- *Corresponding Author:
- Donghui Liang
Zhujiang Hospital, Southern Medical University, Guangzhou, 510280, China
E-mail: dhliang79@smu.edu.cn
| Date of Received | 07 May 2023 |
| Date of Revision | 14 December 2023 |
| Date of Acceptance | 13 August 2024 |
| Indian J Pharm Sci 2024;86(4):1363-1374 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Shenxiong-Xinmaikang decoction has good clinical efficacy in the treatment of stable angina pectoris, but its active compounds and mechanism of action remain unclear. The aim of the study was to explore the potential mechanisms of Shenxiong-Xinmaikang decoction on stable angina pectoris. Shenxiong-Xinmaikang decoction components were obtained from the traditional Chinese medicine systems pharmacology, encyclopedia of traditional Chinese medicine, and SymMap databases, and potential targets were obtained from the Swiss TargetPrediction database. Stable angina pectoris targets were predicted using GeneCards, Online Mendelian Inheritance in Man, and DrugBank databases. The overlapping targets and their network were obtained; subsequently, the 'drugs-active ingredients-targets-disease' network, protein-protein interaction networks, and hub genes network were constructed using Cytoscape. Characterization of biological functions and pathways of potential targets through gene oncology and kyoto encyclopedia of genes and genomes enrichment. Molecular docking was performed using AutoDock 4.2.6 and PyMOL 2.4.0 software. 97 compounds, 829 corresponding targets, 728 stable angina pectoris-related targets, and 183 overlapping targets were identified. The network analysis revealed 9 key components and 14 key targets. Simultaneously, molecular docking analysis confirmed that these compounds had a good affinity with the targets. Gene oncology enrichment analysis showed that most of the common targets were mainly involved in the biological process of mitogen-activated protein kinase cascade regulation and cell response to organic nitrogen compounds, involving the membrane raft, the membrane microdomain and the caveola, and correlated with molecular functions such as related kinase activity and kinase binding. Kyoto encyclopedia of genes and genomes enrichment analysis showed that the overlapping targets were primarily involved in phosphatidylinositol 3-kinase/protein kinase B signaling and mitogen-activated protein kinase signaling. Our study mainly found that Shenxiong-Xinmaikang decoction might treat stable angina pectoris from the perspective of inflammation and lipid regulation, providing a theoretical basis for drug development.
Keywords
Stable angina pectoris, network pharmacology, molecular docking, coronary artery disease, traditional Chinese medicine
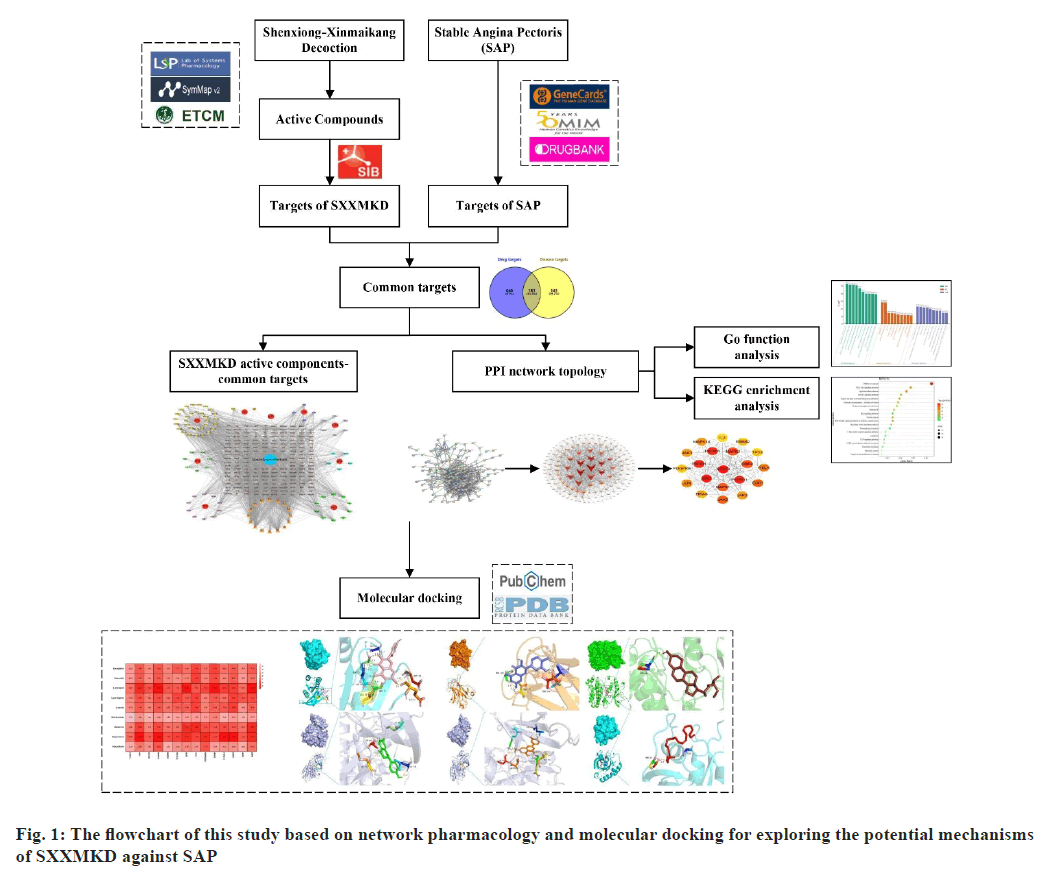
Stable Angina Pectoris (SAP) is part of Coronary Artery Disease (CAD) and is a clinical syndrome characterized by chest pain caused by transient myocardial ischemia[1]. Medication is one of the main treatments for SAP. Western medicine usually uses nitrates, antithrombotic drugs, lipid lowering drugs and renin-angiotensin system inhibitors to relieve the symptoms of SAP[2]. However, most of these drugs only work on a single target and their long-term use has certain side effects, such as impaired liver function[3], reduced platelet counts and increased risk of bleeding[4]. In recent years, many studies have shown that Traditional Chinese Medicine (TCM) is effective in preventing and treating heart diseases such as SAP, with few side effects[5,6]. Shenxiong-Xinmaikang Decoction (SXXMKD) is an empirical formula proposed by our team for the treatment of SAP, consisting of 9 herbs including Dan-Shen (DS), Huang-Qi (HQ), Ge-Gen (GG), San-Qi (SQ), Chuan- Xiong (CX), Mu-Xiang (MX), Tai-Zi-Shen (TZS), Shan-Zha (SZ) and Hong-Jing-Tian (HJT). It has the function of replenishing qi (Yi Qi), invigorating the spleen (Jian Pi), activating blood circulation (Huo Xue), and removing blood congestion to relieve pain (Hua Yu Zhi Tong). Several domestic and international studies have shown that the single drug or single compound in this decoction improves coronary circulation, lowers blood lipids and prevents hypertension. SXXMKD has good clinical efficacy, but the main active ingredients and mechanism of action for the treatment of SAP are not yet known. In addition, as a herbal compound consists of several herbs, it will have a better therapeutic effect than a single drug, which inevitably has more complex mechanisms of action. Network pharmacology is based on the “drug component-target-disease” interaction network to study drugs, their targets and pharmacological activities, and analyses the mechanism of action of drugs from a systematic and comprehensive perspective[7]. To date, several studies have used network pharmacology to explore the complex mechanisms of traditional Chinese medicine compounds in the treatment of SAP and other related diseases, yielding satisfactory results[8-10]. This study aims to explore the active ingredients, key targets and specific mechanisms of SXXMKD for the treatment of SAP through network pharmacology and molecular docking techniques, thereby providing theoretical support for the treatment of SAP and opening up new perspectives for the treatment of SAP (fig. 1).
