- *Corresponding Author:
- Yuliang Zhang
Department of Orthopedics, Fuyang Orthopedics and Traumatology Affiliated Hospital of Zhejiang University of Traditional Chinese Medicine, Hangzhou, Zhejiang 311400, China
E-mail: zyl63313718@163.com
| Date of Received | 30 April 2022 |
| Date of Revision | 04 February 2023 |
| Date of Acceptance | 29 September 2023 |
| Indian J Pharm Sci 2023;85(5):1452-1457 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to investigate the impact of the luteolin-microRNA-335-3p pathway on the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Cell proliferation was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay and osteogenic markers were assayed by Western blotting. Luteolin treatment demonstrated a dose-dependent promotion of bone marrow mesenchymal stem cells proliferation, accompanied by an increase in osteogenic marker content within the cells. Additionally, luteolin treatment dose-dependently up-regulated microRNA-335-3p levels. The restoration of microRNA-335-3p further augmented bone marrow mesenchymal stem cells proliferation and up-regulated osteogenic differentiation markers. Conversely, knockdown of microRNA-335-3p negated the promoting effects of luteolin on bone marrow mesenchymal stem cells. Luteolin facilitates osteogenic differentiation and proliferation in bone marrow mesenchymal stem cells by elevating microRNA-335-3p. This molecular pathway holds promise as a potential therapeutic target for enhancing bone regeneration and combating bone-related disorders.
Keywords
Luteolin, microRNA-335-3p, bone marrow mesenchymal stem cells, proliferation, osteogenic differentiation
Osteoporosis, featured by declined bone mineral density and increased fracture risk, has become a significant public health concern worldwide. The prevalence of osteoporosis continues to rise with age, leading to considerable morbidity and socioeconomic burden[1]. Therefore, exploring novel therapeutic strategies to enhance bone formation and counteract bone loss has emerged as a critical area of research.
Bone Marrow Mesenchymal Stem Cells (BMSCs) play a pivotal role in maintaining skeletal homeostasis through their ability to differentiate into osteoblasts, the bone-forming cells responsible for bone regeneration and repair[2,3]. Various signaling pathways and regulatory factors tightly govern osteogenic differentiation and proliferation of BMSCs, and understanding these mechanisms holds the key to developing effective therapies for bone-related disorders[4,5].
MicroRNAs (miRNAs) have emerged as essential post-transcriptional regulators, capable of influencing various cellular processes, including osteogenic differentiation and proliferation. Among these, miR-335-3p has recently garnered attention for its regulatory role in bone metabolism. Previous studies have manifested that miR-335-3p negatively modulated osteogenesis by targeting key genes involved in the osteogenic pathway[6-9]. However, the potential modulation of miR-335- 3p to promote osteogenic differentiation and proliferation remains an underexplored aspect of bone biology.
Luteolin, a flavonoid abundantly present in various plant-based sources[10], has exhibited diverse biological properties[11-13]. Recent evidence suggests that luteolin may also have beneficial effects on bone health[14]. However, the molecular mechanisms underlying its osteogenic potential and its interaction with miRNAs in BMSCs remain elusive.
In this context, our research article aims to elucidate the function of luteolin in promoting osteogenic differentiation and proliferation in BMSCs. In addition, we hypothesized that luteolin treatment enhances miR-335-3p content, subsequently influencing the expression of osteogenic genes, thus facilitating osteogenic differentiation and proliferation in BMSCs.
Materials and Methods
Cell culture and treatment:
BM-MSCs were purchased from Saiye (Guangdong, China) and cultured in Dulbecco's Modified Eagle Medium (DMEM) and 10 % Fetal Bovine Serum (FBS), 2 mmol/L glutamine, and 1 % antibiotics in a 5 % Carbon dioxide (CO2) incubator at 37° (all from HyClone, Logan, Utah, United States of America (USA)). Cells passages were collected for functional analysis.
BM-MSCs at logarithmic phase (5.0×105) were treated with increasing doses of luteolin (0, 2, 4 or 8 μmol/l) (Chengdu Master Biotechnology Co. Ltd., Sichuan, China) for 24 h.
