- *Corresponding Author:
- X. Cao
Department of Hepatobiliary and Pancreatic Surgery, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Pudong, Shanghai 201203, China
E-mail: sgyycxd@163.com
| Date of Received | 24 July 2022 |
| Date of Revision | 10 June 2023 |
| Date of Acceptance | 11 January 2024 |
| Indian J Pharm Sci 2024;86(1):163-169 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the action of loganin on the oncogenic phenotypes of liver cancer cells. Huh7 cells were treated with loganin, or loganin and 20 μmol/l mitogen-activated protein kinase signaling pathway inhibitor SP600125. 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide and Transwell assays detects cell abilities. Western blot detects E-cadherin, N-cadherin, vimentin, phosphorylated-extracellular signal-regulated kinase, extracellular signal-regulated kinase, phosphorylated-p38, and p38 protein expressions. Cell survival rate, the number of migratory and invasive Huh7 cells were significantly reduced with the increasing dose of loganin. E-cadherin content was increased, and contents of N-cadherin, vimentin, phosphorylated-extracellular signal-regulated kinase, and phosphorylated-p38 proteins were decreased in cells with the increasing dose of loganin. SP600125 could enhance the effects mediated by loganin on Huh7 cells. Loganin could significantly restrain the proliferation and metastasis of liver cancer cells by inhibiting the mitogen-activated protein kinase pathway.
Keywords
Loganin, mitogen-activated protein kinase, migration, invasion, epithelial–mesenchymal transition
Ranking as the 6th in incidence rate and the 3rd in mortality rate, liver cancer seriously threats the human health and life[1]. There have been great advances in treatment, such as radiotherapy, chemotherapy, hepatic artery chemoembolization, etc., however, late recurrence and poor 5 y prognosis are unfavorable factors affecting liver cancer treatment[2,3].
Loganin is an iridoid glycoside isolated from Cornus officinalis, and has been identified to possess neuroprotective, anti-apoptotic, antioxidative, immune regulatory and anti-inflammatory properties[4-6]. Modern pharmacological experiments have shown that loganin has significant functions in various diseases. For example, loganin could suppress sepsis-evoked lung damage by inducing M2 macrophage polarization and impairing Nod-Like Receptor Protein 3 (NLRP3) inflammasome[7]. Loganin alleviated the pathologies and cognitive impairment, as well as molecular deficits in Alzheimer’s disease mouse models[8]. Li et al.[9] showed that loganin inhibited ischemia-reperfusion-evoked myocardial injury by reducing pyroptosis and myocardial infarct. Importantly, loganin impeded cell proliferation, cell cycle and induced death in liver cancer[10]. However, the research mechanism of loganin on liver cancer is still unclear. MAPK cascades are significant pathways involving in modulating cell differentiation, survival, stress responses and Epithelial Mesenchymal Transition (EMT)[11,12]. Moreover, the activation of Mitogen-Activated Protein Kinase (MAPK) pathway is tightly linked with liver cancer progression[13]. Moreover, research results have shown that curcumin could inhibit liver cancer cell survival by inhibiting the MAPK pathway[14]. Here, we hypothesized loganin might affect liver cancer tumorigenesis via MAPK pathway.
Hence, this study mainly explored the action of loganin on liver cancer cell growth and metastasis, and then probed whether the MAPK pathway was implicated in the modulatory action of loganin.
Materials and Methods
Cells and reagents:
Huh7 cell lines, Fetal Bovine Serum (FBS), trypsin and Dulbecco’s Modified Eagle Medium (DMEM) and 3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) kit (Sigma- Aldrich, United States of America (USA)); loganin (Shanghai Yuanye Biotechnology Co., Ltd); Transwell and Matrigel (Corning Corporation, USA); Radio-Immunoprecipitation Assay (RIPA) lysis buffer and Bicinchoninic Acid (BCA) reagent kit (Shanghai Yanjing Biotechnology Co., Ltd); E-cadherin, vimentin, N-cadherin, phosphorylated Extracellular Regulated Kinase (pERK), ERK, phosphorylated (p)-p38, p38, and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) antibodies (CST, USA); Horseradish Peroxidase (HRP)- labeled Immunoglobulin G (IgG) antibodies (Santa Cruz, USA); Polyvinylidene Difluoride (PVDF) membrane and Enhanced Chemiluminescence (ECL) detection reagents (Millipore, USA).