Materials and Methods
Active compounds and targets of SXXMKD:
The active compounds of SXXMKD were acquired from the TCM Systems Pharmacology (TCMSP) (http://tcmspw.com/tcmsp.php)[11], the Encyclopedia of TCM (ETCM) (http://www.tcmip.cn/ETCM/index.php)[12], and the SymMap database (http://www.symmap.org)[13]. Filters for retrieving pharmacokinetic data were set to Oral Bioavailability (OB) ≥30 % and Drug Similarity (DL) ≥0.18 %. After that, we used the PubChem database (https://pubchem.ncbi.nlm.nih.gov)[14] to obtain the normalized name, PubChem Compound Identifier (CID), and the Structure-Data File (SDF) structure format of the 2D structure of the active compounds. The targets were predicted by the Swiss TargetPrediction database (http://www.swisstargetprediction.ch/)[15], and the associated targets belonging to “Homo sapiens” and “probability >0.1” were selected.
Targets of SAP and getting the common targets:
According to databases of GeneCards (https://www.genecards.org/)[16], Online Mendelian Inheritance in Man (OMIM) (https://www.omim.org)[17] and DrugBank database (https://www.drugbank.com/)[18], stable angina, stable angina pectoris and chronic stable angina pectoris were used as the keywords to search for the SAP-related targets. Targets from the GeneCards database were selected based on a score ≥10, and all the targets from the OMIM and DrugBank databases were included. Targets were normalised to the official gene symbols using the UniProt database (https://www.uniprot.org/)[19] with the species restricted to Homo sapiens. After removing the duplicate targets, the SAP-related targets were collected. Finally, the acquired targets of SXXMKD and SAP were imported to draw a Venn diagram using Venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/). The overlapping targets were obtained as the potential targets of SXXMKD in the treatment of SAP.
Network construction:
The drugs-component-targets-disease network diagram was constructed using the Cytoscape 3.8.0 software. And the overlapping targets were selected and imported into the Search Tool for the Retrieval of Interacting Genes (STRING) database (https://string-db.org)[20] for the Protein- Protein Interaction (PPI) information. The species were restricted to “Homo sapiens”. The data with a confidence score ≥0.9 and hide disconnected nodes in the network. The results were imported into Cytoscape 3.8.2 to visualize and construct a PPI network.
The network analyzer tool was used for topology analysis[21], and we selected the first 20 genes according to the degree value. The CytoHubba plugin was then used to analyse the PPI network to obtain the network topology parameters[22]. And the top 20 genes generated by the Maximum Neighbourhood Component (MNC) method were considered as hub genes. Finally, we integrated the key genes using the above two methods and deleted the repeated genes.
Gene Ontology (GO) functional enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis:
The overlapping targets were imported into the Metascape (https://metascape.org/gp/index.html#/main/step3)[23] for bioinformation enrichment analysis, including Biological Process (BP), Cellular Component (CC), Molecular Function (MF), and KEGG pathway enrichment analysis. The GO and KEGG pathway terms with a p value <0.05 were considered to be significant. A histogram was constructed with the first 10 functional categories of BP, CC, and MF. The top 20 signal pathways were plotted as a bubble diagram.
Molecular docking:
The 2D structure of the molecular ligands were downloaded from the PubChem database and the 3D structures obtained from Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) (https://www.rcsb.org/)[24]. The receptor protein was dehydrated using PyMOL 2.4.0 software, and protein hydrogenation and charge calculations were performed using AutoDock tools. We used Open Babel 3.1.1 to convert any file formats that need to be changed[25]. Molecular docking was then performed, the docking scores were collected and and AutoDock was used to construct the docking area using grid box. While the affinity of -7.0 kcal/mol demonstrated a strong binding force, the affinity of -5.0 kcal/mol demonstrated good binding activity[26].
Results and Discussion
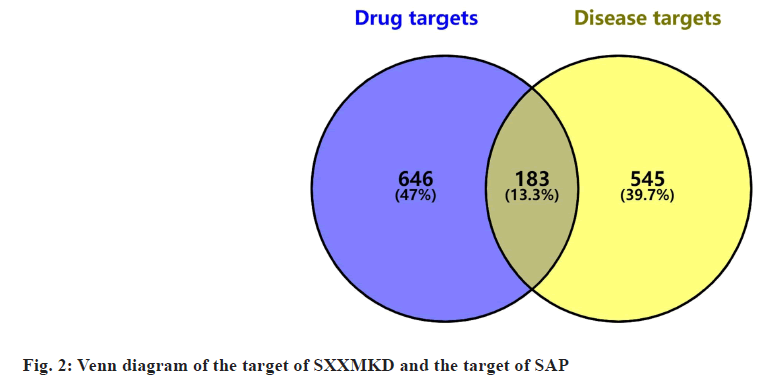
From the TCMSP, ETCM, and SymMap databases, 42 compounds were identified in DS, 22 in HQ, 16 in GG, 13 in SQ, 10 in CX, 11 in MX, 9 in TZS, 3 in SZ and 1 in HJT in SXXMKD. After duplicates were eliminated, 97 active ingredients were retrieved. According to targeted screening, there were 1843 targets in DS, 1194 in HQ, 659 in GG, 834 in SQ, 648 in CX, 379 in MX, 436 in TZS, 187 in SZ and 38 in HJT. After merging and removing duplicates, 829 target genes corresponding to the 97 active ingredients in SXXMKD were finally identified. Disease target searches in GeneCards, OMIM and DrugBank databases yielded 3812 SAP-related targets, of which 728 were retained after removing duplicates. A Venn diagram was then plotted and 183 SXXMKD-SAP common targets were obtained (fig. 2).
