for drugs with short biological half‑lives and narrow therapeutic window. Transdermal patches sustain the constant level of drug for prolonged time period and enhance pharmacological and physiological actions.
Termination of drug is possible at any point of time in case of unwanted effects. Patient compliance is enhanced due to reduced dose frequency and self‑administration [4]. Ketotifen fumarate (KF) has been prophylactically used for chronic asthma. It is an antihistaminergic and stabilizes the mast cells. It is also being used in the treatment of rhinitis and conjunctivitis. Dose size of KF is 1 mg (BD) by oral route. After oral administration, drug is completely absorbed from gastrointestinal tract (GIT). But due to hepatic metabolism, 50% of drug is destroyed reducing amount of drug one‑half at site of action [5]. Salbutamol sulphate (SS) is a β‑adrenergic agonist. Mechanism of action of SS is more specific than other drugs of the same class. It produces pronounced bronchodilation which makes it a suitable part of asthma therapy. The small doze size (2‑8 mg), extensive first pass metabolism and shorter half‑life (2-3 h) of SS makes it a suitable agent to be developed for transdermal drug delivery [6,7]. However, low permeability of these drugs is the main barrier for delivering the drug through skin. Permeation enhancers can be used to break the barrier of skin and increase the permeation of drug through skin [8‑10]. Chemical penetration enhancers work by altering reversibly the structure of stratum corneum. Drug permeation may also be enhanced by increasing its solubility in subject skin [11]. Asthma is a chronic inflammatory disorder of airways. Development of asthma involves many cells and cellular elements particularly mast cells, eosinophils, T‑lymphocytes, macrophages, neutrophils and epithelial cells. They lead to variable airway inflammation and airflow obstruction causing difficulties in breathing [12,13]. The management of chronic diseases like asthma depends on long term compliance to the dosage regimen. More than one pathological disorder may be involved in asthma that usually requires multiple drugs therapy. In such conditions, patient compliance found to be low [14]. To overcome these problems, drugs are to be combined in single dosage regimen. Combination of KF and SS used in allergic asthma is found to enhance antiasthmatic action of SS. A combination of KF (1 mg) and SS (2 mg) is available for treatment of asthma in tablet dosage form (Mastifan‑s East West Pharma, Haridwar) [15].
In this work, novel strategy was applied to develop new combined transdermal antiasthmatic formulations of SS and KF. Different permeation enhancers were used to enhance the drug dissolution and ex vivo permeation through rabbit skin using Franz cell. Physiochemical attributes like clarity, elasticity, folding endurance, tensile strength, brittleness, weight variation, moisture uptake, thickness uniformity and drug content uniformity were also determined. F3 formulation showed superior behaviour in terms of release and permeation of both drugs. Finally, optimized patch formulation was subjected to skin irritation study using human volunteers.
Materials and Methods
Salbutamol sulphate (SS) was obtained as gift sample from Glaxo Smithkline, Karachi, Pakistan. Ketotifen fumarate (KF) was supplied as gift sample from Barrett Hodgson, Karachi, Pakistan. Polyvinyl alcohol (Mol. Wt. 72 000) and eudragit RL 100 were purchased from Sigma‑Aldrich, UK. Eucalyptus oil was obtained from George Rennie, France. Tween 20, isopropyl myristate (IPM), castor oil and span 20 were purchased from BDH, UK. Methanol and propylene glycol (PG) were purchased from Merck, Germany.
Preparation of backing membrane
Backing membrane of patch was prepared using poly vinyl alcohol (PVA). Aqueous 4% w/v PVA solution was prepared in conical flask with continuous stirring on hot plate stirrer at 80°. After cooling, PVA solution was deaerated for 2 min by sonicator. Finally, 15 ml of the prepared solution was poured in glass petri dishes of an area approximately 61 cm2 and then air dried for 24 h [16]. Drying of films was carried out at room temperature. However, temperature and humidity of laboratory were maintained at favorable conditions (25° and 75% RH) for fabrication of transdermal patches. Sharma and Chandy developed a series of membranes in ambient conditions by air‑drying films of PVA. Chitosan blended films were found to have excellent physicochemical properties [17].
PVA forms a water impermeable membrane. PVA‑based membrane can protect transdermal system from external environment. It provides occlusive conditions leading to enhance the permeation of drug through the skin [18]. PVA is the most frequently used polymer in manufacturing of backing membrane [19,20].
Preparation and casting of the matrix solution
Table 1 describes the formulation variables per 100 ml of matrix dispersion. The patch formulation solution was prepared by adding 5 g eudragit to 100 ml methanol in 250 ml conical flask. The flask was sealed and solution was stirred at 500 rpm by magnetic stirrer for 30 min. After thorough mixing, relevant plasticizer and enhancers were added and mixed well for 30 min. 2480 mg SS and 620 mg KF were added and stirred for 30 min to obtain a homogenous dispersion. Addition of the above mentioned amounts of SS and KF in 100 ml of solvent gives 6 mg of SS and 1.5 mg of KF for each patch of 1.5 cm2 size. Then the matrix dispersion was sonicated for 5 min to remove entrapped air. Ten milliliter of above matrix dispersion was poured in petri dishes containing PVA backing membranes. The petri dishes were placed horizontally at room temperature for 24 h covered with inverted funnels to avoid rapid evaporation of solvent. The dried patches containing multiple antiasthmatic drugs were carefully removed from petri dishes, wrapped into aluminum foil and stored at 25°.
