- *Corresponding Author:
- A. Kanugo
SVKM NMIMS School of Pharmacy and Technology Management, Shirpur, Dhule, Maharashtra 425405, India
E-mail: abhi.kanugo09@gmail.com
| Date of Received | 31 May 2020 |
| Date of Revision | 29 March 2022 |
| Date of Acceptance | 09 November 2022 |
| Indian J Pharm Sci 2022;84(6):1417-1428 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Solubility of the active ingredient in the body is the key factor associated with their therapeutic efficacy, in vitro and in vivo correlations. Approximately 90 % of the newly developed active ingredients and 40 % of existing molecules suffered from poor solubility properties and thereby significantly affecting the solubility in the gastrointestinal tract resulted in poor bioavailability. The current aim of the research article is to provide higher bioavailability of ambrisentan by improving its solubility and dissolution rate. Ambrisentan is an endothelin receptor antagonist useful in the treatment of pulmonary arterial hypertension. The solubility of ambrisentan was enhanced by the liquisolid technique using several non-volatile solvents and polyethylene glycol 400 (0.828 mg/ml) was found to be best. The varying concentration of carriers (microcrystalline cellulose, dibasic calcium phosphate) and Aerosil 200 as coating agents was added to develop tablets. The physical interaction study of ambrisentan with non-volatile solvent, carrier and coating agents were performed. The powder blends were evaluated for flow characteristics (Carr’s index and angle of repose). A powder with good flowing characteristics was subjected to tablet manufacturing and evaluated further. The optimization was carried out with Box-Behnken design which provided 32 designs. The carrier, coating agent and superdisintegrants were selected as independent parameters and disintegration and dissolution time as dependent parameters. The optimized batch F2 showed with least disintegration time of 2.12 min and the highest percentage of drug dissolution was 99.61 % within 30 min. Hence, the F2 batch was short-listed and successfully passes the stability testing.
Keywords
Solubility enhancement, ambrisentan, liquisolid compact, hypertension, box-behnken design
The poor solubility of active ingredients in water has great impact on the pharmacokinetic and pharmacodynamic properties. The bonding between the drugs with water is essential for solubilization. Hence, solubility is the prime factor according to the Biopharmaceutical Classification System (BCS)[1]. The poor solubility of active ingredient is the biggest hurdle in the development of dosage form[2]. The absorption and bioavailability of the active ingredients from their dosage forms markedly depends on the solubility, intestinal permeability and dissolution characteristics according to the BCS system[3,4]. The development of new molecules involving drug design, combinatorial chemistry and high-thoughtful screening processes resulted in the formation of lipophilic moieties and ultimately having the poor aqueous solubility. Around 90 % of the new entities and 40 % of the existing molecules in the market have poor solubility[5,6]. Moreover, these molecules failed to elicit a therapeutic response because of non-compliance of criteria of Lipinski’s “rule of five”[7]. The molecule having less than 100 µg/ml is considered as poorly water soluble[8]. Moreover, as per the guidelines stated by the BCS, the active ingredient is considered as highly soluble when the highest therapeutic dose is soluble in 250 ml of the fluid at the pH range of 1-7.4 at 37°±0.5°, otherwise the therapeutic agent is poorly soluble[9,10].
Oral drug delivery is most widely preferred over other routes for their economic, ease of handling, non-invasive, without any pain, patient comfort, compliance and stability. For oral administration of drugs, dissolution is the rate-limiting step which is totally rely on the solubility of active ingredients. The solubility greatly affects release profile of the drugs and disturbs the in vitro in vivo relationship[11,12]. Hence, for accelerating the dissolution of the drugs, there is need of utilizing most appropriate technique. The active ingredients categorized as BCS-II and IV respectively, needs to incorporate for solubility enhancement techniques[13].
Liquisolid compact technique was first utilized by Spirea sp. for improvement of solubility and dissolution rate of the active ingredients. This technique is based on formulating liquid medication of the lipophilic moiety using non-volatile solvent. Further, liquid medicaments are converted into the freely flowing compressible powder by incorporating carrier and coating agents. The liquid load is adsorbed on the surface of carrier agents and addition of coating agents improves the flowing characteristics of the powder. The liquisolid compact system describes the development of tablets for rapid or sustained release by addition of superdisintegrants and lubricants[14,15]. The higher solubility and dissolution of poorly soluble active ingredients are achieved through enhancing the wettability of solid particles with non-volatile liquid, rising drug surface area[16].
Ambrisentan is second generation endothelin receptor antagonist and useful in the therapy of pulmonary arterial hypertension. Ambrisentan is administered orally and approved drug in United States of America (USA), Canada and Europe. The therapeutic efficacy is restricted due to their poor water solubility (0.05 mg/ml), hence current research aim with improvement of solubility and dissolution rate for providing higher bioavailability[17,18].
Materials and Methods
Sorbitan monolaurate, Ambrisentan was received as gift sample from Cipla Pharmaceuticals, Mumbai. Tween 20 (Polysorbate 20, Polyoxyethylene sorbitan monolaurate), Span 20 (Sorbitan monoluarate, sorbitan fatty acid esters), polyethylene glycol (200 and 400 grade) was purchased from Merck chemicals, Mumbai. Anhydrous dibasic calcium phosphate gifted by Nitika pharmaceuticals, Nagpur. All other chemicals and solvents used were of analytical grade only.
Preformulation study:
Ambrisentan was characterized for their appearance, melting point and Loss On Drying (LOD).
Estimation of solubility:
The solubility of ambrisentan in different non-volatile solvents such as Tween 20, Span 20, Propylene Glycol (PG), Polyethylene Glycol (PEG) 200 and PEG 400 were carried out to identify the best suitable solvent. Moreover, saturation solubility study was also performed by the addition of excess amount of the active ingredients in the non-volatile vehicles and subjected for orbital shaker for about 48 h at 37° with continuous vibration. The drug solution was further diluted, filtered through 0.45 µm and analyzed by Ultraviolet (UV) visible spectrophotometer (Shimadzu-1900, Japan). The results were calculated in triplicate[19].