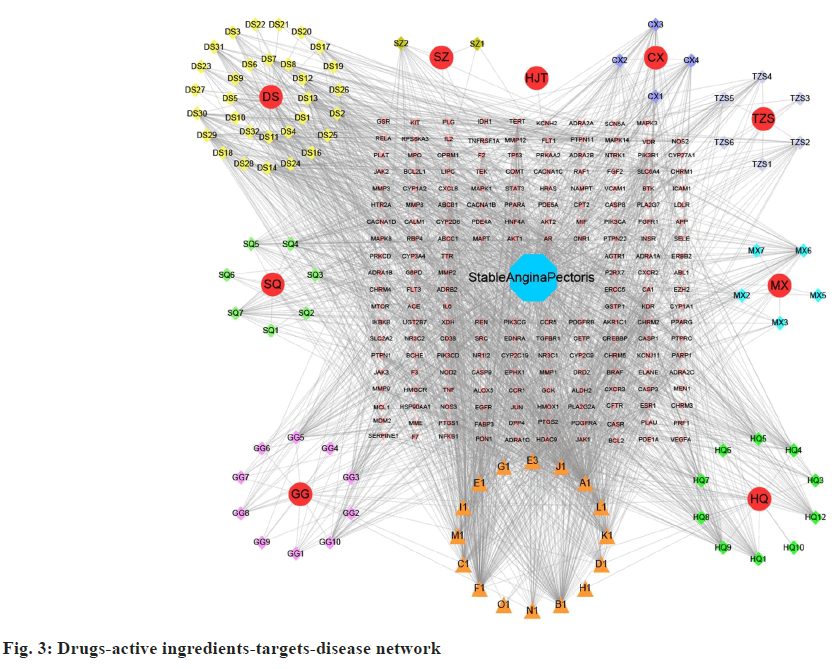
6 unrelated components were removed because the target of some of them did not overlap with the disease target. The network diagram "drugs- active ingredients-targets-disease" consisted of 284 nodes, 2092 edges, 91 active ingredients and 183 potential targets (fig. 3).
The 9 red circles in fig. 3, represent herbs (DS; GG; SQ, SQ; CX; MX; TZS; SZ; HJT). Different colors of diamonds represent different active compounds. Of these, 16 active ingredients were found in more than two drugs (indicated by orange triangles). (“A1” refers to the common active ingredient in HJT, TZS, HQ, and DS; “B1” represents in TZS, CX, SQ, DS, GG, and MX; “C1” refers to the common active ingredient in TZS and DS; “D1” refers to the one in HQ and GG; “E1” refers to the one in HQ and CX; “E3” refers to the one in HQ and CX; “F1” refers to the one in HQ, SQ, DS, and SZ; “G1” refers to the one in HQ and MX; “H1” refers to the one in HQ and DS; “I1” refers to the one in HQ, CX, DS, and GG; “J1” represents in HQ, DS, and GG; “K1” refers to the one in CX and SQ; “L1” refers to the one in SQ and GG; “M1” refers to the one in SQ, DS, and MX; “N” represents SQ, DS, and GG; “O1” refers to the one in DS, and MX). Pink arrows refers to the one core targets, and the blue octagon represents disease.
According to the calculated degree value and previous literature, kaempferol, quercetin, beta- sitosterol, liquiritigenin, luteolin, ethyl linoleate, berberine, stigmasterol and paeoniflorin were selected as the core compounds. Specific information is given in Table 1.
| Mark | Compound | OB (%) | DL | Composition | PubChem CID | CAS. no | Structure |
|---|---|---|---|---|---|---|---|
| A1 | Kaempferol | 41.88 | 0.24 | C15H10O6 | 5280863 | 520-18-3 | 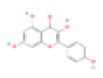 |
| F1 | Quercetin | 46.43 | 0.28 | C15H10O7 | 5280343 | 117-39-5 | 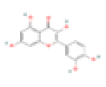 |
| B1 | Beta-sitosterol | 33.94 | 0.7 | C22H22O8 | 222284 | 518-29-6 | 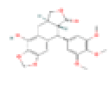 |
| L1 | Liquiritigenin | 32.76 | 0.18 | C15H12O4 | 114829 | 578-86-9 | 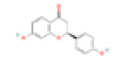 |
| C1 | Luteolin | 36.16 | 0.25 | C15H10O6 | 5280445 | 491-70-3 | 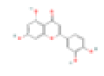 |
| K1 | Ethyl linoleate | 42 | 0.19 | C20H36O2 | 5282184 | 544-35-4 | 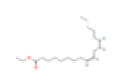 |
| J1 | Berberine | 36.86 | 0.78 | C20H18NO4+ | 2353 | 2086-83-1 | 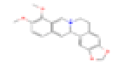 |
| M1 | Stigmasterol | 43.83 | 0.76 | C29H48O | 5280794 | 83-48-7 | 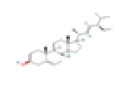 |
| E3 | Paeoniflorin | 53.87 | 0.79 | C23H28O11 | 442534 | 23180-57-6 | 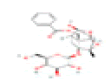 |
Table 1: Chemical Information for the Active Compounds of SAP
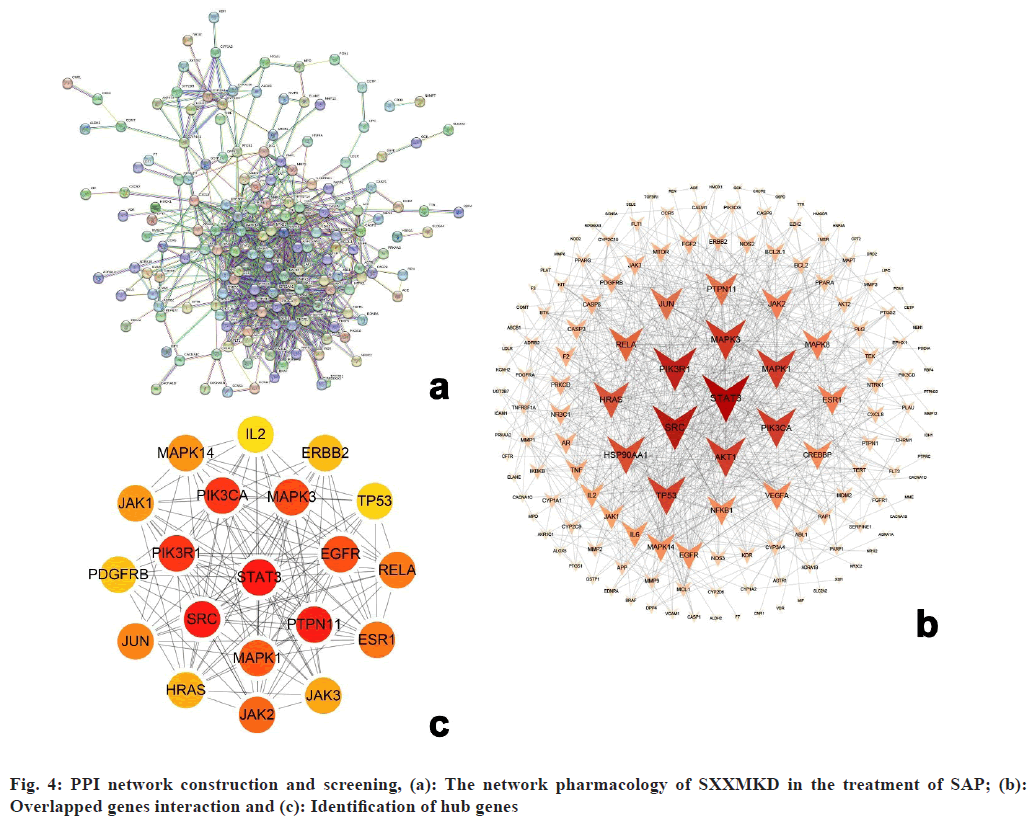