| Ingredients | Formulations | |||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |
| Eudragit RL 100 (g) | 5 | 5 | 5 | 5 | 5 | 5 |
| PG (g) | 1.75 | ‑ | ‑ | ‑ | ‑ | ‑ |
| Tween 20 (g) | ‑ | 5 | ‑ | ‑ | ‑ | ‑ |
| IPM (g) | ‑ | ‑ | 5 | ‑ | ‑ | ‑ |
| Eucalyptus oil (g) | ‑ | ‑ | ‑ | 5 | ‑ | ‑ |
| Castor oil (g) | ‑ | ‑ | ‑ | ‑ | 5 | ‑ |
| Span 20 (g) | ‑ | ‑ | ‑ | ‑ | 5 | |
| Methanol (ml) | 100 | 100 | 100 | 100 | 100 | 100 |
PG=propylene glycol, IPM=isopropyl myristate
Table 1: Formulation variables of transdermal patches
Cutting of patches
The dried films were cut into circular patches with the help of specially designed stainless steel cutter having diameter of 1.5 cm2. Eighteen patches of 1.5 cm2 were obtained from one petri dish.
Physical evaluation of transdermal patches
Physical appearance, weight variation, thickness, texture and colour of various transdermal patches were evaluated. All transdermal patches were visually inspected for the smoothness, clarity and brittleness [3]. Three patches were selected randomly from each formulation and weight uniformity of the dried and cut patches was checked on analytical balance (Shimadzu AUX220, Germany). The thickness of patches was determined by using digital micrometer screw gauge (Sharpfine Type‑A, China). For determining variation in thickness, each patch was checked at three different places and finally mean of three readings was taken.
To check the strength of patches, folding endurance test was performed manually. The test was performed by folding the transdermal film repeatedly at the same place until patch break or crack. Values of folding endurance were given from number of folding at same place without breaking. Three patches were checked and average was reported [21].
Moisture uptake of transdermal patches was evaluated to determine the integrity and stability of the film in humid conditions. In order to determine moisture uptake capacity, 1.5 cm2 of developed patches were weighed and films were placed at room temperature in a desiccator. After 24 h, films were taken and exposed to 84% relative humidity in desiccator. Humid condition was developed by saturated solution of potassium chloride. Patches were weighed repeatedly until a constant weight was achieved. Moisture uptake (%) was calculated by the formula [22], moisture content= [(final weight-initial weight)/initial weight]×100.
Tensile strength of transdermal patches is measured to determine the mechanical properties of the polymeric films. Modified pulley system was used to determine the tensile strength of fabricated transdermal membranes. Three strips of transdermal patches were cut in 2 cm length and 1 cm width and fixed between two jaws of apparatus. Weight was gradually increased until the film break. Three readings were taken for each patch and average was calculated. Tensile strength was measured in kg/cm2 [23].
Drug content
Drug content uniformity was evaluated by the method used by Gupta et al. and Dandagi et al. [24,25]. Patches without drug were also prepared to be used as blank. The films of each formulations containing drugs and without drug were cut into small pieces of an area of 1.5 cm2. The cut pieces of both type of patches, i.e. with drugs and without drug were put in 100 ml of water in conical flask and stirred continuously on magnetic stirrer for 36 h. Then solution was sonicated for 30 min and filtered. After achieving suitable dilutions, the solutions were analysed on double beam UV/Vis Spectrophotometer (Shimadzu‑1601, Germany) at wave length of 300 nm for KF and 276 nm for SS.
In vitro release studies
In vitro drugs release experiments were carried out in dissolution apparatus (PT‑DT7 Pharma Test, Germany). The patch of an area of 1.5 cm2 containing 6 mg of SS and 1.5 mg of KF was placed against the watch glass and retained in position with stainless steel mesh and clips. Both of antiasthmatic drugs (SS and KF) are water soluble. SS is soluble in four parts of water as per the United State Pharmacopoeia [26] and reported solubility for KF is 14.79 mg/ml. Hence, water was used as dissolution medium at ambient temperature. Solubilities of antiasthmatic drugs in sink condition were observed as reported earlier. Vessels were filled with 500 ml of distilled water maintained at 32±0.5° (skin temperature) and paddles were fitted so that the distance between the paddle blade and the surface of disk assembly was 25 mm. The disk assemblies holding the patch were placed at bottom of the vessels with release surfaces facing upward and were centred using a glass rod. The stirring speed was set at 50 rpm and the vessels were covered to minimize evaporation. Auto sampler (PTFC II, Pharma Test, Germany) was used to withdraw 5 ml of release media at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 20 and 24 h after filtering through Millipore filters. The drugs released were measured by spectrophotometric analysis. Five patches of each formulation were tested for in vitro release study and average was calculated.