Determination of load factor:
Powders have limited capability to load the liquid medicaments for retaining good flowability and compressibility. Addition of higher quantity of liquid vehicles resulted in poor flow ability and compressibility. Hence, it was essential to know the maximum loading capacity without compromising compressibility. These were finding out with flowing potential of liquid (Φ) and compressible potential of liquid (¥). The excipient ratio (R) can be calculated by diving the quantity of carrier (Q) to the coating material (q). The liquid load factor was calculated from the following equations.
R=Q/q (1)
Lf=Φca+Φco×1/R (2)
Interaction study:
The physical interactions between the active ingredients and other excipients were analyzed with Fourier-Transform Infrared (FTIR) spectroscopy (IRAffinity-1s, Shimadzu). The accurately weighed quantity of Ambrisentan and carriers (Microcrystalline Cellulose (MCC), Dibasic Calcium Phosphate (DCP)) as well as Liquisolid Compact mixture was characterized for any possible interactions. The mixture was subjected for scanning between the ranges of 4000-400 cm-1[22].
Flowing characteristics of powder:
The variable quantity of carrier and coating materials were added to the liquid medicaments enclosing active ingredient to become it freely flowable. The powder blends were evaluated for their flowing characteristics such as compressibility index and angle of repose (fixed funnel method)[23].
Formulation of tablets from powder blends:
For a batch size of 30 tablets, accurately weighed quantity of the Ambrisentan was transfer in the mortar following by the addition of selected non-volatile solvents. To the liquid medicaments recalculated quantity of carrier agent’s namely anhydrous dibasic calcium phosphate were added. Subsequently, the coating agent Aerosil 200 was added to make the powder blends in their compressible form. Before compression, Crosscarmellose Sodium (CCS), Sodium Steraryl Fumarate (SSF) was added and blended without any friction. The compact mass subjected for compression and tablets were prepared with 10 mm punch size on 12 station multi-tooling machine. (Rimek mini press-II, Karnavati Engineering, Ahmadabad)[24,25]. The formulation components were depicted in Table 1.
| Std | Run | Ambrisentan (mg) | PEG 400 (mg) | Factor 1 | Factor 2 | R | LF | Factor 3 | SSF (mg) | Total (mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| A:DCP (mg) | B:Aerosil 200b (mg) | C:CCS (mg) | ||||||||
| 4 | 1 | 5 | 85 | 260 | 40 | 6.5 | 0.34 | 14 | 4 | 408 |
| 12 | 2 | 5 | 85 | 250 | 40 | 6.25 | 0.36 | 16 | 4 | 400 |
| 5 | 3 | 5 | 85 | 240 | 30 | 8 | 0.375 | 12 | 4 | 376 |
| 14 | 4 | 5 | 85 | 250 | 30 | 8.33 | 0.36 | 14 | 4 | 388 |
| 10 | 5 | 5 | 85 | 250 | 40 | 6.25 | 0.36 | 12 | 4 | 396 |
| 17 | 6 | 5 | 85 | 250 | 30 | 8.33 | 0.36 | 14 | 4 | 388 |
| 9 | 7 | 5 | 85 | 250 | 20 | 12.5 | 0.36 | 12 | 4 | 376 |
| 15 | 8 | 5 | 85 | 250 | 30 | 8.33 | 0.36 | 14 | 4 | 388 |
| 6 | 9 | 5 | 85 | 260 | 30 | 8.66 | 0.34 | 12 | 4 | 396 |
| 11 | 10 | 5 | 85 | 250 | 20 | 12.5 | 0.36 | 16 | 4 | 380 |
| 13 | 11 | 5 | 85 | 250 | 30 | 8.33 | 0.36 | 14 | 4 | 388 |
| 3 | 12 | 5 | 85 | 240 | 40 | 6 | 0.375 | 14 | 4 | 388 |
| 8 | 13 | 5 | 85 | 260 | 30 | 8.66 | 0.34 | 16 | 4 | 400 |
| 16 | 14 | 5 | 85 | 250 | 30 | 8.33 | 0.36 | 14 | 4 | 388 |
| 7 | 15 | 5 | 85 | 240 | 30 | 8 | 0.375 | 16 | 4 | 380 |
| 2 | 16 | 5 | 85 | 260 | 20 | 13 | 0.34 | 14 | 4 | 388 |
| 1 | 17 | 5 | 85 | 240 | 20 | 12 | 0.375 | 14 | 4 | 368 |
Table 1: Formulation of Ambrisentan Tablets.
Optimization study:
Quality by Design (QbD) approach was utilized with the intention of developing the product without any error and saves the materials as well as time[26,27]. The critical quality attributes for designing liquisolid compact was the disintegration and dissolution time, whereas the independent parameters were variations of carrier and coating agents. The 32 Box-Behnken Design (BBD) was applied and suggested 17 runs to find out the optimized batch[21]. The QbD matrix of Ambrisentan tablets was depicted in Table 2.
| Factor | Name | Units | Type | Minimum | Maximum | Coded Low | Coded High | Mean | Standard deviation |
|---|---|---|---|---|---|---|---|---|---|
| A | DCP | mg | Numeric | 240 | 260 | -1↔240.00 | +1↔260.00 | 250.00 | 7.07 |
| B | Aerosil 200 | mg | Numeric | 20 | 40 | -1↔20.00 | +1↔40.00 | 30.00 | 7.07 |
| C | CCS | mg | Numeric | 12 | 16 | -1↔12.00 | +1↔16.00 | 14.00 | 1.41 |
Table 2:QbD Matrix for Liquisolid Compact of Ambrisentan Tablets.
Post compression evaluation parameters:
Weight variation test: The prepared tablets around 20 were randomly picked and accurately weighed. The mean weight of an individual tablets were recorded with standard derivations. The weight variation test passes within 2.5 % variations from the average tablets[28].
Hardness: The crushing strength of tablets from each batch was tested by Monsanto hardness tester and values were reported by average of three[29].