Cell culture and treatment:
Huh7 cells were cultured in 10 % FBS contained DMEM with 5 % Carbon dioxide (CO2) at 37°. For loganin treatment, per well was added with 0, 50, 100, 150 μg/ml of loganin and incubated with Huh7 cells for 48 h, namely Negative Control (NC), 50, 100, or 150 μg/ml group. In addition, Huh7 cells were co-treated with 150 μg/ml loganin and 20 μmol/l SP600125 (the inhibitor of MAPK pathway), namely loganin+SP600125 group, the cells treated with 150 μg/ml loganin were used as the control (loganin group).
MTT assay:
After indicated treatment, Huh7 cells were incubated with 20 μl (5 mg/ml) MTT for 4 h in a 96 well plate, followed by adding with 150 μl Dimethyl Sulfoxide (DMSO) into per well for 2 h. Lastly, Optical Density (OD) value at 490 nm was examined.
Transwell assay:
For cell migration experiment, 100 μl Huh7 cell supernatant (1×106/ml) adjusting by complete culture medium without serum were inoculated into the upper chamber of Transwell. 24 h later, migrated cells were observed and counted under an inverted microscope after crystal violet dyeing. For cell invasion experiment, the Matrigel was diluted with a complete culture solution without serum at 1:8, and 60 μl was taken and spread to the bottom of the upper chamber of Transwell. After drying, other steps were consistent with the above migration experiment.
Western blot:
The extracted protein samples were denatured by boiling water, and transferred to a PVDF membrane after separating. Then the antibody diluents were incubated overnight at 4° with membranes for 12 h. After secondary incubation (dilution 1:500) for 2 h at 37°, the protein bands were analyzed by ECL incubation, and processed by ImageJ software.
Statistical analysis:
The data were presented by x͞ ±s. Analysis of Variance (ANOVA) or t-test was adopted for the comparison of multiple groups or two groups. p<0.05 indicated significant difference.