Cell transfection:
30 nm miR-335-3p mimic (miR-335-3p), inhibitor (anti-miR-335-3p) or the control (miR-NC or anti-miR-NC) (Gene Pharma, Shanghai, China) were transfected into BM-MSCs for 24 h. After the validation of transfection efficiency, cells were treated with 8 μmol/l luteolin for further investigation.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl-2HTetrazolium Bromide (MTT) assay:
BM-MSCs were inoculated into a 96-well plate for 48 h and 72 h, then per well was added with 10 μl MTT reagent (Solarbio, Beijing, China) and incubated for 4 h. Following 150 μl dimethyl sulfoxide reaction, the absorbance was tested at 490 nm.
Western blot:
Radioimmunoprecipitation Assay (RIPA) protein lysis solution was used to extract total cell proteins. The solution was boiled in a boiling water bath for 5 min, and then separated by 12 % Sodium Dodecyl- Sulfate Polyacrylamide Gel Electrophoresis (SDSPAGE), followed by shifting to the Polyvinylidene Difluoride (PVDF) membrane. Then primary incubation and secondary incubation with corresponding antibodies were performed. The Enhanced Chemiluminescence (ECL) substrate kit was utilized to detect protein signals and ImageJ software was applied to determine the gray values. All primary antibodies included Runt-Related Transcription Factor 2 (RUNX2) (ab76956, 1:1000), Osteocalcin (OCN) (ab93876, 1:1000), Osteopontin (OPN) (ab214050, 1:1000) and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (ab8245, 1:5000) were provided by Abcam (Cambridge, United Kingdom)
Quantitative ReverseTranscription-Polymerase Chain Reaction (qRT-PCR):
The Trizol reagent was applied to incubate with BM-MSCs to extract total Ribonucleic Acid (RNA), which were then synthesized to complementary Deoxyribonucleic Acid (cDNA). Then amplification reaction using SYBR Green PCR Master Mix was conducted (Invitrogen) with U6 as an internal reference. The 2-ΔΔCt method was applied to assess miR-335-3p content. The primers for qRT-PCR, miR-335-3p: Forward 5ʹ-UUUUUCAUUAUUGCUCCUGACC-3ʹ and reverse 5ʹ-CCAGTCTCAGGGTCCGAGGTATTC- 3ʹ; U6: Forward 5ʹ-CTCGCTTCGGCAGCACA-3ʹ and reverse 5ʹ-AACCGCTTCACGAATTTGCGT- 3ʹ.
Statistical analysis:
All experimental data are manifested as the mean±- standard deviation. The comparison was conducted using Analysis of Variance (ANOVA) in multiple groups or t test in two groups. p<0.05 was considered statistically significant difference.