Friability: The tablets were subjected for friability using Roche friabilator. The tablets from each batch were weighed accurately equivalent to 6.5 g and further kept in the friabilator which was rotated at a speed of 25 rpm for 100 rotations. After rotation, tablets were collected and reweighed. The percentage of friabilator was calculated by subtracting the weight of initial from final weight[30].
Disintegration: The randomly selected 6 tablets were kept in the disintegration test apparatus and time required to pass all the particles from the sieves was noted. This test was carried out at 37°±0.5° using 900 ml of simulated gastric fluid[31].
In vitro dissolution: The dissolution study of Ambrisentan tablets were performed with United States Pharmacopeia (USP) dissolution apparatus II (Paddle) using pH 7.4 phosphate buffer. The paddle was allowed to rotate at a speed of 50 rpm, at 37°±0.5°. The samples were withdrawn at an interval of 5 min, diluted, filter through 0.45 µm membrane filter and analyzed spectrophotometrically at 264 nm[32,33].
Content uniformity: The prepared tablets (10) were randomly selected and converted into the powder after crushing. The average weight of tablet containing a powder was taken and dissolved with pH 7.4 phosphate buffer. The solution was further diluted and filters through 0.45 µ membrane filter and analyzed spectrophotometrically at 264 nm[34].
Stability study: The stability study of an optimized formulation was carried out according to the International Council on Harmonisation (ICH) guidelines. The optimized batch was kept at 40° and 75 % relative humidity for about 3 mo. The samples were withdrawn at an interval of 1 mo and estimated for drug content, disintegration and dissolution time[35].
Results and Discussion
Ambrisentan is available as crystalline powder. The melting point was observed at 165°-167° and LOD value was 0.34 %. The weight quantity of Ambrisentan was transferred to the solution of different non-volatile solvents and best suitable solvent for improvement of solubility and dissolution was identified. PEG 400 was selected as best one in which Ambrisentan solubilizes completely in comparison with other non-volatile solvents. The solubility of Ambrisentan in water is 0.05 mg/ml, whereas solubility in PEG 400 recorded as 147 mg/ml. The solubility of Ambrisentan in various non-volatile solvents was depicted in Table 3.
| S.no | Solvents | Solubility (mg/ml) |
|---|---|---|
| 1 | Tween 20 | 62±0.54 |
| 2 | Tween 80 | 84±0.39 |
| 3 | Span 20 | 78±0.67 |
| 4 | Span 80 | 129±1.08 |
| 5 | Polyethylene glycol 400 | 147±1.44 |
| 6 | Propylene glycol | 96±0.89 |
Table 3: Solubility of Ambrisentan in various Solvents.
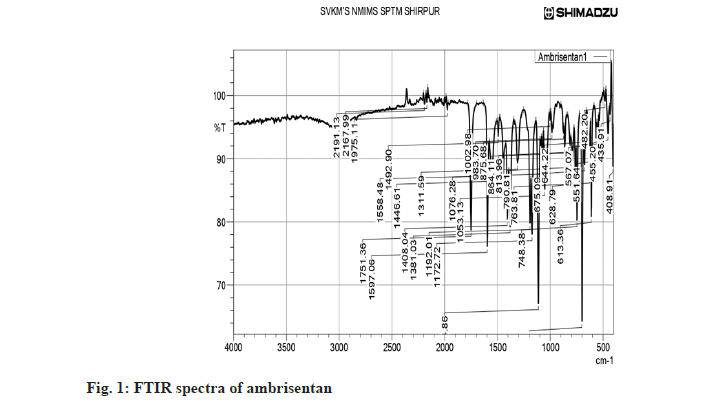
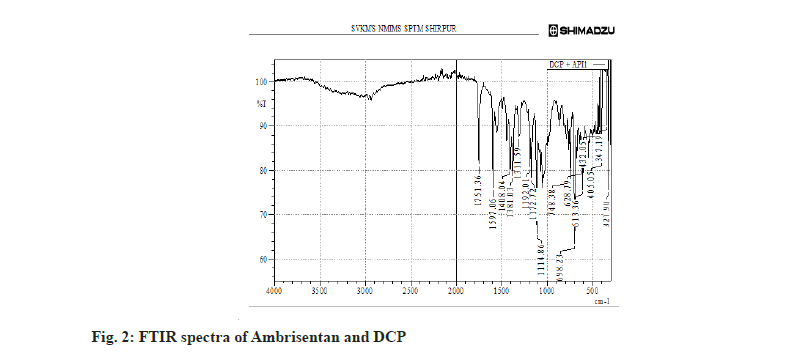
The FTIR spectra of pure Ambrisentan and its interactions was carried out and depicted in fig. 1. Moreover, the physical interaction of Ambrisentan with other selected excipients was check for any possible interactions and indicated no such interaction. The Ambrisentan was found compatible with DCP (fig. 2). The C-O-C stretching was observed at 1172 and 1192 cm-1, aromatic carbon ring at 1567, 1751 and 1975 cm-1, methylene C-H bending at 1446 cm-1, C=C at 1558, 1597 cm-1, Aromatic C-H at 702, 875 cm-1 and C=N at 1558, 1597 cm-1.