The PPI network consisted of 183 nodes and 742 edges with an average node degree value of 8.11 (fig. 4a). The top 20 genes were selected based on the value of the interaction relationship between the targets (fig. 4b). Meanwhile, a network of 20 hub genes in highly connected dense regions[27] was screened using cytoHubba (fig. 4c).
Finally, after integrating the key genes using the above two methods and deleting the repetitive genes, 14 key targets, including Signal Transducer and Activator of Transcription 3 (STAT3), SRC, Tyrosine-Protein Phosphatase Non-receptor type 11 (PTPN11), Phosphoinositide-3-Kinase Regulatory subunit 1 (PIK3R1), Phosphatidylinositol-4,5- bisphosphate 3-Kinase Catalytic subunit Alpha (PIK3CA), Mitogen-Activated Protein Kinase 3 (MAPK3), Epidermal Growth Factor Receptor (EGFR), MAPK1, Janus Kinase 2 (JAK2), Protein kinase B (AKT1), Heat Shock Protein 90 Alpha family class A member 1 (HSP90AA1), HRAS, Estrogen Receptor 1 (ESR1) and TP53 were obtained. More details can be found in Table 2.
| Gene symbol | Description | PDB ID | Uniprot ID | Degree | Betweenness centrality | Closeness centrality |
|---|---|---|---|---|---|---|
| STAT3 | Signal transducer and activator of transcription 3 | 6NJS | P40763 | 45 | 0.110236425 | 0.473354232 |
| SRC | Proto-oncogene tyrosine-protein kinase Src | 2SRC | P12931 | 42 | 0.107606621 | 0.473354232 |
| PIK3R1 | Phosphatidylinositol 3-kinase regulatory subunit alpha | 3I5R | P27986 | 40 | 0.028435003 | 0.421787709 |
| MAPK3 | Mitogen-activated protein kinase 3 | 4QTB | P27361 | 37 | 0.039472609 | 0.453453453 |
| MAPK1 | Mitogen-activated protein kinase 1 | 8AOJ | P28482 | 36 | 0.038521014 | 0.452095808 |
| PIK3CA | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform | 7JIU | P42336 | 36 | 0.020157343 | 0.42776204 |
| AKT1 | RAC-alpha serine/threonine-protein kinase | 4GV1 | P31749 | 36 | 0.036250043 | 0.448071217 |
| TP53 | Cellular tumor antigen p53 | 6GGC | P04637 | 35 | 0.143221008 | 0.457575758 |
| HSP90AA1 | Heat shock protein HSP 90-alpha | 6GR5 | P07900 | 34 | 0.066983219 | 0.442815249 |
| HRAS | GTPase HRas | 2RGE | P01112 | 32 | 0.026760351 | 0.431428571 |
| PTPN11 | Tyrosine-protein phosphatase non-receptor type 11 | 5DF6 | Q06124 | 28 | 0.008836305 | 0.405913978 |
| EGFR | Epidermal growth factor receptor | 5UG8 | P00533 | 23 | 0.022816527 | 0.419444444 |
| JAK2 | Tyrosine-protein kinase JAK2 | 6E2P | O60674 | 26 | 0.031387563 | 0.405913978 |
| ESR1 | Estrogen receptor | 6CHZ | P03372 | 25 | 0.035343025 | 0.436416185 |
Table 2: Detailed Information of 14 Target Genes
After GO and KEGG enrichment analysis of the 183 selected proteins, BP analysis yielded 5736, CC yielded 483, and MF yielded 1037. Histograms of the GO terms provided the top ten significant terms (fig. 5a). Meanwhile, the results of the KEGG analysis showed 280 pathways with a threshold of p<0.05 and bubble plots drawn from the top 20 pathways (fig. 5b). The KEGG pathway of SXXMKD on SAP was mainly associated with cancer, PI3K-AKT signaling pathway, lipid and atherosclerosis and MAPK signaling pathway.