Permeation study through rabbit skin
In the permeation study of combined antiasthmatic patches, Franz diffusion cell (Permegear, Bethlehem USA) with an area of 1.5 cm2 and volume of receptor compartment 12 ml was used. Due to the difficulty of obtaining human skin samples, rabbit skin was used. The receptor medium (distilled water) was filled in the receptor compartment. The receptor was chosen on the basis of compatibility with the membrane formulation and on the basis of physicochemical properties of drugs used. In Franz diffusion cell, the temperature of outer jacket was set at 37±1° in order to provide a temperature of 32±1° in the receptor compartment [27]. Actually, the loss of heat occurs in plastic tubes that connect the Franz cell with the thermostatic water bath (Brookfield, USA). The rabbit skin membrane was carefully placed over the open end of the receptor compartment. Patch of an area of 1.5 cm2 was placed over the membrane. The glass disk of donor compartment was placed over receptor compartment and both compartments were kept in position with the help of the stainless steel clamp. To avoid evaporation, the junction of two compartments was wrapped with adhesive tape. To provide occlusive conditions, the patches were covered with aluminium foil and petroleum jelly. The whole assembly was kept on magnetic stirrer and the receptor fluid was kept stirring continuously during test by using magnetic stirrer at a speed of 500 rpm. Sample of 1 ml was withdrawn at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 20 and 24 h from the sampling port with the help of long needle syringe and replaced by an equal volume of receptor fluid at each sampling time. Dissolution medium (distilled water) was used to take the blank reading. The collected samples were suitably diluted and analysed spectrophotometrically at a wave length of 300 nm for KF and 276 nm for SS to measure permeated contents of antiasthmatic drugs. Spectrophotometric method is being successfully used for measuring the permeated concentration of drug in receptor compartment, during the in vitro permeation studies [28,29].
Skin irritation test
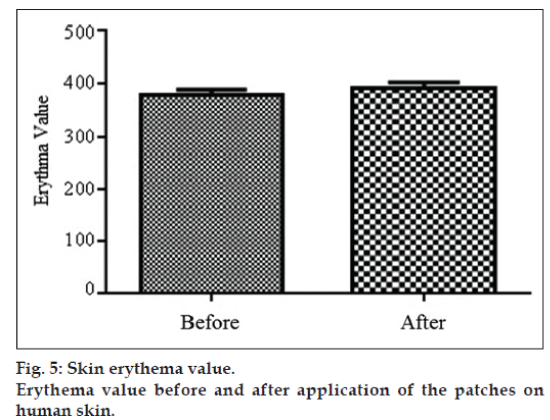
Formulation showing best results during in vitro studies was further evaluated for the presence or absence of hazards of irritation. This test was performed to ensure the safety of formulation for the application on the intact skin. Irritation study of formulated patches was performed on 10 healthy human volunteers, weighing 60‑80 kg and in the age of 21-27 years. Before the application of patches, erythema readings were taken from the inner arm of volunteers by Mexameter™ (Courage and Khazaka, Germany) and considered as control reading. Patches were applied on the inner arm of the volunteer for a period of 8 h. After specified time, patches were removed and readings were taken again and compared with control reading by statistical paired sample t‑test [30].
Results and Discussion
The aim of this study was to develop matrix type patches of combined antiasthmatic drugs SS and KF by solvent casting or plate casting method followed by their in vitro and ex vivo permeation evaluation. For this purpose, a series of matrix patches of combined antiasthmatic drugs were prepared using eudragit RL100 as polymer. Different enhancers, tween 20, IPM, eucalyptus oil, castor oil and span 20 were employed. PG was applied as plasticizer. Effects of different permeability enhancers on in vitro dissolution and ex vivo permeation through rabbit skin were evaluated.
The results of visual inspection showed that the patches containing tween 20, IPM, eucalyptus oil, castor oil and span 20 as permeation enhancers were smooth and transparent and do not need plasticizer to be added (Table 2). This may be attributed to plasticising effect of permeation enhancers. Results of weight variation test are presented in Table 3. Low values of standard deviations proved that the patches containing combined antiasthmatic drugs possess uniformity in weight. To ensure the uniformity in thickness of transdermal patches, each patch was checked at three places and averaged. Thickness values of various patch formulations are shown in Table 3. Thickness with low standard deviation values ensures uniformity of thickness in films prepared by solvent casting method. PG was primarily used as additive and plasticizer in manufacturing of transdermal patches. It reduces brittleness and rigidity of membrane leading to improved smoothness, physical stability, plasticity and appearance of films [31]. Other formulations having tween 20, isopropyl myristate, eucalyptus oil, castor oil and span 20 as permeability enhancers, showed satisfactory physical and mechanical properties without plasticizer. Tween 20, isopropyl myristate, eucalyptus oil, castor oil, and span 20 when used as enhancers produce smooth, clear, transparent and flexible patch membranes even without any addition of plasticizer. These enhancers possess suitable physiochemical properties to act as plasticizer.