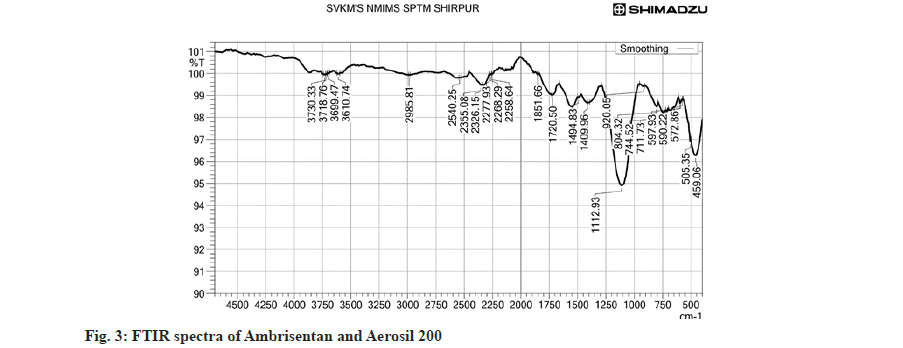
The powder blends were evaluated for bulk density, tapped density, compressibility index and angle of repose. All the prepared batches pass the flowability test. The compressibility index was found 12.11±0.13 % to 16.2±0.06 %, angle of repose in the range of 24.2±0.31 to 29.4±0.13 and Hausner’s ratio as 1.06±0.06 to 1.23±0.10. The results of all batches were depicted in Table 4. The optimization study was performed with Design Expert software (Version 11). The independent parameters such as DCP, Aerosil 200 and CCS with their low and high values and disintegration time and folding endurance as dependable parameters fitted into the Design of experiments (DOE) and accordingly 32 BBD was applied. The BBD predicted 17 runs depicted in Table 5 and fig. 3. The BBD showed similarity in 4 batches and hence only 13 batches were considered for formulation of Ambrisentan tablets.
| Batch | Bulk density | Tapped density | Carr’s index (%) | Angle of repose (?) | Hausner’s index |
|---|---|---|---|---|---|
| F1 | 0.45±0.01 | 0.39±0.05 | 13.33±0.21 | 24.2±0.12 | 1.08±0.02 |
| F2 | 0.42±0.09 | 0.36±0.07 | 14.28±0.13 | 23.4±0.29 | 1.16±0.07 |
| F3 | 0.41±0.04 | 0.34±0.08 | 17.07±0.13 | 29.47±0.14 | 1.20±0.06 |
| F4 | 0.40±0.01 | 0.32±0.12 | 15.2±0.04 | 27.6±0.11 | 1.21±0.11 |
| F5 | 0.42±0.01 | 0.36±0.17 | 14.28±0.12 | 26.61±0.13 | 1.2±0.12 |
| F6 | 0.41±0.03 | 0.35±0.21 | 14.63±0.06 | 27.15±0.14 | 1.17±0.06 |
| F7 | 0.42±0.021 | 0.36±0.12 | 14.28±0.05 | 26.56±0.02 | 1.18±0.16 |
| F8 | 0.45±0.11 | 0.38±0.08 | 15.55±0.23 | 26.76±0.13 | 1.18±0.17 |
| F9 | 0.42±0.04 | 0.35±0.08 | 16.66±0.13 | 30.7±0.14 | 1.20±0.06 |
| F10 | 0.43±0.01 | 0.38±0.12 | 13.4±0.04 | 24.06±0.11 | 1.15±0.11 |
| F11 | 0.43±0.01 | 0.36±0.17 | 16.27±0.12 | 27.87±0.13 | 1.15±0.12 |
| F12 | 0.44±0.03 | 0.39±0.21 | 15.2±0.06 | 27.48±0.14 | 1.06±0.06 |
| F13 | 0.48±0.07 | 0.41±0.20 | 18.75±0.10 | 30.62±0.21 | 1.17±0.09 |
Note: All value are n=3±SD.
Table 4: The Evaluation of Flow Characteristics of Powder Blends.
| Std | Run | Batch | Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 |
|---|---|---|---|---|---|---|---|
| A: DCP (mg) | B: Aerosil 200 (mg) | C: CCS | DT (min) | Dissolution (%) | |||
| 4 | 1 | F1 | 260 | 40 | 14 | 2.19 | 98.7 |
| 12 | 2 | F2 | 250 | 40 | 16 | 2.15 | 98.9 |
| 5 | 3 | F3 | 240 | 30 | 12 | 2.24 | 98.37 |
| 14 | 4 | F4 | 250 | 30 | 14 | 2.22 | 97.26 |
| 10 | 5 | F5 | 250 | 40 | 12 | 2.27 | 97.59 |
| 17 | 6 | - | 250 | 30 | 14 | 2.21 | 98.12 |
| 9 | 7 | F6 | 250 | 20 | 12 | 2.32 | 98.48 |
| 15 | 8 | - | 250 | 30 | 14 | 2.25 | 97.56 |
| 6 | 9 | F7 | 260 | 30 | 12 | 2.26 | 97.6 |
| 11 | 10 | F8 | 250 | 20 | 16 | 2.11 | 98.22 |
| 13 | 11 | - | 250 | 30 | 14 | 2.23 | 98.05 |
| 3 | 12 | F9 | 240 | 40 | 14 | 2.21 | 97.66 |
| 8 | 13 | F10 | 260 | 30 | 16 | 2.15 | 99.56 |
| 16 | 14 | - | 250 | 30 | 14 | 2.24 | 97.87 |
| 7 | 15 | F11 | 240 | 30 | 16 | 2.16 | 97.83 |
| 2 | 16 | F12 | 260 | 20 | 14 | 2.17 | 97.2 |
| 1 | 17 | F13 | 240 | 20 | 14 | 2.2 | 98.52 |
Table 5: Box-Behnken design for Ambrisentan Tablets.
The factor coding is coded. Sum of square is Type III-Partial. The model F-value of 7.79 implies the model is significant. There is only a 0.65 % chance that an F-value this large could occur due to noise. p<0.0500 indicate model terms are significant. In this case C is a significant model term. Values greater than 0.1000 indicate the model terms are not significant. If there are many insignificant model terms (not counting those required to support hierarchy), model reduction may improve your model (Table 6). The model F-value of 5.12 implies the model is significant. There is only a 2.13 % chance that an F-value this large could occur due to noise. p<0.0500 indicate model terms are significant. In this case C, AB, AC, C² are significant model terms. Values greater than 0.1000 indicate the model terms are not significant. If there are many insignificant model terms (not counting those required to support hierarchy), model reduction may improve your model (Table 7).