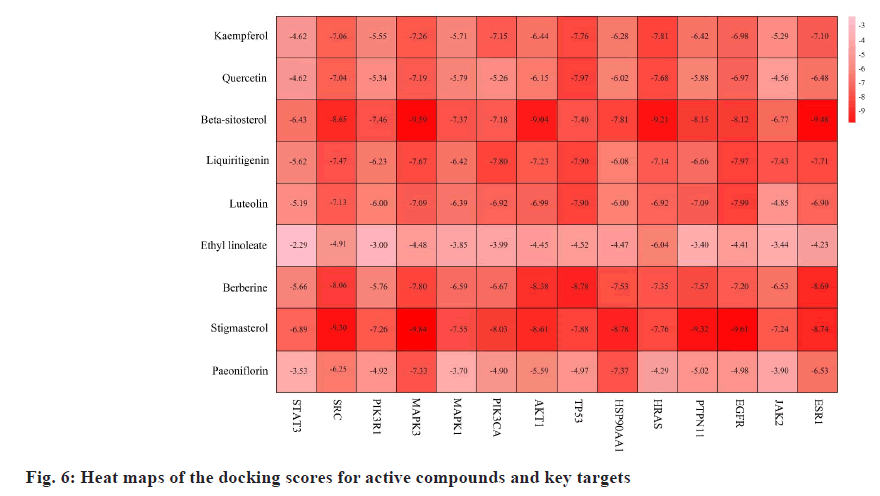
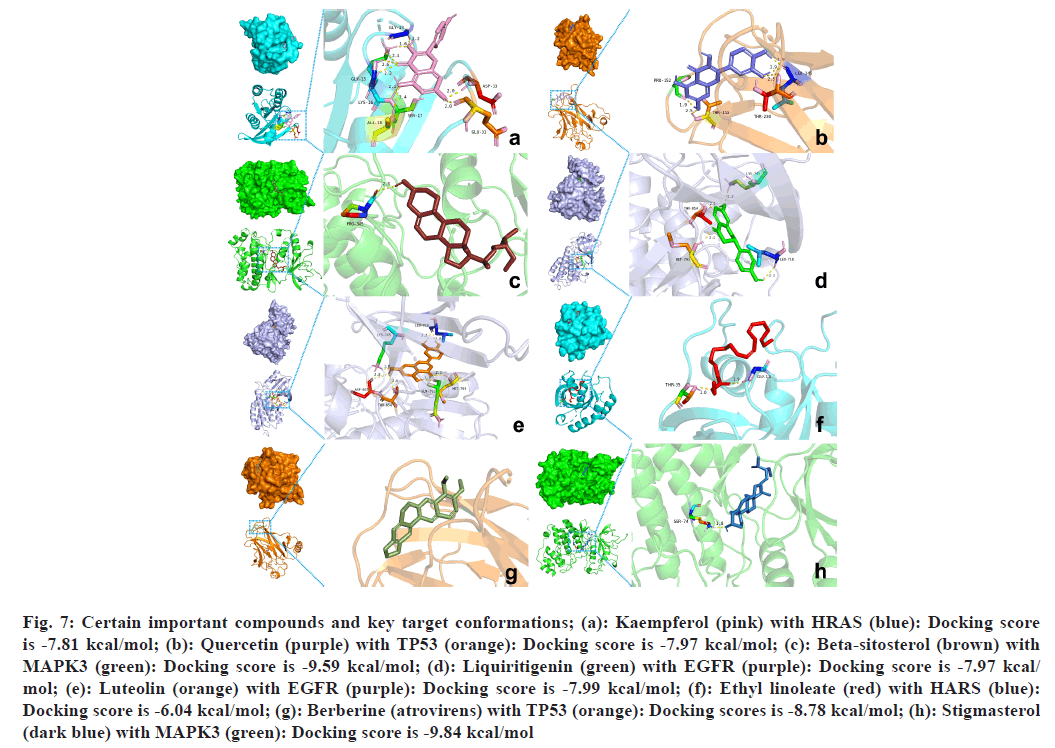
Molecular docking and binding capability prediction were performed between 9 core active compounds (including kaempferol, quercetin, beta- sitosterol, liquiritigenin, luteolin, ethyl linoleate, berberine, stigmasterol, and paeoniflorin) and 14 key targets (including STAT3, SRC, PTPN11, PIK3R1, PIK3CA, MAPK3, EGFR, MAPK1, JAK2, AKT1, HSP90AA1, HRAS, ESR1, and TP53), respectively. Finally, 126 groups of ligand- receptor docking results were obtained, 101 with an affinity <-5 kcal/mol and 63 of the 101 groups had hydrogen bonds ≥2. The binding free energies are shown in fig. 6. Several docking results with good activity were selected and visualized (fig. 7).
Fig. 7: Certain important compounds and key target conformations; (a): Kaempferol (pink) with HRAS (blue): Docking score is -7.81 kcal/mol; (b): Quercetin (purple) with TP53 (orange): Docking score is -7.97 kcal/mol; (c): Beta-sitosterol (brown) with MAPK3 (green): Docking score is -9.59 kcal/mol; (d): Liquiritigenin (green) with EGFR (purple): Docking score is -7.97 kcal/ mol; (e): Luteolin (orange) with EGFR (purple): Docking score is -7.99 kcal/mol; (f): Ethyl linoleate (red) with HARS (blue): Docking score is -6.04 kcal/mol; (g): Berberine (atrovirens) with TP53 (orange): Docking scores is -8.78 kcal/mol; (h): Stigmasterol (dark blue) with MAPK3 (green): Docking score is -9.84 kcal/mol
Medication, Percutaneous Coronary Intervention (PCI), and Coronary Artery Bypass Graft (CABG) surgery are the main treatment options for SAP[28]. Patients with moderate to severe symptoms who have failed medical therapy typically undergo surgery for single-vessel disease, but drug therapy remains the mainstay of care for SAP patients[29]. Western medicine uses long-term oral antiplatelet drugs, coronary vasodilators and other therapies to treat angina symptoms. Although these drugs can reduce angina attacks to some extent, angina attacks can still occur, and long-term use can have some adverse side effects, such as impaired liver function[3], reduced platelet count, and increased risk of bleeding[4]. SAP is also caused by a variety of factors. New multi-target drugs need to be developed because single-target drugs often do not provide the necessary therapeutic efficacy. In addition, single-target drugs do not always provide satisfactory efficacy in the treatment of SAP caused by multiple factors, so there is a need to develop multi-target drugs with fewer side effects. The SXXMKD is derived from the addition or subtraction of an empirical decoction that regulates fat metabolism, which is proposed by our team to improve the proliferation of endothelial cells by regulating the imbalance of Matrix Metalloproteinase (MMP-9)/Tissue Inhibitors of Metalloproteinase (TIMP)-1, thereby reducing lipid deposition in the vessel wall and reducing the degree of AS, and is considered beneficial to invigorate qi and spleen, eliminate phlegm and promote blood circulation in TCM[30]. In our daily clinical use, we have found that the SXXMKD can reduce the frequency of angina attacks and improve the quality of life of patients with SAP to some extent. However, its specific active ingredients and mechanism of action are still unclear. This study investigated and analysed the entire formulation using network pharmacology and molecular docking.