| Visual appearance | ||||
|---|---|---|---|---|
| Formulation | Smoothness | Clarity | Brittleness | Overall appearance |
| F1 | + | + | × | Satisfied |
| F2 | ++ | × | ++ | Satisfied |
| F3 | ++ | ++ | ++ | Satisfied |
| F4 | ++ | × | + | Satisfied |
| F5 | ++ | ++ | ++ | Satisfied |
| F6 | + | × | + | Satisfied |
+=level of satisfaction, ×=level of dissatisfaction
Table 2: Visual appearance of transdermal patches
| Formulation | Physicochemical properties | ||||
|---|---|---|---|---|---|
| Weight variation (mg) | Thickness (μm) | Folding endurance | Moisture uptake (%) | Tensile strength (kg/cm2) | |
| F1 | 30.57±0.01 | 18±0.0055 | 175±1.02 | 3.4±0.08 | 0.76±0.023 |
| F2 | 30.21±0.0 | 20±0.006 | 162±2.09 | 2.8±0.10 | 0.58±0.067 |
| F3 | 29.6±0.01 | 22±0.0031 | 158±1.61 | 3.1±0.06 | 0.62±0.071 |
| F4 | 28.9±0.003 | 25±0.004 | 167±1.05 | 2.6±0.05 | 0.65±0.038 |
| F5 | 31.00±0.007 | 27±0.009 | 180±0.98 | 2.9±0.09 | 0.79±0.042 |
| F6 | 30.5±0.00 | 30±0.01 | 172±2.13 | 3.0±0.15 | 0.6±0.035 |
+=level of satisfaction, ×=level of dissatisfaction
Table 3: Physicochemical properties of transdermal patches
Plasticizers act by interposing forces that holds the polymer chains, causing softening and extending the formulated matrix membrane [32]. All prepared patches possess suitable folding endurance. The results are presented in Table 3. Moisture uptake study was carried out at 84% relative humidity. Results of study are shown in Table 3. Low values of moisture uptake are attributed to hydrophobic nature of eudragit RL 100. Low moisture uptake favours formulation stability for long term storage and reduces brittleness. Low moisture uptake also keeps the product safe from microbial contamination [33]. Results of tensile strength reveal that patches have suitable strength and elasticity. Results are presented in Table 3.
All patches showed uniform distribution of both drugs. Among different patches, difference in percent uniformity of contents of both drugs was not significant. The drug contents in different membrane formulations are placed in Table 4. The results showed that the plate casting method of producing patches was capable of providing uniform drug distribution in patches.
| Formulation | % Contents | (mean±SD) |
|---|---|---|
| SS | KF | |
| F1 | 98.58±0.009 | 97.62±0.002 |
| F2 | 100.49±0.001 | 101.73±0.001 |
| F3 | 101.25±0.008 | 101.98±0.004 |
| F4 | 97.32±0.002 | 99.09±0.006 |
| F5 | 98.57±0.007 | 98.27±0.004 |
| F6 | 102.29±0.003 | 101.11±0.006 |
The values are expressed as mean±SD, where SD is standard deviation
Table 4: Content uniformity of ss and kf in transderml patches
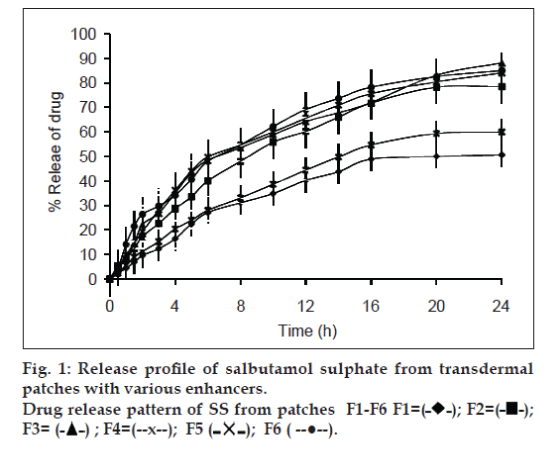
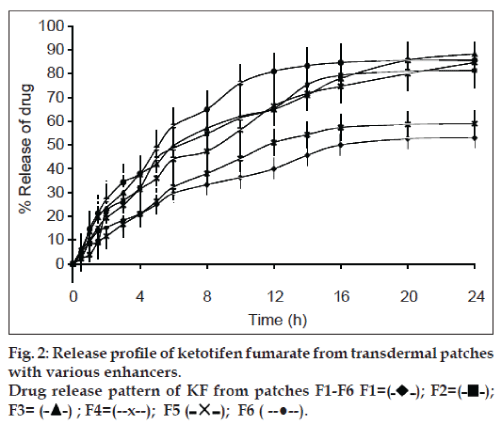
The percent cumulative release of both drugs after 24 h dissolution experiments are presented in Tables 5 and 6. Highest percent cumulative release for both drugs was observed from F3 containing IPM as permeation enhancer. This significant amount of drug release form the patches within 24 h is due to the presence of quaternary ammonium group in eudragit RL 100, which make the polymer hydrophilic to some extent. When formulation comes in contact with dissolution medium, polymer absorb fluid followed by hydration and swelling of patches leading to the release of drug from polymeric device [34]. Further, addition of plasticizer and enhancers increases the release rate of drug from the patches during dissolution study (figs. 1 and 2). High release rate of drugs is associated with the presence of plasticizer and enhancer. The presence of enhancer improves flexibility and smoothness of eudragit RL 100 matrix of patches. Water molecules permeate easily in formulated film and cause the film to swell. This phenomenon ultimately increases the amount of drug release from patches [35]. The highest release of F3 is due to solubilising effect of IPM. Least amounts of percent cumulative release for both drugs were observed from F1. This aspect of F1 can be associated with the absence of permeability enhancer. Other formulations containing enhancers showed relatively higher cumulative release of both drugs.