| Source | Sum of squares | Df | Mean square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 0.0411 | 9 | 0.0046 | 7.79 | 0.0065 | Significant |
| A-DCP | 0.0005 | 1 | 0.0005 | 0.7683 | 0.4098 | |
| B-Aerosil 200 | 0.0008 | 1 | 0.0008 | 1.37 | 0.2808 | |
| C-CCS | 0.0338 | 1 | 0.0338 | 57.71 | 0.0001 | |
| AB | 0.002 | 1 | 0.002 | 3.46 | 0.1053 | |
| AC | 0.0002 | 1 | 0.0002 | 0.3841 | 0.555 | |
| BC | 0.002 | 1 | 0.002 | 3.46 | 0.1053 | |
| A² | 0.0005 | 1 | 0.0005 | 0.9098 | 0.3719 | |
| B² | 6.6E-06 | 1 | 6.6E-06 | 0.0112 | 0.9186 | |
| C² | 0.0011 | 1 | 0.0011 | 1.9 | 0.2107 | |
| Residual | 0.0041 | 7 | 0.0006 | |||
| Lack of fit | 0.0031 | 3 | 0.001 | 4.13 | 0.102 | Not significant |
| Pure error | 0.001 | 4 | 0.0003 | |||
| Total | 0.0452 | 16 |
Table 6: DOE containing ANOVA for response 1: DT.
| Source | Sum of squares | Df | Mean square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 5.36 | 9 | 0.5958 | 5.12 | 0.0213 | significant |
| A-DCP | 0.0578 | 1 | 0.0578 | 0.4966 | 0.5038 | |
| B-Aerosil 200 | 0.0231 | 1 | 0.0231 | 0.1986 | 0.6693 | |
| C-CCS | 0.7626 | 1 | 0.7626 | 6.55 | 0.0376 | |
| AB | 1.39 | 1 | 1.39 | 11.96 | 0.0106 | |
| AC | 1.56 | 1 | 1.56 | 13.43 | 0.008 | |
| BC | 0.6162 | 1 | 0.6162 | 5.29 | 0.0549 | |
| A² | 0.0888 | 1 | 0.0888 | 0.7632 | 0.4113 | |
| B² | 0.0445 | 1 | 0.0445 | 0.3819 | 0.5561 | |
| C² | 0.7525 | 1 | 0.7525 | 6.47 | 0.0385 | |
| Residual | 0.8147 | 7 | 0.1164 | |||
| Lack of fit | 0.2996 | 3 | 0.0999 | 0.7756 | 0.5655 | Not significant |
| Pure error | 0.5151 | 4 | 0.1288 | |||
| Total | 6.18 | 16 |
Table 7: DOE model containing ANOVA for response 2: Dissolution.
The model equations for both dependable parameters were depicted below.
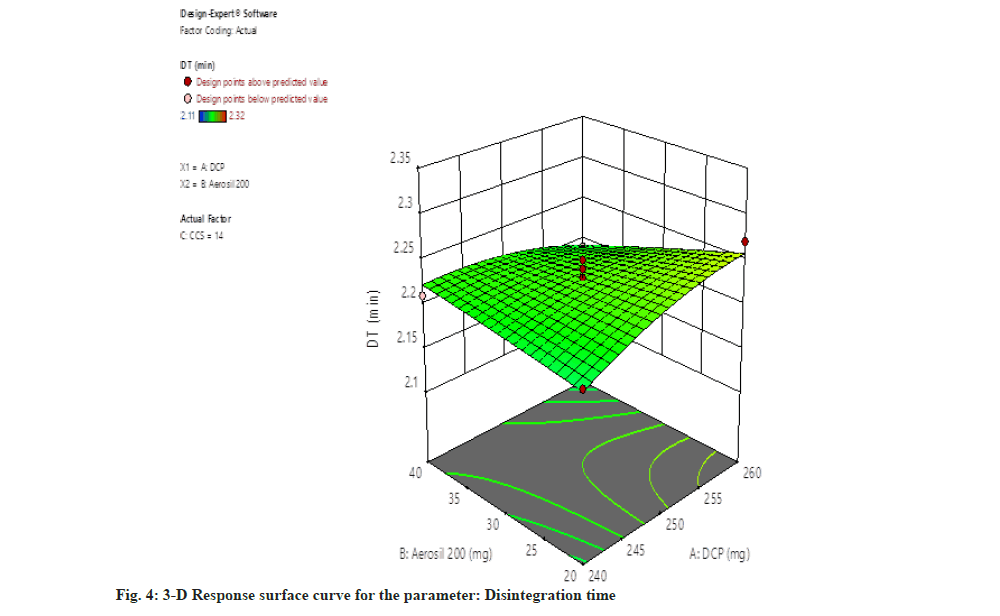
For disintegration time (DT)=2.23+0.0075, A-0.0100, B-0.0650, C-0.0225, AB-0.0075, AC+0.0225, BC-0.0113, A2-0.0013 B2-0.0162 C2.
The independent parameter dibasic calcium phosphate concentration gradually increases then the disintegration time also increases. Whereas raising the concentration of Aerosil 200 resulted in reduction of disintegration time (fig. 4).