According to the analysis results of the “drugs-active ingredients-targets-disease” network, most of the active ingredients of the drugs came from DS, HQ, GG, and SQ. They are the main ingredients of many proprietary Chinese medicines for cardiovascular disease, and related preparations are widely used in the clinic to treat angina pectoris and myocardial infarction[31]. After integrating the existing database information from different sources of the above 9 drugs, the main active ingredients with high degree values were kaempferol, quercetin, beta-sitosterol, liquiritigenin, luteolin, ethyl linoleate, berberine, stigmasterol and paeoniflorin. The 9 main active ingredients may affect SAP in the following ways; kaempferol may have an anti- atherosclerotic effect by increasing antioxidant capacity, reducing the release of TNF-α and IL-1β, and downregulating gene and protein expression of pro-atherosclerotic molecules[32]; by reducing levels of Total Cholesterol (TC), Low-Density Lipoprotein Cholesterol (LDL-C), Oxidized LDL (Ox-LDL) and cytoplasmic lipid droplets, quercetin helps to control lipid metabolism and reduce the symptoms of atherosclerosis[33]; in vivo and in vitro studies of beta-sitosterol have shown that it has anti-inflammatory, anti-angiogenic and cholesterol-lowering properties[34-36]; liquiritigenin may improve cardiac function by reducing inflammatory responses by reducing Reactive Oxygen Species (ROS) production and lipid accumulation[37,38]; luteolin has been found to can reduce ox-LDL-induced inflammatory responses in vitro, possibly by inhibiting STAT3, thereby delaying atherosclerosis[39]; ethyl linoleate[40], berberine[41], and stigmasterol[42] also have lipid- regulating properties; via the MAPK/ Nuclear Factor kappa B (NF-κB), JAK2/STAT3, and PI3K/ AKT/ mammalian Target Of Rapamycin (mTOR) pathways, paeoniflorin controls the activation of immune cells and reduces the production of inflammatory mediators[43]. It also activates the Liver X Receptor (LXR) pathway to control cholesterol metabolism for anti-atherosclerosis[44].
Based on the above, we suggest that SXXMKD may play a better role in the treatment of SAP characterized by an immuno-inflammatory response or lipid metabolism disorders, which is consistent with some previous studies[45-47]. Therefore, further elucidation of the regulatory mechanisms of anti- inflammation and lipid metabolism may provide new therapeutic targets for the treatment of SAP. Molecular docking between 14 selected target genes and nine core active compounds showed that 80.2 % (101/126) of the groups had a good binding affinity (binding energy <-5 kcal/mol), further suggesting that SXXMKD may prevent and treat SAP mainly by regulating the expression of these genes. In addition, the molecular docking results also showed that the affinity of these targets after binding to related active compounds was relatively low, suggesting that we can further explore their potential in the treatment of SAP. The top common target BP in the GO enrichment analysis is regulation of the MAPK cascade and cellular response to organonitrogen compounds, meaning that most of the top targets are involved in both BPs. The CC is associated with the membrane raft, the membrane microdomain, and the caveola, suggesting that most of the targets of interest may be located at the plasma membrane, and MF results in protein kinase activity, phosphotransferase activity, and kinase binding, suggesting that the targets may depend on different kinase activities and kinase binding. KEGG enrichment analysis indicated that SXXMKD may affect SAP by simultaneously targeting multiple pathways, including the cancer pathway, the PI3K-AKT signaling pathway, the lipid and atherosclerosis signaling pathway, and the MAPK signaling pathway.
The PI3K-AKT pathway plays a critical role in regulating cardiomyocyte survival and function[48]. By mediating dendritic cells to intervene in the immune response, it initiates inflammatory immunity[49], affects chemokines and promotes the activation of inflammatory cells[50], which is closely linked to the development of atherosclerosis. In macrophages without PI3K, the activation of inflammatory cells was significantly attenuated or even abolished. In addition, inhibition of this pathway can reduce lipid deposition in vascular smooth muscle cells and increase lipid efflux to ameliorate lipid metabolism disorders, thereby reducing the likelihood of plaque formation[51]. A study showed that rats treated with the MAPK inhibitor U0126 had significantly reduced levels of MAPK and NF-kB, as well as atherosclerotic plaques and macrophages[52]. This suggests that activation of the MAPK pathway is involved in the regulation of the atherosclerotic inflammatory response[53]. Based on these results, we suggest that the PI3K-AKT pathway and the MAPK pathway are the two main mechanisms of action of SAP in the treatment of SXXMKD.
The treatment effect of a combination of multiple drugs is more evident than that of individual drugs for the prevention and treatment of heart conditions such as angina pectoris[5,6,54], as multiple systems of the body can be targeted to control symptoms and treat underlying problems. SAP belongs to the category of chest pain in TCM and deficiency, phlegm dampness, and stasis are the important pathological factors. The prescription of Chinese herbal compounds follows the principle of compatibility of TCM, which combines several medicinal materials, including different active ingredients. There have been a number of studies using network pharmacology to explore the relationship between drug, chemistry, target and disease from a holistic perspective, including using SAP[8-10]. TCM advocates selecting and prescribing different treatments according to different symptoms.
There are some limitations to the study. First, the network pharmacology technique is based on existing databases and experimental data, and those compounds or targets that are unidentified and undocumented may not be included in our analysis. Secondly, the nine compounds are considered to be the major bioactive components of SXXMKD for the treatment of SAP, but are not fully representative of SXXMKD and further validation of the results of this study by molecular biology experiments is required. Finally, this study only considered SAP due to CAD caused by atherosclerosis and did not consider other induced SAP, such as hypertrophic or dilated cardiomyopathy and aortic stenosis. Although the effects and mechanisms of these potential agents on SAP have not been explained and validated, this study still provides reasonable confidence and evidence to support the treatment of SAP with TCM drugs. There remains much to be done in this area.
In conclusion, we have explored the material basis and potential mechanisms of SXXMKD for the treatment of SAP through network pharmacology. The core components of SXXMKD may improve the symptoms of SAP patients mainly through anti- inflammation and regulation of lipid metabolism. The above can provide a theoretical basis for subsequent basic research and for developing new drugs.
Acknowledgments:
This work was supported by the GuangDong Basic and Applied Basic Research Foundation [grant number 2022A1515012188].