| Time (h) | Amount released (%) | |||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |
| 0 | 0.00±00 | 0.00±00 | 0.00±00 | 0.00±00 | 0.00±00 | 0.00±00 |
| 0.5 | 2.44±0.7 | 5.33±0.45 | 4.44±0.81 | 4.89±0.36 | 2.44±0.85 | 4.67±0.74 |
| 1 | 4.44±0.12 | 8.22±0.57 | 8.22±0.64 | 9.78±0.45 | 5.56±0.62 | 14.22±0.51 |
| 1.5 | 6.89±0.97 | 12.44±0.48 | 14.44±0.75 | 14.67±0.62 | 8.89±0.41 | 21.56±0.73 |
| 2 | 9.56±1.2 | 17.33±0.36 | 22.22±0.93 | 19.33±0.57 | 11.33±0.73 | 26.44±0.97 |
| 3 | 12.44±0.59 | 22.67±0.62 | 26.89±1.4 | 28.22±0.61 | 15.33±0.95 | 29.78±0.82 |
| 4 | 16.44±0.47 | 28.67±0.73 | 35.11±0.83 | 36.22±0.82 | 20.44±0.86 | 34.22±0.64 |
| 5 | 22.44±0.83 | 33.56±0.82 | 43.33±0.78 | 44.00±0.95 | 24.00±0.38 | 40.67±0.75 |
| 6 | 27.11±0.45 | 40.00±0.91 | 48.44±0.97 | 49.78±0.48 | 28.00±1.2 | 48.00±0.59 |
| 8 | 31.11±1.4 | 48.00±0.8 | 53.56±0.67 | 54.67±0.77 | 33.11±0.97 | 54.67±0.85 |
| 10 | 34.89±0.93 | 55.78±0.76 | 58.89±1.1 | 60.00±0.58 | 38.67±0.78 | 62.22±0.91 |
| 12 | 40.22±0.84 | 60.22±0.85 | 64.22±0.85 | 65.56±0.67 | 44.44±0.55 | 69.11±0.51 |
| 14 | 43.78±0.75 | 66.00±0.88 | 67.78±0.62 | 70.89±1.2 | 49.78±0.23 | 73.67±0.43 |
| 16 | 48.89±0.41 | 71.78±0.53 | 72.00±0.76 | 75.56±0.9 | 54.67±0.88 | 78.22±0.22 |
| 20 | 50.00±0.44 | 78.22±0.54 | 83.11±0.93 | 80.44±0.8 | 59.33±0.61 | 82.56±1.5 |
| 24 | 50.67±0.65 | 78.44±0.9 | 88.11±0.7 | 84.00±0.55 | 60.00±1.01 | 85.00±0.38 |
Values are presented as mean±SD. SD=Standard deviation, SS=salbutamol sulphate
Table 5: Amount of ss released from transdermal patches
| Time (h) | Amount released (%) | ||||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | ||
| 0 | 0.00±00 | 0.00±00 | 0.00±00 | 0.00±00 | 0.00±00 | 0.00±00 | 0.00±00 |
| 0.5 | 2.67±0.7 | 5±0.63 | 6±0.9 | 5.67±0.57 | 2.33±0.81 | 4.67±0.63 | 4.67±0.63 |
| 1 | 9.33±0.8 | 8.33±0.91 | 13.67±0.89 | 10±0.39 | 3.87±0.76 | 14.67±0.81 | 14.67±0.81 |
| 1.5 | 13.67±0.6 | 9.5±0.63 | 19.67±0.65 | 15.33±0.74 | 9±0.58 | 21.33±0.65 | 21.33±0.65 |
| 2 | 15.33±0.9 | 22±0.43 | 23.67±0.56 | 19.33±0.43 | 11.67±0.87 | 27±0.72 | 27±0.72 |
| 3 | 18.33±0.5 | 26.67±0.81 | 30±0.43 | 24.67±0.64 | 16.67±0.45 | 34.33±0.54 | 34.33±0.54 |
| 4 | 21±0.4 | 32±0.76 | 37.67±0.87 | 31.33±0.86 | 21±0.64 | 38±0.38 | 38±0.38 |
| 5 | 25±0.2 | 44.33±0.83 | 42±0.78 | 36±1.02 | 26.33±0.69 | 48.67±0.47 | 48.67±0.47 |
| 6 | 29.67±0.9 | 48.67±0.79 | 49.67±0.56 | 44±0.72 | 32.33±0.55 | 58±0.77 | 58±0.77 |
| 8 | 33.33±1.1 | 54.67±0.93 | 57±0.52 | 47.33±0.69 | 38±0.71 | 65±0.83 | 65±0.83 |
| 10 | 36.33±0.6 | 61.33±0.53 | 62±0.32 | 56.33±0.77 | 44.33±0.76 | 76±0.9 | 76±0.9 |
| 12 | 40±0.91 | 65.33±0.49 | 65±0.49 | 66.33±0.99 | 51±0.89 | 81±0.95 | 81±0.95 |
| 14 | 45.67±0.85 | 75.33±0.38 | 71±0.58 | 71.67±0.56 | 54.33±0.9 | 83.33±0.45 | 83.33±0.45 |
| 16 | 50±0.76 | 79.33±0.98 | 78±0.81 | 74.67±0.61 | 57.33±0.35 | 84.67±0.55 | 84.67±0.55 |
| 20 | 52.67±0.82 | 81±0.76 | 85.67±0.94 | 80±0.83 | 58.67±0.45 | 85.67±0.61 | 85.67±0.61 |
| 24 | 53±0.92 | 81.33±0.67 | 88.33±0.73 | 84.67±0.92 | 59±0.51 | 85.67±0.82 | 85.67±0.82 |
Values are presented as mean±SD. SD=Standard deviation, KF=ketotifenfumarate
Table 6: Amount of kf released from transdermal patches
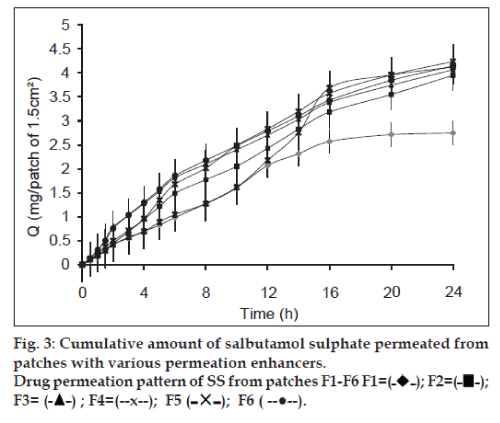
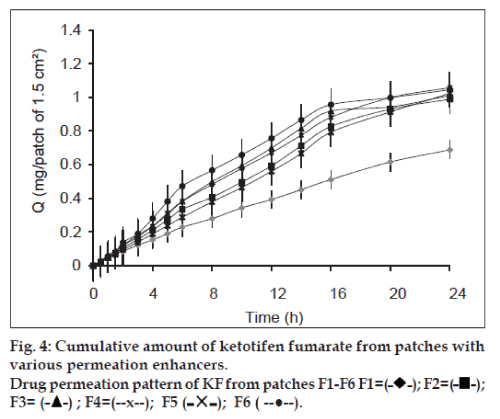