For dissolution;
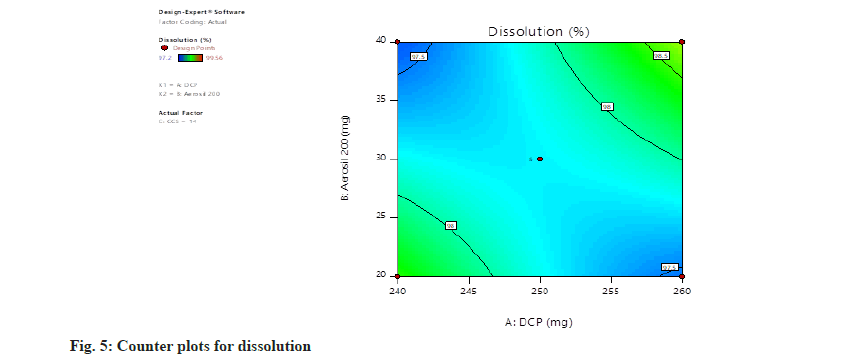
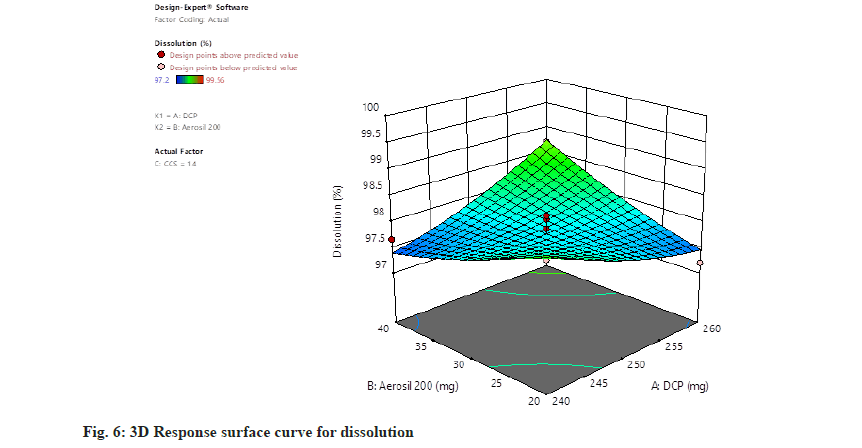
Dissolution=97.77+0.0850, A+0.0538, B+0.3087, C+0.5900, AB+0.6250, AC+0.3925, BC+0.1452, A2+0.1027, B2+0.4228 C2. The concentration of all independent parameters has positive impact on the rate of dissolution (fig. 5 and fig. 6).
The formulated tablets of Ambrisentan were evaluated for weight variation, hardness, friability, disintegration time and content uniformity. All the batches were passed the weight variation test. The hardness of tablets varies from 4.1±0.30 to 4.6±0.26 kg/cm2. The thickness of all the tablets was recorded in the range of 3.7 to 3.9 mm. The friability of tablets from all the batches was observed in the range of 0.39 to 0.57 %. Moreover, the disintegration time for the batches was showed in the range of 2.11 to 2.32 min. The content uniformity of the several tablets was identified in the range of 98.42 to 99.45 %. The in vitro drug dissolution studies for Ambrisentan tablets were performed with using USP type II dissolution apparatus using pH 7.4 phosphate buffers as dissolution media. The 3 tablets were placed in the dissolution apparatus and frequent sampling was carried out at an interval of 5 min. After withdrawing of 5 ml of sample from dissolution apparatus immediately add the pH 7.4 phosphate buffer to maintain the stock solution. The samples were diluted, filter through 0.45µ filter paper (Whatman filter paper no. 41) and analyzed spectrophotometrically at 264 nm (Table 8).
| Batch | Weight variation (mg) | Hardness (kg/cm2) | Thickness (mm) | Friability (%) | Disintegration (m) | Content uniformity (%) |
|---|---|---|---|---|---|---|
| F1 | 405±0.93 | 4.4±0.12 | 3.8±0.08 | 0.44±0.01 | 2.19±0.07 | 98.42±0.50 |
| F2 | 398±0.84 | 4.2±0.03 | 3.9±0.06 | 0.54±0.12 | 2.15±0.05 | 99.45±0.37 |
| F3 | 373±0.56 | 4.5±0.02 | 3.8±0.09 | 0.40±0.06 | 2.24±0.10 | 98.65±0.68 |
| F4 | 388±0.92 | 4.4±0.11 | 3.8±0..05 | 0.41±0.26 | 2.22±0.05 | 98.78±0.72 |
| F5 | 395±0.78 | 4.5±0.07 | 3.7±0..09 | 0.43±0.01 | 2.27±0.07 | 99.22±0.90 |
| F6 | 377±0.59 | 4.6±0.01 | 3.8±0.03 | 0.39±0.17 | 2.32±0.06 | 99.35±0.30 |
| F7 | 394±0.96 | 4.4±0.12 | 3.9±0.05 | 0.43±0.01 | 2.26±0.05 | 98.48±0.49 |
| F8 | 376±0.63 | 4.1±0.03 | 3.8±0.06 | 0.65±0.12 | 2.11±0.09 | 98.94±0.85 |
| F9 | 388±0.68 | 4.3±0.02 | 3.8±0.08 | 0.49±0.06 | 2.21±0.11 | 99.37±0.89 |
| F10 | 399±0.77 | 4.2±0.11 | 3.7±0.04 | 0.52±0.26 | 2.15±0.13 | 98.70±0.77 |
| F11 | 380±0.85 | 4.2±0.07 | 3.8±0.05 | 0.57±0.01 | 2.16±0.20 | 99.30±0.91 |
| F12 | 386±0.94 | 4.3±0.01 | 3.8±0.06 | 0.50±0.17 | 2.17±0.08 | 98.45±0.60 |
| F13 | 366±0.54 | 4.3±0.06 | 3.7±0.04 | 0.48±0.20 | 2.20±0.12 | 98.80±0.23 |
Note: All values in n=3 ±SD.
Table 8: The Post Compression Evaluations of Ambrisentan Tablets.
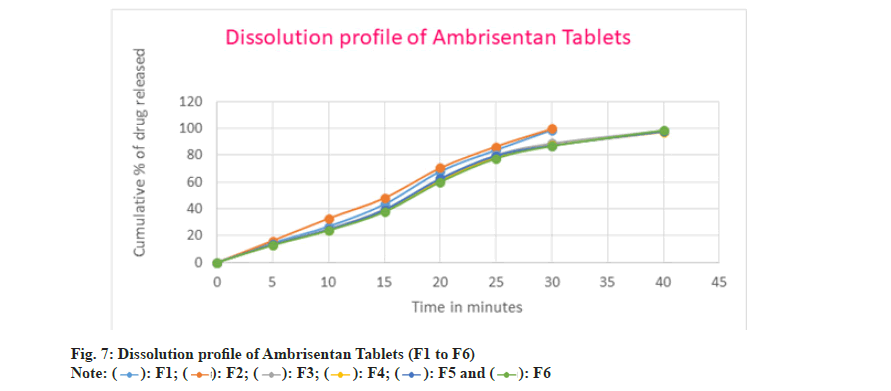
The prepared batches of Ambrisentan were subjected for in vitro dissolution testing. In all the batches, the release of ambrisentan was rapid due to the conversion of solid form of the drug in the liquid medication. The drug release from the F1 batch was found to be 98.70 % after 30 min whereas, the cumulative amount of 99.61 % of Ambrisentan was recored in F2 batch. The release rate was slighly more in the F2 batch comparatively with the F1. The rapid release of Ambrisentan was attributed due to the greater concentration of superdisintegrants in the batch F2. Similarly, the cumulative percentage of drug released from the batches F3 to F6 were 98.37 %, 97.26 %, 97.59 5 and 98.45 % respectively. Among all these batches the slight variations in the drug release was due to the composition and hardness of the tablets. The release rate of batches F3 to F5 were observed in 30 min and 40 min for the batch F6.