Results and Discussion
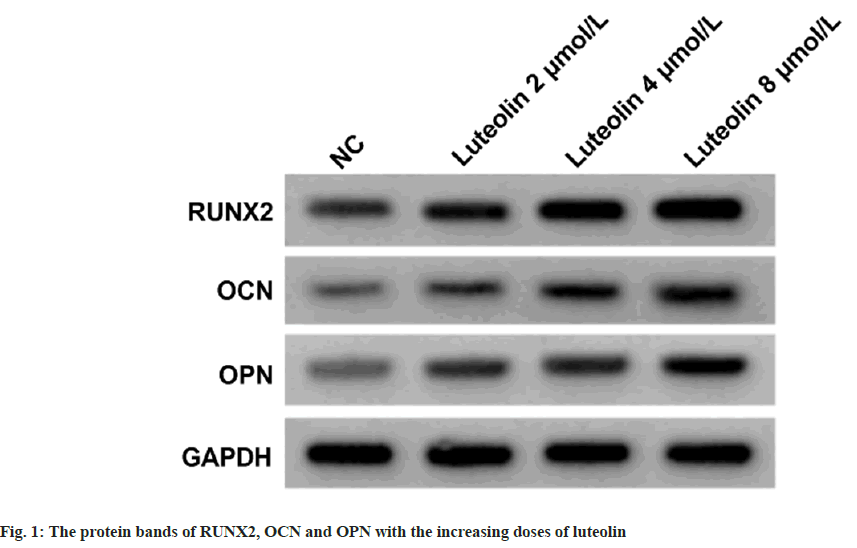
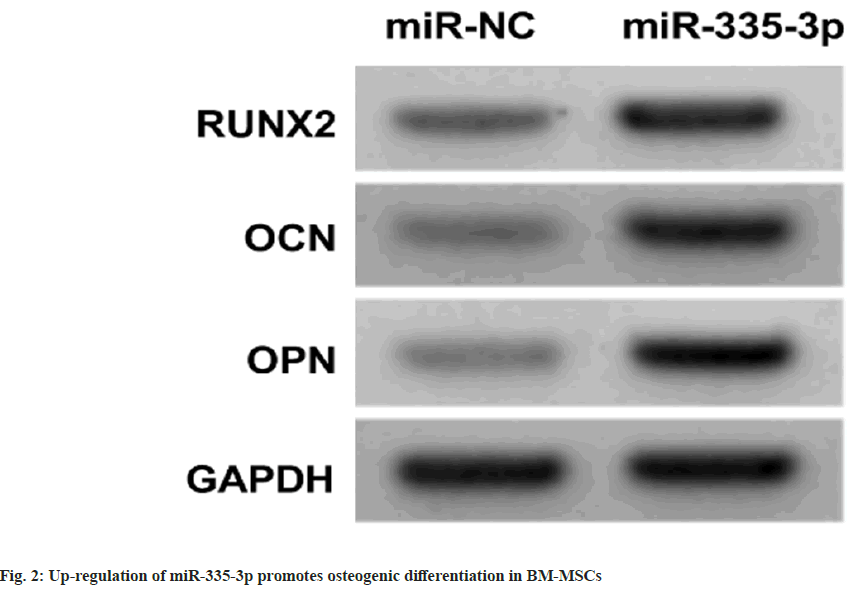
As shown in Table 1, the proliferation of BM-MSCs was dose-dependently increased after treating with 2, 4, or 8 μmol/l luteolin relative to the untreated cells (Negative Control (NC) group). The contents of RUNX2, OCN and OPN proteins in BM-MSCs were dose-dependently elevated with the treatment of increasing doses of luteolin compared with the untreated cells (NC group) as shown in fig. 1 and Table 2. As exhibited in Table 3, the treatment of luteolin increased miR-335-3p expression in BMMSCs at concentrations of 2, 4, or 8 μmol/l luteolin. Compared with the introduction of miR-NC, miR- 335-3p transfection markedly elevated its level in BM-MSCs (Table 4). Thereafter, it was found that miR-335-3p mimic induced proliferation in BMMSCs (Table 4).
| Luteolin | OD (490 nm) | |
|---|---|---|
| 48 h | 72 h | |
| NC | 0.38±0.05 | 0.45±0.04 |
| 2 μmol/l | 0.47±0.05* | 0.52±0.05* |
| 4 μmol/l | 0.68±0.07* | 0.73±0.09* |
| 8 μmol/l | 0.92±0.07* | 1.14±0.10* |
| F | 140.655 | 156.216 |
| p | 0.000 | 0.000 |
Note: Relative to the NC group, *p<0.05
Table 1: The Effects of Luteolin on BM-MSCs Proliferation
| Luteolin | RUNX2 | OCN | OPN |
|---|---|---|---|
| NC | 0.41±0.04 | 0.35±0.03 | 0.25±0.04 |
| 2 μmol/l | 0.53±0.05* | 0.44±0.04* | 0.37±0.04* |
| 4 μmol/l | 0.69±0.04* | 0.58±0.05* | 0.48±0.05* |
| 8 μmol/l | 0.86±0.07* | 0.71±0.03* | 0.65±0.03* |
| F | 129.821 | 152.542 | 157.591 |
| p | 0.000 | 0.000 | 0.000 |
Note: Relative to the NC group, *p<0.05
Table 2: The Effects of Luteolin on BM-MSCs Osteogenic Differentiation
| Luteolin | miR-335-3p |
|---|---|
| NC | 1.00±0.10 |
| 2 μmol/l | 1.59±0.15* |
| 4 μmol/l | 1.84±0.19* |
| 8 μmol/l | 2.45±0.22* |
| F | 111.036 |
| p | 0.000 |
Note: Relative to the NC group, *p<0.05
Table 3: The Effects of Luteolin On miR-335-3p Expression in BM-MSCs
| Group | miR-335-3p | OD (490 nm) | |
|---|---|---|---|
| 48 h | 72 h | ||
| miR-NC | 0.99±0.08 | 0.32±0.05 | 0.41±0.04 |
| miR-335-3p | 2.88±0.32* | 0.68±0.04* | 0.92±0.07* |
| t | 17.19 | 16.687 | 18.977 |
| p | 0.000 | 0.000 | 0.000 |
Note: Relative to the NC group, *p<0.05
Table 4: The Effects of miR-335-3p on BM-MSCs Proliferation
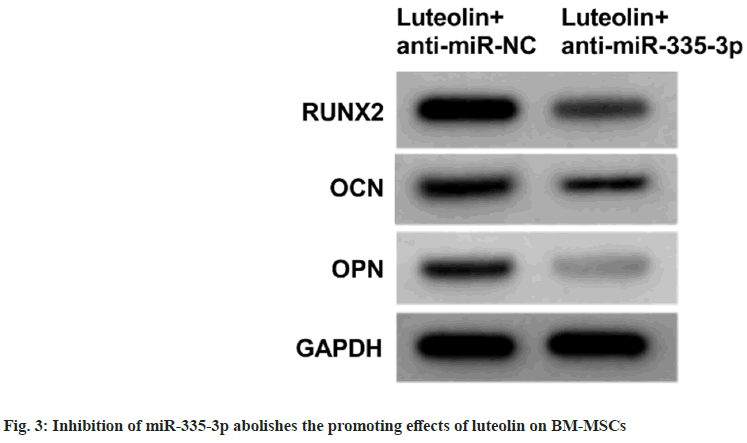