Results and Discussion
MTT assay showed that the survival rate, as well as migratory and invasive abilities of Huh7 cells were decreased after treating with 50, 100 or 150 μg/ml loganin (p<0.05, Table 1).
| Group | Survival rate (%) | Migration | Invasion |
|---|---|---|---|
| NC | 100.55±7.35 | 125.61±9.46 | 112.34±9.27 |
| 50 μg/ml loganin | 83.79±5.12* | 107.78±8.67* | 97.61±8.33* |
| 100 μg/ml loganin | 65.38±6.38* | 90.44±7.32* | 81.92±7.52* |
| 150 μg/ml loganin | 40.56±3.76* | 67.69±4.85* | 58.73±5.61* |
| F | 176.351 | 91.018 | 77.816 |
| p | 0.000 | 0.000 | 0.000 |
Note: Relative to the NC group, *p<0.05
Table 1: Effects of Loganin on Liver Cancer Cells
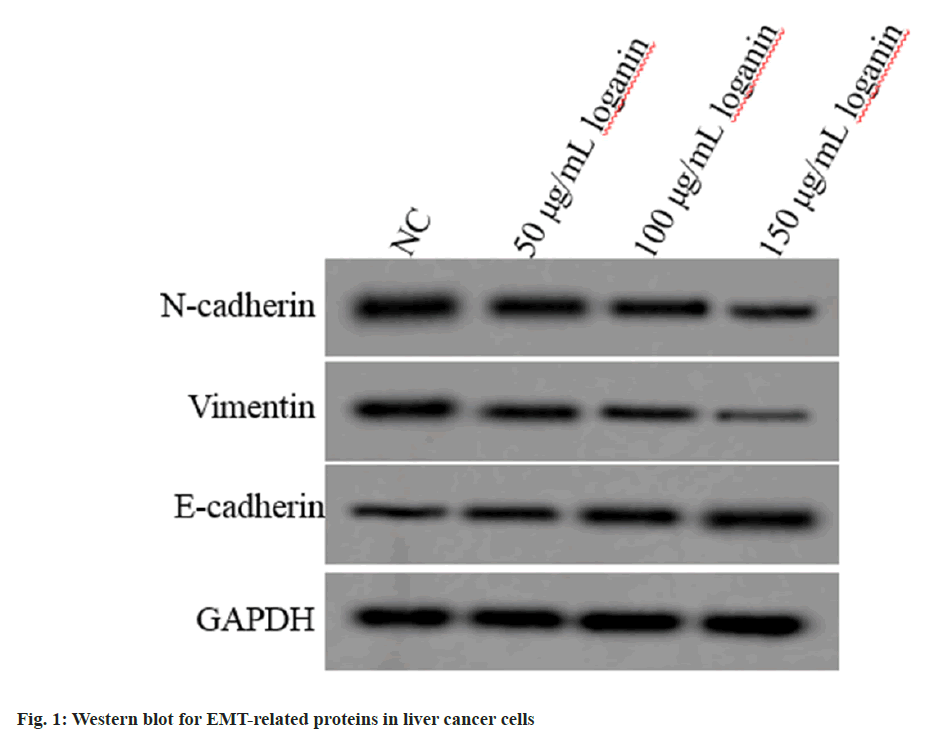
Relative to the NC group, E-cadherin level was increased, while N-cadherin and vimentin levels were declined in Huh7 cells of 50, 100 or 150 μg/ml loganin group (p<0.05, Table 2 and fig. 1), suggesting that loganin suppressed the EMT progression.
| Group | E-cadherin | N-cadherin | Vimentin |
|---|---|---|---|
| NC | 0.23±0.03 | 0.74±0.07 | 0.68±0.06 |
| 50 μg/ml loganin | 0.38±0.04* | 0.59±0.06* | 0.54±0.05* |
| 100 μg/ml loganin | 0.52±0.05* | 0.45±0.05* | 0.41±0.05* |
| 150 μg/ml loganin | 0.68±0.06* | 0.30±0.03* | 0.28±0.02* |
| F | 154.988 | 107.496 | 117.967 |
| p | 0.000 | 0.000 | 0.000 |
Note: Relative to the NC group, *p<0.05
Table 2: Effects of Loganin on the EMT of Liver Cancer Cells
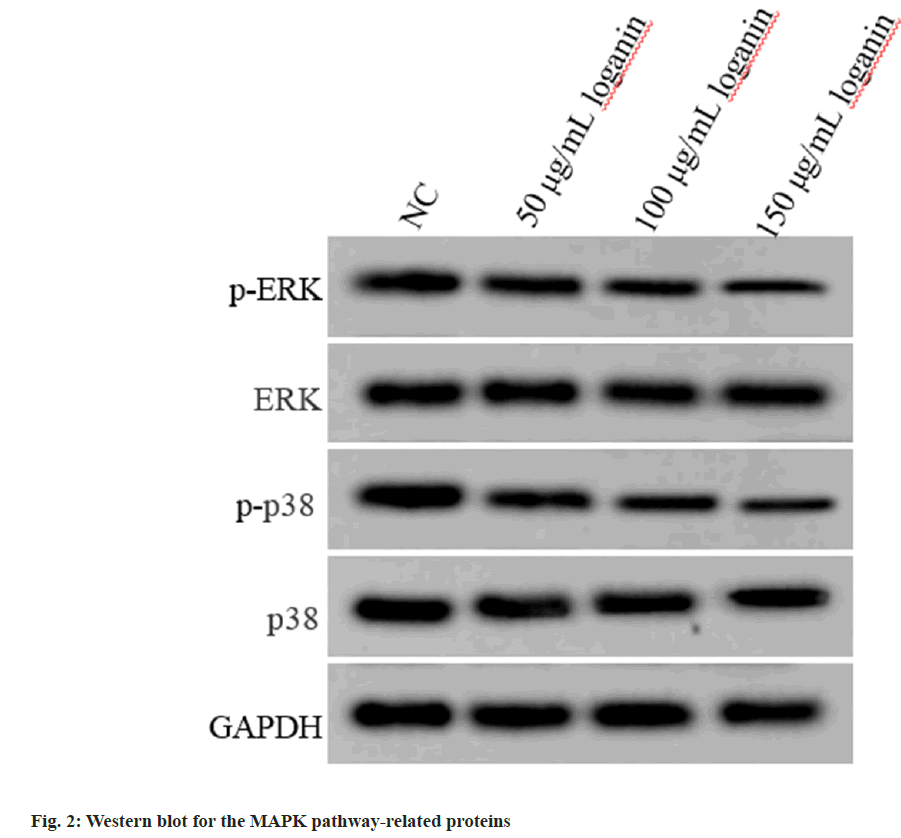
Relative to the NC group, p-ERK and phosphorylated (p)-p38 contents were decreased in Huh7 cells of 50, 100 or 150 μg/ml loganin group, while no changes were observed in ERK and p38 protein levels (p<0.05, Table 3 and fig. 2), suggesting that loganin suppressed the activation of MAPK pathway.