The in vitro permeation study gives prediction about the in vivo performance of the formulation. The permeation study was carried out on Franz Diffusion cell using rabbit skin membrane. Graphs representing cumulative amounts of SS and KF permeated vs. time were constructed (figs. 3 and 4). An increase in concentration of both drugs was found in receptor compartment with passage of time. Each formulated patch contained 6 mg of SS and 1.5 mg of KF. The amounts of both drugs permeated from transdermal patches at the end of 24 h permeation study in Franz Cell were (2.747 mg SS and 0.688 mg KF) from formulation F1, (3.949 mg SS and 0.989 mg KF) from F2, (4.235 mg SS and 1.057 mg KF) from F3, (4.121 mg SS and 1.023 mg KF) from F4, (2.787 mg SS and 0.697 mg KF) from F5 and (4.144 mg SS and 1.044 mg KF) from F6. Formulation F1 having no permeability enhancer showed minimum amount of drug permeation. Similar effect has been reported for transdermal patches of diclofenac sodium subjected to permeation study through hairless rate skin [36]. F2 has tween 20 as permeability enhancer and showed sufficient amount of drug permeated during ex vivo permeation study. Tween 20 is nonionic surfactant used to enhance the permeability of drug though stratum corneum. The permeation promoting activity of nonionic surfactant (tween) may be due to the reduction in surface tension, improvement in wetting of skin and enhanced distribution of the drug [37]. F3 showed the highest amounts of both drugs permeated through rabbit skin. F3 patches have IPM as drug permeability promoter. This can be explained on the fact that the solubility parameter of IPM (16.40MPa1/2) is similar to that of skin (20.01MPa1/2), which leads to high affinity of IPM for SC. The high affinity of IPM for SC results in the formation of a pool that drives the drugs eventually into the SC, thereby reducing the SC barrier function [38]. Similarly, F3 also showed the highest amounts of cumulative release for both drugs. The results are in good agreement of expected results as per dissolution study. Transdermal patches of F4 contain eucalyptus oil as enhancer. The permeation enhancing effect of eucalyptus oil is primarily believed to be due to the promotion of membrane vehicle partitioning tendency of the drug with the oils. It is believed that penetration of the vegetable oil into the intracellular lipid phase of the membrane may increase the degree of fluidity resulting in decreased resistance to permeation. This mechanism can increase flux of drugs [39]. Formulation F6 contains span 20 as permeability enhancer. The permeation results of this formulation are comparable with those reported by Vora et al. [40]. Flux was calculated to determine the quantity of drugs permeated per centimeter square of patch per hour. Values of Flux are presented in Table 7. Formulation F3 showed maximum flux both for SS and KF through the rabbit skin membrane. The formulation showed this pronounced flux due to the presence of IPM as permeation enhancer. The lag time of both drugs was separately calculated from the intercept on the time axis in the plot of cumulative amount permeated vs. time and lag times for various formulations are given in Table 7.