miR-335-3p mimic introduction in BM-MSCs relative to miR-NC markedly elevated the contents of RUNX2, OCN and OPN proteins as shown in fig.2 and Table 5. Luteolin treatment in BMMSCs increased the proliferation and contents of RUNX2, OCN and OPN proteins, while these effects were abolished after the inhibition of miR- 335-3p as shown in fig. 3 and Table 6.
| Group | RUNX2 | OCN | OPN |
|---|---|---|---|
| miR-NC | 0.37±0.02 | 0.32±0.03 | 0.22±0.04 |
| miR-335-3p | 0.54±0.05* | 0.49±0.07* | 0.45±0.05* |
| t | 9.47 | 6.697 | 10.776 |
| p | 0.000 | 0.000 | 0.000 |
Note: Relative to the NC group, *p<0.05
Table 5: miR-335-3p Effects on Osteogenic Differentiation in BM-MSCs
| Group | miR-335-3p | OD (490 nm) | RUNX2 | OCN | OPN | |
|---|---|---|---|---|---|---|
| 48 h | 72 h | |||||
| Luteolin+anti-miR-NC | 1.02±0.09 | 0.89±0.06 | 1.16±0.12 | 0.84±0.07 | 0.73±0.06 | 0.64±0.07 |
| Luteolin+anti-miR-335-3p | 0.32±0.05* | 0.62±0.04* | 0.93±0.05* | 0.62±0.05* | 0.58±0.04* | 0.47±0.03* |
| t | 20.397 | 11.233 | 5.308 | 7.672 | 6.24 | 6.697 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Relative to luteolin+anti-miR-NC group, *p<0.05
Table 6: miR-335-3p Deficiency Abolishes the Promoting Effects of Luteolin on BM-MSCs
Osteoporosis, in severe cases, it can cause fractures in patients, which can have adverse effects on their daily lives[15]. BM-MSCs are crucial for maintaining bone resorption and bone formation balance, and are closely related to the pathogenesis of osteoporosis[16]. Luteolin is widely used in medicine[17,18]. Luteolin has been manifested that can impact osteogenic differentiation[19]. Luteolin was able to protect against high glucose-evoked oxidative injury in osteoblasts[20]. According to the findings of Nash et al., the luteolin isolated from tea up-regulated the content of the mineral in human osteoblasts[21]. All the data suggested the possible suppressing effects of luteolin on osteoporosis. In our work, we found that the proliferation of BM-MSCs was dose-dependently increased after treating with 2, 4, or 8 μmol/l luteolin. Moreover, the contents of osteogenic markers RUNX2, OCN and OPN were dose-dependently elevated with the treatment of increasing doses of luteolin in BMMSCs. RUNX2 is essential for bone development and osteodifferentiation, and is involved in encouraging the expression of osteoblast secretion proteins OPN and OCN[22-24]. Therefore, we confirmed that luteolin promoted osteogenic differentiation in BM-MSCs.