| Group | p-ERK/ERK | p-p38/p38 |
|---|---|---|
| NC | 0.86±0.07 | 0.78±0.05 |
| 50 μg/ml loganin | 0.71±0.06* | 0.63±0.06* |
| 100 μg/ml loganin | 0.56±0.05* | 0.50±0.05* |
| 150 μg/ml loganin | 0.41±0.04* | 0.32±0.03* |
| F | 107.143 | 144.600 |
| p | 0.000 | 0.000 |
Note: Relative to the NC group, *p<0.05
Table 3: Effects of Loganin on the MAPK Pathway of Liver Cancer Cells
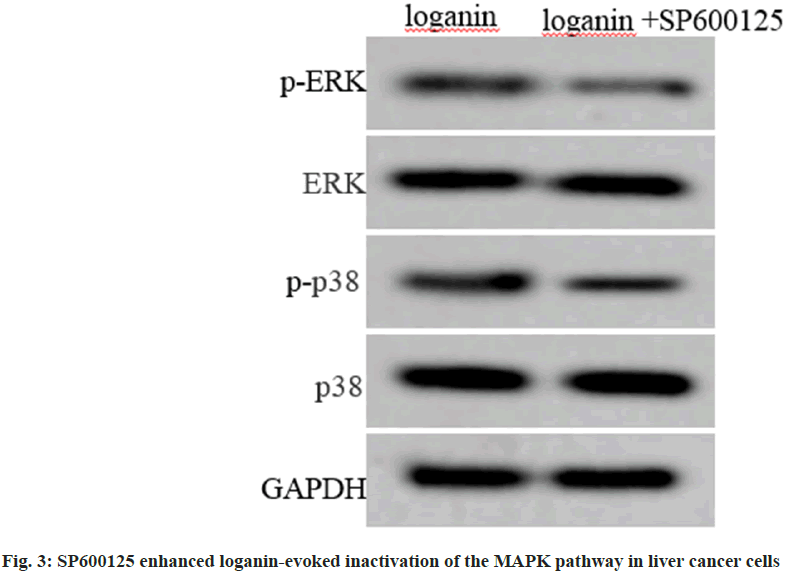
Relative to the loganin group, p-ERK and p-p38 levels were decreased in Huh7 cells of loganin+SP600125 group, and levels of ERK and p38 protein between the two groups had no changes (p<0.05, Table 4 and fig. 3).
| Group | p-ERK/ERK | p-p38/p38 |
|---|---|---|
| Loganin | 0.43±0.04 | 0.35±0.03 |
| Loganin+SP600125 | 0.28±0.04* | 0.22±0.02* |
| t | 7.955 | 10.817 |
| p | 0.000 | 0.000 |
Note: Relative to the loganin group, *p<0.05
Table 4: SP600125 Enhanced Loganin-Evoked Inactivation of the MAPK Pathway in Liver Cancer Cells
The survival rate as well as migrated and invaded Huh7 cells in loganin+SP600125 group relative to the loganin group were markedly decreased (p<0.05, Table 5).