Conflict of interests:
The authors declared no conflicts of interest.
References
- Joshi PH, De Lemos JA. Diagnosis and management of stable angina: A review. Jama. 2021;325(17):1765-78.
[Crossref] [Google Scholar] [PubMed]
- Valgimigli M, Biscaglia S. Stable angina pectoris. Curr Atheroscler Rep 2014;16:1-10.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Li J, Wang T, Zhang Z, Li Q, Ma D, et al. Associations between statins and adverse events in secondary prevention of cardiovascular disease: Pairwise, network, and dose-response meta-analyses of 47 randomized controlled trials. Front Cardiovasc Med 2022;9:929020.
[Crossref] [Google Scholar] [PubMed]
- Walker J, Cattaneo M, Badimon L, Agnelli G, Chan AT, Lanas A, et al. Highlights from the 2019 International aspirin foundation scientific conference, Rome, 28 June 2019: Benefits and risks of antithrombotic therapy for cardiovascular disease prevention. Ecancermedicalscience 2020;14.
[Crossref] [Google Scholar] [PubMed]
- Wei J, Ma T, Zhou C, Hao P, Li B, Wang X, et al. Efficacy and safety of Shexiang Baoxin Pill for stable coronary artery disease: A systematic review and meta-analysis of 42 randomized controlled trials. Front Pharmacol 2022;13:1002713.
[Crossref] [Google Scholar] [PubMed]
- Huang P, Li Z, Chen L, Zeng J, Zhao S, Tang Y, et al. The comparative effects of oral Chinese patent medicines combined with western medicine in stable angina: A systematic review and network meta-analysis of 179 trials. Front Pharmacol 2022;13:918689.
[Crossref] [Google Scholar] [PubMed]
- Li Shao LS, Zhang Bo ZB. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin J Nat Med 2013;11(2):110-20.
[Crossref] [Google Scholar] [PubMed]
- Chai LL, Sun MY, Yao HZ, Li YH, Gao R. The mechanism of guanxining tablet in treating stable angina pectoris of coronary heart disease based on network pharmacology. J Tradit Chin Med 2019;41(4):933-36.
- Zhang GX, Zhang YY, Zhang XX, Wang PQ, Liu J, Liu Q, et al. Different network pharmacology mechanisms of Danshen-based Fangjis in the treatment of stable angina. Acta Pharmacol Sin 2018;39(6):952-60.
[Crossref] [Google Scholar] [PubMed]
- Chen YY, Nan JY, Li HX, Liu Q, Li B, Liu J, et al. Deciphering potential pharmacological mechanisms of Danhong injection to treat chronic stable angina based on drug response-related modules and genes. J Ethnopharmacol 2022;291:115125.
[Crossref] [Google Scholar] [PubMed]
- Ru J, Li P, Wang J, Zhou W, Li B, Huang C, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 2014;6:1-6.
[Crossref] [Google Scholar] [PubMed]
- Xu HY, Zhang YQ, Liu ZM, Chen T, Lv CY, Tang SH, et al. ETCM: An encyclopaedia of traditional Chinese medicine. Nucleic Acids Res 2019;47(D1):D976-82.
[Crossref] [Google Scholar] [PubMed]
- Wu Y, Zhang F, Yang K, Fang S, Bu D, Li H, et al. SymMap: An integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Res 2019;47(D1):D1110-7.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Xiao J, Suzek TO, Zhang J, Wang J, Bryant SH. PubChem: A public information system for analyzing bioactivities of small molecules. Nucleic Acids Res 2009;37(suppl_2):W623-33.
[Crossref] [Google Scholar] [PubMed]
- Gfeller D, Grosdidier A, Wirth M, Daina A, Michielin O, Zoete V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res 2014;42(W1):W32-8.
[Crossref] [Google Scholar] [PubMed]
- Safran M, Dalah I, Alexander J, Rosen N, Iny Stein T, Shmoish M, et al. GeneCards Version 3: The human gene integrator. Database 2010;2010:baq020.
[Crossref] [Google Scholar] [PubMed]
- Amberger JS, Hamosh A. Searching Online Mendelian Inheritance in Man (OMIM): A knowledgebase of human genes and genetic phenotypes. Curr Protoc Bioinformatics 2017;58(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, et al. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 2006;34:D668-72.
[Crossref] [Google Scholar] [PubMed]
- Wu C, Nebert DW. Update on genome completion and annotations: Protein information resource. Hum Genomics 2004;1(3):229-33.
[Crossref] [Google Scholar] [PubMed]
- von Mering C, Jensen LJ, Snel B, Hooper SD, Krupp M, Foglierini M, et al. STRING: Known and predicted protein–protein associations, integrated and transferred across organisms. Nucleic Acids Res 2005;33:D433-7.
[Crossref] [Google Scholar] [PubMed]
- Luo H, Wang M, Xu K, Peng Q, Zou B, Yin S, et al. Effect of fushengong decoction on PTEN/PI3K/AKT/NF-κB pathway in rats with chronic renal failure via dual-dimension network pharmacology strategy. Front Pharmacol 2022;13:807651.
[Crossref] [Google Scholar] [PubMed]
- Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst Biol 2014;8:1-7.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 2019;10(1):1523.
[Crossref] [Google Scholar] [PubMed]
- Bittrich S, Bhikadiya C, Bi C, Chao H, Duarte JM, Dutta S, et al. RCSB protein data bank: Efficient searching and simultaneous access to one million computed structure models alongside the PDB structures enabled by architectural advances. J Mol Biol 2023;435(14):167994.
[Crossref] [Google Scholar] [PubMed]
- O'Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open babel: An open chemical toolbox. J Cheminform 2011;3:1-4.
[Crossref] [Google Scholar] [PubMed]
- Zhu R, Du B, Yuan J, Yan S, Shao M, Sang F, et al. Potential mechanisms of biejiajian pill in the treatment of diabetic atherosclerosis based on network pharmacology, molecular docking, and molecular dynamics simulation. Evid Based Complement Alternat Med 2022;2022(1):3296279.
[Crossref] [Google Scholar] [PubMed]
- Kotni MK, Zhao M, Wei DQ. Gene expression profiles and protein-protein interaction networks in amyotrophic lateral sclerosis patients with C9orf72 mutation. Orphanet J Rare Dis 2016;11:1-9.
[Crossref] [Google Scholar] [PubMed]
- Park H, Kang DY, Lee CW. Functional angioplasty: Definitions, historical overview, and future perspectives. Korean Circ J 2022;52(1):34-46.