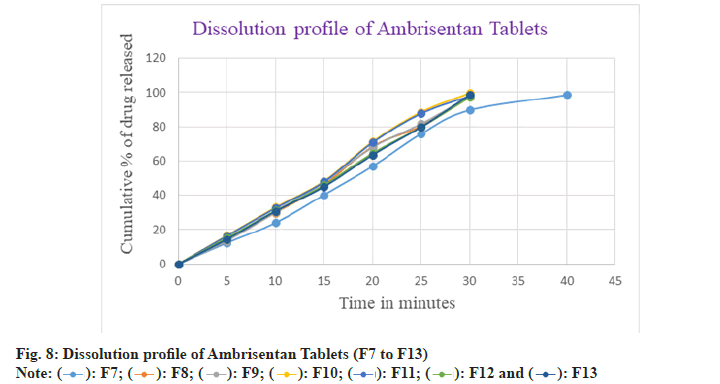
In F7 batch, the drug dissolution was comparatively slow with the other batches and found to be 98.49 % after 40 min. Moreover, the drug released within 30 min in the batches of F8 to F13 and observed as 98.22 %, 97.66 %, 99.56 %, 97.83 %, 97.20 % and 98.52 % respectively. Among all the batches F2 batch was considered as optimized because of rapid and greater amount of drug released in 30 min as well as showed the better flow characteristics amongst the others batches. The in vitro dissolution profile of Ambrisentan was depicted in the fig. 7 and fig. 8 respectively. The optimized batch F2 was tested for their stability and passes the test. The results were depicted in Table 9.
| Parameters | After 1 mo | After 2 mo | After 3 mo |
|---|---|---|---|
| Physical appearance | No change | No change | No change |
| Hardness (kg/cm2) | 4.2±0.05 | 4.1±0.06 | 4.0±0.08 |
| Friability (%) | 0.56±0.18 | 0.61±0.23 | 0.63±0.27 |
| Disintegration (min) | 2.11±0.07 | 2.10±0.06 | 2.09±0.09 |
| Drug content (%) | 99.35 ±0.49 | 99.07±0.54 | 98.83±0.37 |
Note: n=3, ±SD
Table 9: Stability study of an Optimized Batch F2.
Improvement in the solubility and dissolution is the prerequisite for designing of dosage form. Ambrisentan belongs to BCS-II and therefore needs to enhance the solubility thereby higher bioavailability was achieved. Liquisolid Compact is most convenient and economical method for improving the solubility of poor solubility of drugs. For enhancing solubility of Ambrisentan, PEG 400 was found to be best solvent. Anhydrous dibasic calcium phosphate having higher potential than MCC to load liquid and does not affect the flow ability and compression characteristics. The marked rise in the solubility and dissolution rate of Ambrisentan is due to conversion of lipophilic drug into liquid medicaments with greater aqueous solubility. The higher wettability, surface area and conversion of crystalline drug into amorphous forms are the mechanisms responsible for higher solubility and dissolution rate. Hence, liquisolid compact technique successfully enhances the solubility and dissolution as well as bioavailability of Ambrisentan. The best formulation batch F2 was selected on the basis of disintegration, dissolution release profile and the minimum quantity of material utilized in the development of the tablets without compromising flow characteristics.
Acknowledgement:
Authors are thankful to SVKM NMIMS for providing all necessary facilities for the research work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Rodriguez-Aller M, Guillarme D, Veuthey JL, Gurny R. Strategies for formulating and delivering poorly water-soluble drugs. J Drug Deliv Sci Technol 2015;30:342-51.
- Sayyad FJ, Tulsankar SL, Kolap UB. Design and development of liquisolid compact of candesartan cilexetil to enhance dissolution. J Pharm Res 2013;7(5):381-8.
- Asare-Addo K, Walton K, Ward A, Totea AM, Taheri S, Alshafiee M, et al. Direct imaging of the dissolution of salt forms of a carboxylic acid drug. Int J Pharm 2018;551(1-2):290-9.
[Crossref] [Google Scholar] [PubMed]
- Kanugo A. Liquisolid-pellets technique: A recent technique for enhancing solubility and bioavailability of drugs. Int J Appl Pharm 2020;12(6):34-40.
- Butt S, Hasan SM, Hassan MM, Alkharfy KM, Neau SH. Directly compressed rosuvastatin calcium tablets that offer hydrotropic and micellar solubilization for improved dissolution rate and extent of drug release. Saudi Pharm J 2019;27(5):619-28.
[Crossref] [Google Scholar] [PubMed]
- Thakral NK, Meister E, Jankovsky C, Li L, Schwabe R, Luo L, Chen S. Prediction of in vivo supersaturation and precipitation of poorly water-soluble drugs: Achievements and aspirations. Int J Pharm 2021;600:120505.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 2001;46(1-3):3-26.
[Crossref] [Google Scholar] [PubMed]
- Bazzo GC, Pezzini BR, Stulzer HK. Eutectic mixtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs. Int J Pharm 2020;588:119741.
- Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 1995;12(3):413-20.
[Crossref] [Google Scholar] [PubMed]
- Papich MG, Martinez MN. Applying biopharmaceutical classification system (BCS) criteria to predict oral absorption of drugs in dogs: Challenges and pitfalls. AAPS J 2015;17(4):948-64.