| Group | Survival rate (%) | Migration | Invasion |
|---|---|---|---|
| Loganin | 43.68±4.24 | 68.90±7.21 | 56.14±4.79 |
| Loganin+SP600125 | 30.12±2.85* | 46.35±4.33* | 40.62±3.53* |
| t | 7.963 | 8.044 | 7.825 |
| p | 0.000 | 0.000 | 0.000 |
Note: Relative to the loganin group, *p<0.05
Table 5: Loganin Suppressed Liver Cancer Cell Oncogenic Phenotypes by Blocking MAPK Pathway
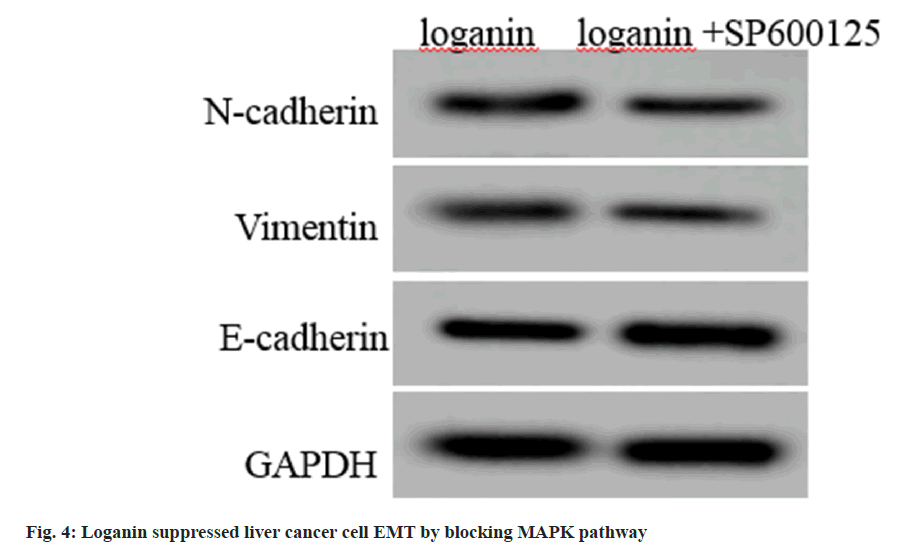
Compared with the loganin group, E-cadherin contents were rose, while contents of N-cadherin and vimentin were declined in Huh7 cells in loganin+SP600125 group (p<0.05, fig. 4 and Table 6).
| Group | E-cadherin | N-cadherin | Vimentin |
|---|---|---|---|
| Loganin | 0.65±0.06 | 0.33±0.04 | 0.30±0.03 |
| Loganin+SP600125 | 0.83±0.06* | 0.19±0.02* | 0.17±0.02* |
| t | 6.364 | 9.391 | 10.817 |
| p | 0.000 | 0.000 | 0.000 |
Note: Relative to the loganin group, *p<0.05
Table 6: Loganin Suppressed Liver Cancer Cell EMT by Blocking Mapk Pathway
The pathogenesis of liver cancer is affected by many factors, such as staying up late for a long time, irregular diet, long-term alcohol and coffee drinking, genetic factors, etc., resulting in a trend of younger liver cancer patients in recent years[15]. Moreover, liver cancer patients at advanced stage show poor prognosis and unsatisfactory survival rate, therefore, it is very important to find new treatment and diagnosis methods for liver cancer[16]. At present, the main treatment methods of Western medicine have achieved certain therapeutic effects in clinical practice. However, there are problems such as high recurrence and metastasis rates and multiple adverse reactions after treatment. Traditional Chinese medicine combined with Western medicine treatment at different stages of liver cancer can achieve the goals of controlling tumor development, improving clinical efficacy, reducing adverse reactions, and preventing recurrence and metastasis. The therapeutic effect is quite good.