In the present work, we also found luteolin increased miR-335-3p levels in BM-MSCs. miRNAs are widely discovered in eukaryotic cells, and can regulate signal transduction pathways and gene expression, thereby involving in development of various cells including BM-MSCs[25-27]. A study showed forced expression of miR-335- 5p promoted osteogenic differentiation in BMMSCs[ 28], which was consistent with our findings with the increased RUNX2, OPN and OCN protein levels after miR-335-5p restoration. Besides, miR- 335-5p restoration triggered proliferation in BMMSCs. In the meanwhile, we also found that miR- 335-5p deficiency abolished the action of luteolin on BM-MSCs.
In all, luteolin accelerates the osteogenic differentiation and proliferation in BM-MSCs through elevation miR-335-5p, bringing to light the possible mechanisms implicated in the function of luteolin in osteoporosis treatment.
Funding:
This work was supported by Zhejiang Traditional Chinese Medicine science and technology plan project(NO.2020ZQ047).
Conflict of interests:
The authors declared no conflict of interests.
References
- Aspray TJ, Hill TR. Osteoporosis and the ageing skeleton. Subcell Biochem 2019;91:453-76.
[Crossref] [Google Scholar] [PubMed]
- Sleeman A, Clements JN. Abaloparatide: A new pharmacological option for osteoporosis. Am J Health-System Pharm 2019;76(3):130-5.
[Crossref] [Google Scholar] [PubMed]
- Zhou W, Lin J, Zhao K, Jin K, He Q, Hu Y, et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med 2019;47(7):1722-33.
[Crossref] [Google Scholar] [PubMed]
- Lin H, Sohn J, Shen H, Langhans MT, Tuan RS. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019;203:96-110.
[Crossref] [Google Scholar] [PubMed]
- Arthur A, Gronthos S. Clinical application of bone marrow mesenchymal stem/stromal cells to repair skeletal tissue. Int J Mol Sci 2020;21(24):9759.
[Crossref] [Google Scholar] [PubMed]
- Lin YP, Liao LM, Liu QH, Ni Y, Zhong Y, Yu S. miRNA-128-3p induces osteogenic differentiation of bone marrow mesenchymal stem cells via activating the Wnt3a signaling. Eur Rev Med Pharmacol Sci 202;25(3).
[Crossref] [Google Scholar] [PubMed]
- Yang Q, Zhou Y, Wang T, Cai P, Fu W, Wang J, et al. miRNA‐1271‐5p regulates osteogenic differentiation of human bone marrow‐derived mesenchymal stem cells by targeting forkhead box O1 (FOXO1). Cell Biol Int 2021;45(7):1468-76.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Liu S, Li J, Zhao S, Yi Z. Roles for miRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells. Stem Cell Res Ther 2019;10(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Avendaño-Félix M, Fuentes-Mera L, Ramos-Payan R, Aguilar-Medina M, Pérez-Silos V, Moncada-Saucedo N, et al. A novel osteomirs expression signature for osteoblast differentiation of human amniotic membrane-derived mesenchymal stem cells. Biomed Res Int 2019;2019:8987268.
[Crossref] [Google Scholar] [PubMed]
- Wu G, Li J, Yue J, Zhang S, Yunusi K. Liposome encapsulated luteolin showed enhanced antitumor efficacy to colorectal carcinoma. Mol Med Rep 2018;17(2):2456-64.