[Crossref] [Google Scholar] [PubMed]
- Agarwal M, Mehta PK, Merz CN. Nonacute coronary syndrome anginal chest pain. Med Clin North Am 2010;94(2):201-16.
[Crossref] [Google Scholar] [PubMed]
- Huang ZY, Huang MP, Liang DH, Liu YY, Jiang WQ, Zhang W. The effects of Zhengzhifang on reversing atherosclerosis by regulating MMP-9 and TIMP-1. Guangdong Med J 2016;37(15):2224-27.
- Cheng TO. Danshen: A versatile Chinese herbal drug for the treatment of coronary heart disease. Int J Cardiol 2006;113(3):437-8.
[Crossref] [Google Scholar] [PubMed]
- Kong L, Luo C, Li X, Zhou Y, He H. The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis 2013;12:1-2.
[Crossref] [Google Scholar] [PubMed]
- Jia Q, Cao H, Shen D, Li S, Yan L, Chen C, et al. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARγ, LXRα and ABCA1. Int J Mol Med 2019;44(3):893-902.
[Crossref] [Google Scholar] [PubMed]
- Loizou S, Lekakis I, Chrousos GP, Moutsatsou P. β?Sitosterol exhibits anti?inflammatory activity in human aortic endothelial cells. Mol Nutr Food Res 2010;54(4):551-8.
[Crossref] [Google Scholar] [PubMed]
- Moon EJ, Lee YM, Lee OH, Lee MJ, Lee SK, Chung MH, et al. A ncovel angiogenic factor derived from Aloe vera gel: β-sitosterol, a plant sterol. Angiogenesis 1999;3:117-23.
[Crossref] [Google Scholar] [PubMed]
- Zak A, Zeman M, Vitkova D, Hrabak P, Tvrzicka E. Beta-sitosterol in the treatment of hypercholesterolemia. Cas Lek Cesk 1990;129(42):1320-3.
[Google Scholar] [PubMed]
- Zhang M, Qi J, He Q, Ma D, Li J, Chu X, et al. Liquiritigenin protects against myocardial ischemic by inhibiting oxidative stress, apoptosis, and L?type Ca2+ channels. Phytother Res 2022;36(9):3619-31.
[Crossref] [Google Scholar] [PubMed]
- Xu Z, Hu Z, Xu H, Zhang L, Li L, Wang Y, et al. Liquiritigenin alleviates doxorubicin-induced chronic heart failure via promoting ARHGAP18 and suppressing RhoA/ROCK1 pathway. Exp Cell Res 2022;411(2):113008.
[Crossref] [Google Scholar] [PubMed]
- Ding X, Zheng L, Yang B, Wang X, Ying Y. Luteolin attenuates atherosclerosis via modulating signal transducer and activator of transcription 3-mediated inflammatory response. Drug Des Devel Ther 2019:3899-911.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Hua J, Huang Z. Effects of beer, wine, and baijiu consumption on non-alcoholic fatty liver disease: Potential implications of the flavor compounds in the alcoholic beverages. Front Nutr 2023;9:1022977.
[Crossref] [Google Scholar] [PubMed]
- Li XY, Zhao ZX, Huang M, Feng R, He CY, Ma C, et al. Effect of Berberine on promoting the excretion of cholesterol in high-fat diet-induced hyperlipidemic hamsters. J Transl Med 2015;13:278
[Crossref] [Google Scholar] [PubMed]
- Hussein HA, Abdullah MA. Anticancer compounds derived from marine diatoms. Mar Drugs 2020;18(7):356.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Wei W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol Ther 2020;207:107452.
[Crossref] [Google Scholar] [PubMed]
- Yu W, Ilyas I, Hu X, Xu S, Yu H. Therapeutic potential of paeoniflorin in atherosclerosis: A cellular action and mechanism-based perspective. Front Immunol 2022;13:1072007.
[Crossref] [Google Scholar] [PubMed]
- Kruk ME, Gage AD, Joseph NT, Danaei G, García-Saisó S, Salomon JA. Mortality due to low-quality health systems in the universal health coverage era: A systematic analysis of amenable deaths in 137 countries. Lancet 2018;392(10160):2203-12.
[Crossref] [Google Scholar] [PubMed]
- Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res 2016;118(4):535-46.
[Crossref] [Google Scholar] [PubMed]
- Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, et al. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules 2018;8(3):80.
[Crossref] [Google Scholar] [PubMed]
- Rong R, Xijun X. Erythropoietin pretreatment suppresses inflammation by activating the PI3K/Akt signaling pathway in myocardial ischemia-reperfusion injury. Exp Ther Med 2015;10(2):413-8.
[Crossref] [Google Scholar] [PubMed]
- Monaci S, Coppola F, Giuntini G, Roncoroni R, Acquati F, Sozzani S, et al. Hypoxia enhances the expression of RNASET2 in human monocyte-derived dendritic cells: Role of PI3K/AKT pathway. Int J Mol Sci 2021;22(14):7564.
[Crossref] [Google Scholar] [PubMed]
- Graves DT, Milovanova TN. Mucosal immunity and the FOXO1 transcription factors. Front Immunol 2019;10:2530.
[Crossref] [Google Scholar] [PubMed]
- Pi S, Mao L, Chen J, Shi H, Liu Y, Guo X, et al. The P2RY12 receptor promotes VSMC-derived foam cell formation by inhibiting autophagy in advanced atherosclerosis. Autophagy 2021;17(4):980-1000.
[Crossref] [Google Scholar] [PubMed]
- Jiang ZM, Wu T, Xue YT, Li Y, Li GH, Huang K, et al. Decoding the mechanism of shixiao powder in treating coronary heart disease based on network pharmacology and molecular docking. Evid Based Complement Alternat Med 2022;2022(1):3756668.
[Crossref] [Google Scholar] [PubMed]
- Meng T, Li X, Li C, Liu J, Chang H, Jiang N, et al. Natural products of traditional Chinese medicine treat atherosclerosis by regulating inflammatory and oxidative stress pathways. Front Pharmacol 2022;13:997598.
[Crossref] [Google Scholar] [PubMed]
- Han YC, Xie HZ, Lu B, Xiang RL, Li JY, Qian H, et al. Effect of berberine on global modulation of lncRNAs and mRNAs expression profiles in patients with stable coronary heart disease. BMC Genomics 2022;23(1):400.
[Crossref] [Google Scholar] [PubMed]

 MF
MF