[Crossref] [Google Scholar] [PubMed]
- Kanikkannan N. Technologies to improve the solubility, dissolution and bioavailability of poorly soluble drugs. J Anal Pharm Res 2018;7(1):00198.
- El-Sayyad NM, Badawi A, Abdullah ME, Abdelmalak NS. Dissolution enhancement of leflunomide incorporating self emulsifying drug delivery systems and liquisolid concepts. Bull Faculty Pharm Cairo Univ 2017;55(1):53-62.
- Hentzschel CM, Alnaief M, Smirnova I, Sakmann A, Leopold CS. Enhancement of griseofulvin release from liquisolid compacts. Eur J Pharm Biopharm 2012;80(1):130-5.
[Crossref] [Google Scholar] [PubMed]
- Liquisolid systems and methods of preparing same. US6423339B1 Google Patent. 2021.
- Liquisolid systems and methods of preparing same. US5800834A Google Patent. 2021.
- Ghadi R, Dand N. BCS class IV drugs: Highly notorious candidates for formulation development. J Control Release 2017;248:71-95.
[Crossref] [Google Scholar] [PubMed]
- Ambrisentan: Uses, Interactions, Mechanism of Action|DrugBank Online [Internet]. [cited 2021 Sep 19];Available from: https://go.drugbank.com/drugs/DB06403
- Deshmane S, Deshmane S, Shelke S, Biyani K. Enhancement of solubility and bioavailability of ambrisentan by solid dispersion using Daucus carota as a drug carrier: Formulation, characterization, in vitro and in vivo study. Drug Dev Ind Pharm 2018;44(6):1001-11.
[Crossref] [Google Scholar] [PubMed]
- Javadzadeh Y, Jafari-Navimipour B, Nokhodchi A. Liquisolid technique for dissolution rate enhancement of a high dose water-insoluble drug (carbamazepine). Int J Pharm 2007;341(1-2):26-34.
[Crossref] [Google Scholar] [PubMed]
- Lu M, Xing H, Jiang J, Chen X, Yang T, Wang D, et al. Liquisolid technique and its applications in pharmaceutics. Asian J Pharm Sci 2017;12(2):115-23.
[Crossref] [Google Scholar] [PubMed]
- Bonthagarala B, Dasari V, Kotra V, Swain S, Beg S. Quality-by-Design based development and characterization of pioglitazone loaded liquisolid compact tablets with improved biopharmaceutical attributes. J Drug Deliv Sci Technol 2019;51:345-55.
- Taghizadeh Z, Rakhshani S, Jahani V, Rajabi O, Haghighi HM, Abbaspour M. Preparation and in vitro characterization of carvacrol pellets by combination of liquisolid technique and extrusion-spheronization. J Drug Deliv Sci Technol 2021;61:102232.
- Mostafa M, Gardouh AR, Abogresha NM, Gad S. Factorial design, formulation, in vitro and in vivo evaluation of rapid orally disintegrating tablets prepared by sublimation technique using captopril as a model drug. J Drug Deliv Sci Technol 2020;57:101635.
- Prajapat MD, Butani SB, Gohel MC. Liquisolid: A promising technique to improve dissolution efficiency and bioavailability of poorly water soluble nimodipine. J Drug Deliv Sci Technol 2019;53:101135.
- Kanugo AY. Design and evaluation of enteric compression-coated tablet for chronotherapeutic drug delivery. Asian J Pharm 2017;11(3).
- Krstic M, Djuris J, Petrovic O, Lazarevic N, Cvijic S, Ibric S. Application of the melt granulation technique in development of lipid matrix tablets with immediate release of carbamazepine. J Drug Deliv Sci Technol 2017;39:467-74.
- Krstic M, Djuris J, Petrovic O, Lazarevic N, Cvijic S, Ibric S. Application of the melt granulation technique in development of lipid matrix tablets with immediate release of carbamazepine. J Drug Deliv Sci Technol 2017;39:467-74.
[Crossref] [Google Scholar] [PubMed]
- Hellberg E, Westberg A, Appelblad P, Mattsson S. Evaluation of dissolution techniques for orally disintegrating mini-tablets. J Drug Deliv Sci Technol 2021;61:102191.
- Kadota K, Terada H, Fujimoto A, Nogami S, Uchiyama H, Tozuka Y. Formulation and evaluation of bitter taste-masked orally disintegrating tablets of high memantine hydrochloride loaded granules coated with polymer via layering technique. Int J Pharm 2021;604:120725.
- Kokott M, Lura A, Breitkreutz J, Wiedey R. Evaluation of two novel co-processed excipients for direct compression of orodispersible tablets and mini-tablets. Eur J Pharm Biopharm 2021;168:122-30.
- Dholakiya A, Dudhat K, Patel J, Mori D. An integrated QbD based approach of SMEDDS and liquisolid compacts to simultaneously improve the solubility and processability of hydrochlorthiazide. J Drug Deliv Sci Technol 2021;61:102162.
- Han X, Shan X, Du Y, Pang S, Hu L. Development and evaluation of novel innovative multi-channel aripiprazole orally disintegrating tablets. J Drug Deliv Sci Technol 2020;55:101446.
- Kanugo AY, Kochar NI, Chandewar AV. Development of Pulsatile drug delivery for chronotherapeutics of hypertension. Int J Drug Deliv Technol 2017;7(3):184-9.
- Jaipakdee N, Limpongsa E, Sripanidkulchai BO, Piyachaturawat P. Preparation of Curcuma comosa tablets using liquisolid techniques: In vitro and in vivo evaluation. Int J Pharm 2018;553(1-2):157-68.
[Crossref] [Google Scholar] [PubMed]
- Huanbutta K, Yunsir A, Sriamornsak P, Sangnim T. Development and in vitro/in vivo evaluation of tamarind seed gum-based oral disintegrating tablets after fabrication by freeze drying. J Drug Deliv Sci Technol 2019;54:101298.
 .
.
 .
.