Loganin belongs to the iridoid glycoside class. It can enhance non-specific immune function, promote macrophage phagocytosis and delay senescence, it has good anticancer and anti- radiation effects, and is widely used in clinical medicine as a new anticancer drug. It has good anti-inflammatory and antibacterial effects, and is commonly used as a raw material for Chinese medicine. In addition, it also has antitussive, expectorant and other functions. Previous research results have shown that loganin has suppressing effects on cancer progression. For example, loganin declined B-Cell Lymphoma 2 (Bcl-2) level to induce the cell death of colorectal cancer[17]. In gastric cancer, the growth and metastasis of cells were hindered by oganin[18]. Loganin induced cycle arrest and repressed the proliferation in melanoma cells by ERK1/2 pathway[19]. Loganin retrained the cell survival and mobility by blocking Protein Kinase B (AKT)/mammalian Target of Rapamycin (mTOR) pathway in pancreatic cancer[20]. However, the action of loganin on Hepatocellular Carcinoma (HCC) remains unclear. In our study, we discovered that loganin could suppress the proliferative, invasive and migratory capacities of live cancer cells, in addition, it was discovered that loganin reduced N-cadherin and vimentin level, and increased E-cadherin levels in cells, thus suppressing EMT process. In all, loganin retrained liver cancer cell proliferation and metastasis.
MAPKs including ERK1/2, JNK, SAPK, and p38 pathways, are closely related to the research on anti-tumor effects of traditional Chinese medicine[21,22]. For example, tanshinone II could block the p38 signaling pathway to inhibit liver cancer cell movement and growth[23]. Zhao et al.[24] showed that a biflavonoid isolated from Resina Draconis exerted anti-tumor effects by activating the MAPK pathway to repress HCC cell survival and migration[25]. Moreover, aconitine has inhibitory effects on HCC cell mobility and viability, and its mechanism was linked with p38 MAPK pathway. The above data indicates the implication of the MAPK pathway in liver cancer. In the present study, the treatment with different concentrations of loganin could reduce p-ERK and p-p38 proteins, and this reduction was enhanced by SP600125 (the inhibitor of MAPK pathway), indicating that loganin blocked the activation of MAPK pathways. Functionally, SP600125 boosted loganin-induced inhibition of cell proliferative, migratory and invasive capacities and EMT progression in live cancer, implying that loganin could affect liver cancer by MAPK pathway.
In summary, loganin suppressed cell proliferation and metastasis by blocking MAPK pathway. However, there were also shortcomings in this study. Further exploration is needed on the functions of loganin on liver cancer growth and metastasis in vivo in order to provide reasonable theoretical support for the study of loganin.
Author’s contributions:
Jiajun Chen and Yan Qu have contributed equally to this work.
References
- Nayagam S, Chan P, Zhao K, Sicuri E, Wang X, Jia J, et al. Investment case for a comprehensive package of interventions against hepatitis B in China: Applied modeling to help national strategy planning. Clin Infect Dis 2021;72(5):743-52.
[Crossref] [Google Scholar] [PubMed]
- Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ 2020;371:m3544.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Pu X, Jiang L, Huang Y, Yan L, Yang J, et al. Living donor liver transplantation or hepatic resection combined with intraoperative radiofrequency ablation for Child–Pugh A hepatocellular carcinoma patient with Multifocal Tumours Meeting the University of California San Francisco (UCSF) criteria. J Cancer Res Clin Oncol 2021;147(2):607-18.
[Crossref] [Google Scholar] [PubMed]
- Cheng YC, Chu LW, Chen JY, Hsieh SL, Chang YC, Dai ZK, et al. Loganin attenuates high glucose-induced schwann cells pyroptosis by inhibiting ROS generation and NLRP3 inflammasome activation. Cells 2020;9(9):1948.
[Crossref] [Google Scholar] [PubMed]
- Liu S, Shen H, Li J, Gong Y, Bao H, Zhang J, et al. Loganin inhibits macrophage M1 polarization and modulates sirt1/NF-κB signaling pathway to attenuate ulcerative colitis. Bioengineered 2020;11(1):628-39.
[Crossref] [Google Scholar] [PubMed]
- Yao L, Peng SX, Xu YD, Lin SL, Li YH, Liu CJ, et al. Unexpected neuroprotective effects of loganin on 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced neurotoxicity and cell death in zebrafish. J Cell Biochem 2017;118(3):615-28.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Wang C, Wang H, Li X, Xu J, Yu K. Loganin alleviates sepsis-induced acute lung injury by regulating macrophage polarization and inhibiting NLRP3 inflammasome activation. Int Immunopharmacol 2021;95:107529.