| Formulations | Flux=J (mg/cm2/h) | Lag time (h) | ||
|---|---|---|---|---|
| SS | KF | SS | KF | |
| F1 | 0.0851±0.023 | 0.0178±0.019 | 1.62±0.081 | 1.51±0.12 |
| F2 | 0.1096±0.015 | 0.0272±0.027 | 0.85±0.095 | 0.87±0.074 |
| F3 | 0.1423±0.020 | 0.0374±0.031 | 0.74±0.073 | 0.72±0.091 |
| F4 | 0.0830±0.018 | 0.0263±0.016 | 1.46±0.120 | 1.50±0.063 |
| F5 | 0.0920±0.021 | 0.0206±0.020 | 2.18±0.085 | 2.00±0.055 |
| F6 | 0.1168±0.032 | 0.0325±0.015 | 0.75±0.061 | 0.69±0.100 |
Values are presented as mean±SD. SD=Standard deviation, KF=ketotifen fumarate, SS=salbutamol sulphate
Table 7: Flux and lag time of ss and kf from transdermal patches
Formulation F3, which showed the best release rate and permeation profile during in vitro and ex vivo evaluations, was further subjected for skin irritation test. Erythema values were taken before and 8 h after the application of patches compared by t‑test in Graph pad, keeping the confidence interval 95%. Results of study give P value 0.37 and difference between the readings before and after application of patches was found insignificant (fig. 5). Results of irritation study revealed that F3 transdermal patches possess high compatibility and showed no sign of erythema or irritation during the period of study [41].
In present study, KF and SS were combined and evaluated successfully in transdermal patches. Formulated patches were found to possess satisfactory physicochemical properties and uniform dispersion of drug. In vitro release and ex vivo permeation rates were optimised by adding various permeation enhancers. Formulation F3 having IPM as enhancer was superior in performance and showed the highest amounts of both drugs released from patches and permeated through the rabbit skin membrane. Irritation study revealed that formulation was free from the hazard of irritation and safe for application on intact skin. Patches having no enhancer showed very low permeability. The study provides a comprehensive data to develop and optimize transdermal patches of combined antiasthmatic drugs by applying various chemical enhancers.
References
- Jain NK. Advances in controlled and novel drug delivery. 1st ed. New Delhi: CBS Publishers and Distributors; 2001. p. 108‑10.
- Gupta JR, Tripathi P, Irchhiaya R, Garud N, Dubey P, Patel JR. Formulation and evaluation of matrix type transdermal patches of Glibenclamide. Int J Pharm Sci Drug Res 2009;1:46‑50.
- Sanap GS, Dama GY, Karpe AS, Nalawade SV, Kakadi RS, Jadhav UY. Preparation of transdermal monolithic systems of indapamide by solvent casting method and the use of vegetable oils as permeation enhancer. Int J Green Pharm 2008;2:129‑33.
- Hadgraft J, Guy R. Transdermal Drug Delivery. New York: Marcel Dekker Inc.; 1989. p. 296.
- Hemangi JP, Jitendra SP, Keyur DP. Transdermal patch for ketotifenfumarate (ktf) as Asthmatic drug. Int J PharmTech Res 2009;1:1297‑304.
- Mofizur R, Qamrul A, Mithilesh KJ. Development and in vitro evaluation of sustained release matrix tablets of salbutamol sulphate using methocel k100mcr polymer. Int J Pharm Res Dev 2011;11:105‑15.
- Uttam B, Panna T. Effect of chemical enhancers on in vitro release of salbutamol sulphate from transdermal patches. Kathmandu Univ J SciEngTechnol 2005;1:1‑8.
- Guy RH. Current status and future prospects of transdermal drug delivery. J Pharm Res 1996;13:1765‑9.
- Trommer H, Neubert RH. Overcoming the stratum corneum, the modulation of skin penetration. A review. Skin PharmacolPhysiol 2006;19:106‑21.
- Purdon GH, Azzi GG, Zhang J, Smith EW, Maibach HI. Penetration enhancement of transdermal delivery‑current permutation and limitations. Crit Rev Ther Drug Carrier Syst 2004;21:97‑132.
- Wang YP, Thakur R, Fan QX, Michniak B. Transdermal Iontophoresis combination strategies to improve trnsdermaliontophoretic drug delivery. Eur J Pharm Biopharm 2005;60:179‑91.
- Lugogo NL, MacIntyre NR. Life‑threatening asthma: Pathophysiology and management. Respir Care 2008;53:726‑35.
- Nizar NJ, Elizabeth AB. Pathogenesis of asthma. J Med Clin N Am 2002;86:925‑36.
- Abhijit NM, Rahul KG, Smita KP, Bhanudas SK, Prakash NK, Prasad PT, et al. Formulation and in vitro-in vivo evaluation of salbutamol sulphatesustained release tablets. Der Pharm Lett 2010;2:546‑52.
- Satya VS, Prachi K, Ritu K, Siddheshwar G, Preeti K, Nargund LV. Spectrophotometric estimation of ketotifen and salbutamol by validated analytical method from tablet dosage form. J Chem Pharm Res 2013;1:123‑7.
- Biswajit M, Sushmita M, Ritu G, Balaram P. A comparison between povidone‑ethylcellulose and povidone‑eudragit transdermal dexamethasone matrix patches based on in vitro skin permeation. Eur J Pharm Biopharm 2005;59:475‑8.