[Crossref] [Google Scholar] [PubMed]
- Nabavi SF, Braidy N, Gortzi O, Sobarzo-Sanchez E, Daglia M, Skalicka-Woźniak K, et al. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res Bull 2015;119:1.
[Crossref] [Google Scholar] [PubMed]
- Wang JS, He Y, Zhang WJ, Zhang P, Huang QL, Hua ZC. Advances in studies on pharmacological effects of luteolin. Chin Bull Life Sci 2013;25(6):560-5.
- Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed Pharmacother 2019;112:108612.
[Crossref] [Google Scholar] [PubMed]
- Jing Z, Wang C, Yang Q, Wei X, Jin Y, Meng Q, et al. Luteolin attenuates glucocorticoid‐induced osteoporosis by regulating ERK/Lrp‐5/GSK‐3β signaling pathway in vivo and in vitro. J Cell Physiol 2019;234(4):4472-90.
[Crossref] [Google Scholar] [PubMed]
- Johnston CB, Dagar M. Osteoporosis in older adults. Med Clin 2020;104(5):873-84.
[Crossref] [Google Scholar] [PubMed]
- Berthelot JM, Le Goff B, Maugars Y. Bone marrow mesenchymal stem cells in rheumatoid arthritis, spondyloarthritis and ankylosing spondylitis: Problems rather than solutions? Arthritis Res Ther 2019;21:239.
[Crossref] [Google Scholar] [PubMed]
- Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets 2008;8(7):634-46.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Zeng M, Wang Z, Qin F, Chen J, He Z. Dietary luteolin: A narrative review focusing on its pharmacokinetic propertyes and effects on glycolipid metabolism. J Agric Food Chem 2021;69(5):1441-54.
[Crossref] [Google Scholar] [PubMed]
- Kwon SM, Kim S, Song NJ, Chang SH, Hwang YJ, Yang DK, et al. Antiadipogenic and proosteogenic effects of luteolin, a major dietary flavone, are mediated by the induction of DnaJ (Hsp40) Homolog, Subfamily B, Member 1. J Nutr Biochem 2016;30:24-32.
[Crossref] [Google Scholar] [PubMed]
- Khosravi A, Aidy A, Shafiei M. Biphasic response to luteolin in MG-63 osteoblast-like cells under high glucose-induced oxidative stress. Iranian J Med Sci 2016;41(2):118-25.
[Google Scholar] [PubMed]
- Liu L, Peng Z, Huang H, Xu Z, Wei X. Luteolin and apigenin activate the Oct‐4/Sox2 signal via NFATc1 in human periodontal ligament cells. Cell Biol Int 2016;40(10):1094-106.
[Crossref] [Google Scholar] [PubMed]
- Park KR, Kim S, Cho M, Yun HM. Limonoid triterpene, obacunone increases runt-related transcription factor 2 to promote osteoblast differentiation and function. Int J Mol Sci 2021;22(5):2483.
[Crossref] [Google Scholar] [PubMed]
- Vimalraj S, Arumugam B, Miranda PJ, Selvamurugan N. Runx2: Structure, function and phosphorylation in osteoblast differentiation. Int J Biol Macromol 2015;78:202-8.
[Crossref] [Google Scholar] [PubMed]
- Komori T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol 2018;149(4):313-23.
[Crossref] [Google Scholar] [PubMed]
- Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signaling 2014;7(332):ra63.
[Crossref] [Google Scholar] [PubMed]
- Rui J, Fang S, Wang Y, Lv B, Yu B, Li S. Inhibition of microRNA-16 protects mesenchymal stem cells against apoptosis. Mol Med Rep 2018;18(1):902-10.
[Crossref] [Google Scholar] [PubMed]
- Lin Z, He H, Wang M, Liang J. microRNA‐130a controls bone marrow mesenchymal stem cell differentiation towards the osteoblastic and adipogenic fate. Cell Proliferation 2019;52(6):e12688.
[Crossref] [Google Scholar] [PubMed]
- Huang Z, Cai Z, Qian J, Wang J, Hu N. Effect of micro RNA-335-5p regulating bone morphogenetic protein 2 on osteogenic differentiation of human bone marrow mesenchymal stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2020;34(6):781-6.
[Google Scholar] [PubMed]