[Crossref] [Google Scholar] [PubMed]
- Nie L, He K, Xie F, Xiao S, Li S, Xu J, et al. Loganin substantially ameliorates molecular deficits, pathologies and cognitive impairment in a mouse model of Alzheimer’s disease. Aging (Albany NY) 2021;13(20):23739-56.
[Crossref] [Google Scholar] [PubMed]
- Li W, Fan P, Wang X, Tang H. Loganin alleviates myocardial ischemia–reperfusion injury through GLP-1R/NLRP3-mediated pyroptosis pathway. Environ Toxicol 2023;38(11):2730-40.
[Crossref] [Google Scholar] [PubMed]
- Zhang C, Hu N, Li S. Effect of maggianin on proliferation and apoptosis of hepatocellular carcinoma cells HepG2 and its mechanism. Chin Pharm 2020;31(7):782-8.
- Moon H, Ro SW. MAPK/ERK signaling pathway in hepatocellular carcinoma. Cancers 2021;13(12):3026.
[Crossref] [Google Scholar] [PubMed]
- Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med 2020;19(3):1997-2007.
[Crossref] [Google Scholar] [PubMed]
- Jin C, Chen Z, Shi W, Lian Q. Tropomodulin 3 promotes liver cancer progression by activating the MAPK/ERK signaling pathway. Oncol Rep 2019;41(5):3060-8.
[Crossref] [Google Scholar] [PubMed]
- Li HX, Yang H, Zhang HB. Curcumin induces apoptosis of human hepatocellular carcinoma SMMC-7721 cells through MAPK signaling pathway. Nat Prod Res Dev 2014;26(3):329-34.
- Yang WS, Zeng XF, Liu ZN, Zhao QH, Tan YT, Gao J, et al. Diet and liver cancer risk: A narrative review of epidemiological evidence. Br J Nutr 2020;124(3):330-40.
[Crossref] [Google Scholar] [PubMed]
- George ES, Sood S, Broughton A, Cogan G, Hickey M, Chan WS, et al. The association between diet and hepatocellular carcinoma: A systematic review. Nutrients 2021;13(1):172.
[Crossref] [Google Scholar] [PubMed]
- Hu XH, Wang ML, Chen JC. Effect of maggianin on proliferation of colon cancer SW480 cells and its mechanism. J Guangdong Pharm Univ 2015;31(1):80-3.
- Zhang Y. Effect of margin on epithelial-mesenchymal transformation of gastric cancer cells and its mechanism. Hebei Univ 2020.
- Dang WJ. Effect and mechanism of margin on human malignant melanoma A375 cells. Heilongjiang Univ Chin Med 2016.
- Ge FM, Yang L, Chen ZQ. Study on the antitumor effect of margannin on human pancreatic cancer cells BXPC3 and its mechanism. Chin J Mod Appl Pharm 2022;37(19):2323-7.
- Bigeard J, Hirt H. Nuclear signaling of plant MAPKs. Front Plant Sci 2018;9:469.
[Crossref] [Google Scholar] [PubMed]
- Hong ZD, Mo ZX. 11 signaling pathways in the anti-tumor mechanism of traditional Chinese medicine. Chin J Exp Formula 2018;24(21):205-18.
- Wang Y, Li Q, Fan ZZ. Tanshinone IIA mediates p38/MAPK signal pathway to induce apoptosis of human liver cancer cells. Int J Chin Dig 2009;17(2):124-9.
- Zhao YN, Yang AL, Pang DR. Anti-tumor activity of HIS-4,a biflavonoid from Resina Draconis, on human hepatoma HepG2 and SK-HEP-1 cells. Chin J Tradit Chin Med 2019;44(7):1442-9.
- Xiong HS, Jiang C, Gao R. Effects of aconitine on the growth, invasion and migration of MHCC97 cells in hepatocellular carcinoma. Chin J Immunol 2018;34(5):688-92.