- Chandy T, Sharma CP. Prostaglandin E1‑immobilized poly (vinyl alcohol)‑blended chitosan membranes: Blood compatibility and permeability properties. J ApplPolymSci 1992;44:2145‑56.
- Wiwat P, Jirapornchai S, Prapaporn B, Thanaporn A, Wirach T, Garnpimol CR. Nicotine transdermal patches using polymeric natural rubber as the matrix controlling system: Effect of polymer and plasticizer blends. J MembrSci 2012;411:81‑90.
- Arijit D, Sibaji G, Biplab KD, Sudip D. A novel technique for treating the type‑ll diabetes by transdermal patches prepared by using multiple polymer complexes. Int J Pharma Res Dev 2010;9:195‑204.
- Viswanatha RM, Jayashankar RV, Ramesh Y, Venkateswarlu I. Formulation and evaluation of fuconazole transdermal patches.Int J InstPharma Life Sci 2011;1:18‑29.
- Arora P, Mukherjee B. Design development of physicochemical in vitro and in vivo evaluation of transdermal patches containing diclofenacdiethylammonium salt. Indian J Pharm Sci 2002;91:2076‑89.
- Kusum DV, Saisivam S, Maria GR, Depti PU. Design and evaluation of matrix diffusion controlled transdermal patches of verapamil hydrochloride. Drug DevInd Pharm 2003;29:495‑503.
- Murthy SN, Hiremath SR. Preformulation studies of transdermal films of hydroxypropyl methylcellulose and sodium carboxymethyl cellulose. Int J Pharm Excip 2002;1:34‑8.
- Gupta SP, Jain SK. Effective and controlled transdermal delivery of metoprolol tartrate. Indian J Pharm Sci 2005;67:346‑50.
- Dandagi PM, Manavi FV, Gadag AP, Mastiholimath VS, Jagdeesh T. Formulation of transdermal drug delivery of ketotifenfumarate. Indian J Pharm Sci 2003;65:239‑43.
- United States Pharmacopoeia 27/NF 22. Toronto, Canada: The United States Pharmacopoeia convention, Inc. Webcom Limited; 2004.
- Narasimha M, Shobharani R, Hiremath SR. Physical and chemical permeation enhancers in transdermal delivery of terbutalineSulphate. AAPS PharmSciTech 2001;2:1‑5.
- Ansari K, Singhai AK, Saraogi GK, Patil S. Transdermal drug delivery of salbutamol sulphate with different concentration of polymers. Int J Res Pharm Sci 2011;1:50‑65.
- Hemangi JP, Jitendra SP, Keyur DP. Transdermal patch for ketotifenfumarate (ktf) as asthmatic drug. Int J Pharm Tech Res 2009;1:1297‑304.
- Kantarci G, Ozguney I, Karasulu HY, Arzik S, Guneri T. Comparison of different water/oil microemulsions containing diclofenac sodium: Preparation, characterization, release rate, and skin irritation studies. AAPS PharmSciTech 2007;8:75‑81.
- Jitendra B, Subhash P, Pathak AK. Development and optimization of transdermal system of lisinopril dehydrate: Employing permeation enhancers. Iran J Pharm Sci 2010;6:245‑51.
- Entwistle CA, Rowe RE. Plasticization of cellulose ethers used in the film coating of tablets. J Pharm Pharmacol 1979;31:269‑72.
- Mutalik S, Udupa N. Glibenclamide transdermal patches: Physicochemical, pharmacodynamic, and pharmacokinetic evaluations. J Pharm Sci 2004;93:1577‑94.
- Meenakshi B, Rajesh KN, Mahip B. Development and characterization of transdermal patches of Metoprolol tartrate. Asian J Pharm Clin Res 2010;3:130‑4.
- Lim LY, Wan LS. The effect of plasticizers on the properties of polyvinyl alcohol patches. Drug DevInd Pharm 1994;20:1007‑20.
- Chauhan R, Mehta N, Jain A, Jain S, Jain AK, Gupta MK. Optimization of plasticizer for diclofenac sodium transdermal film, permeation enhancement. Asian J Pharm Clin Res 2011;4:178‑80.
- Tanwar YS. Formulation and evaluation of transdermal films of salbutamol sulphate. Dhaka Univ J Pharm Sci 2005;4:93‑7.
- Panchagnula R, Desu H, Jain A, Khandavilli S. Feasibility studies of dermal delivery of paclitaxel with binary combinations of ethanol and isopropyl myristate, role of solubility, partitioning and lipid bilayer perturbation. Farmaco 2005;60:894‑9.
- Walters KA, Walker M, Olejnik O. Non‑ionic surfactant effects on hairless mouse skin permeability characteristics. J Pharm Pharmacol 1988;40:525‑9.
- Vora B, Khopade AJ, Jain NK. Proniosome based transdermal delivery of levonorgestrel for effective contraception. J Control Release 1997;54:149‑65.
- Dangi AA, Sheth ZP, Janki J. Formulation and evaluation of transdermal ondansetron hydrochloride matrix patch: In vitro skin permeation and irritation study. Int J Pharm Res Allied Sci 2012;1:26‑34.