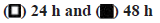- *Corresponding Author:
- Chun-Hua Wang
School of Medicine, Tzu Chi University, Hualien 970, Taiwan
E-mail: dermawang@gmail.com
| Date of Received | 01 August 2022 |
| Date of Revision | 6 March 2023 |
| Date of Acceptance | 26 July 2024 |
| Indian J Pharm Sci 2024;86(4):1316-1323 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Hair loss is a common symptom in dermatology. While it is not fatal, it can significantly affect an individual's mental well-being. There are many products derived from traditional Chinese medicine formulas on the market that advertise the efficacy of hair growth and hair care, but there is no scientific evidence establishing the efficacy of these traditional Chinese medicine formulas. Peach kernel (Tao Ren) is used in traditional Chinese medicine for removing blood stasis, laxative and cough, and its extracted oil is also often used for hair growth and hair care. We used human hair follicle dermal papilla cells, which control epidermal hair growth, as experimental materials to explore the effect of the methanol extract of peach kernel on the growth of human hair follicle dermal papilla cells. Our results found that methanol extract of peach kernel can increase human hair follicle dermal papilla cells viability and promote cell proliferation. When the methanol extract of peach kernel was added to human hair follicle dermal papilla cells, it increased the production of cell cycle-regulated proteins and increased the cell population in the S phase of the cell cycle. We further found that the methanol extract of peach kernel could activate extracellular signal-regulated kinase and protein kinase B signal transduction in human hair follicle dermal papilla cells, with the extracellular signal-regulated kinase pathway playing an important role in regulating the growth of these cells. The above results suggest that the efficacy of peach kernel for hair growth and hair care is related to its ability to promote the growth of human hair follicle dermal papilla cells.
Keywords
Peach kernel, human hair follicle dermal papilla cells, cell proliferation
Hair loss is common on the scalp or body, and the symptoms may be temporary or long-lasting. Symptoms of hair loss may differ between men and women and can be caused by a family history of genetic disorders, hormonal changes, infection, medication, medical treatments (e.g., radiation therapy), or stress[1]. The reasons for hair loss are different, and the methods used in medical treatment are also different. Dermatological drugs such as minoxidil are used primarily to regrow hair, slow hair loss, or both. Some drugs, like Propecia, inhibit androgenetic baldness induced by male hormones. Permanent hair loss is often treated with a hair transplant. Hair growth consists of four phases: The anagen phase (the longest growth phase), the catagen phase (transitional), the telogen phase (resting), and the exogen phase (shedding)[2]. After the telogen phase, the exogen phase begins, leading to hair loss. However, a new anagen phase typically starts during this time, with new hair growing to replace the lost hair. Abnormal hair loss occurs when too much hair is shed or when the anagen growth phase is disrupted. This transition from telogen to anagen occurs near the dermal papilla, where stem cells rapidly proliferate to form new hair follicles. Therefore, Dermal Papilla Cells (DPCs) play a crucial role in the hair growth cycle and are often used as target cells to study the cellular mechanisms of hair follicle growth.
At present, there are several theoretical bases for traditional Chinese medicine to regulate hair loss according to different causes of hair loss. For example, unprocessed Rehmannia root or Moutan cortex radicis is used to reduce fever caused by heart-fire hyperactivity due to stress[3]. Shen Ying Yang Zheng Dan (nourishing bolus), which contains Angelica sinensis, Gastrodia elata, Ligusticum chuanxiong Hort., Rhizoma et Radix Notopterygii, Paeonia lactiflora Pall., and Rehmannia glutinosa, is used to nourish the liver and kidneys, treat itchy dandruff, and act as a hematinic. "Renshen Yangrong Decoction (including Panax ginseng C. A. Mey., Paeonia lactiflora Pall., Atractylodes macrocephala Koidz., Citrus reticulata Blanco, Ziziphus jujuba Mill., Angelica sinensis (Oliv.) Diels., Glycyrrhiza uralensis Fisch., etc,.) and the Tongqiao Huoxue decoction (consisting of Paeonia lactiflora Pall., Ligusticum chuanxiong Hort., Prunus persica (L.) Batsch, Carthamus tinctorius L., Zingiber officinale (Willd.) Rosc.) are used for blood obstruction due to chronic illness or during the postpartum period[4-7]. The curative effect targets of current Chinese medicines for promoting blood circulation and removing blood stasis have been described in many studies. For example, Angelica sinensis (Oliv.) Diels, used for promoting blood circulation and removing blood stasis, has been shown to promote the growth of hair follicle cells and inhibit cell death in vivo[8]. Lycium barbarum (boxthorn), used as an anti-aging product, has been shown to increase the activity of superoxide dismutase in tissues, enhance collagen expression, and increase hair growth in mice in vivo[9].
Many plant extracts and traditional Chinese medicines are marketed today as health care products for hair care and growth, including safflower oil (HT26), the Panax notoginseng derivative Orich-37, and Salvia miltiorrhiza. There is currently no scientific evidence that these products have hair growth properties, and their targets of action in hair tissue or cells have not been defined. The peach kernel (Tao Ren) is the dried mature seed of the plant Prunus persica or the mountain peach Prunus davidiana. The peach kernel is promoted in many commercial products and is advertised as protecting hair and improving the microenvironment of hair[10-12]. However, whether it has a direct effect on hair follicle cells remains unknown. This study aimed to investigate whether the Methanol Extract of Peach kernel (PME) affects the growth of Dermal Papilla Cells (DPCs) and to clarify the intracellular signaling pathways involved in PME-mediated cell proliferation.
Materials and Methods
Herbal powder extracts of peach kernel were obtained from Sun Ten Pharmaceutical Company (New Taipei City, Taiwan). The extraction of peach kernel was described as described previously[13]. Briefly, peach kernel powder was added to methanol to achieve a concentration of 10 mg/ml. After 15 min of ultrasonic vibration, the mixture was placed at 4° for 24 h. Ultrasonic vibration was performed again for 15 min. After incubating at 70° for 30 min, the mixture was centrifuged at 14 000×g for 10 min to obtain the supernatant for subsequent experiments.
Cell culture:
HFDPCs were obtained from PromoCell (Heidelberg, Germany), and the cell culture conditions were based on the previous study[14,15]. Cells with 4-10 population doublings were used for the research. The cell appearance and intracellular alkaline phosphatase activity did not change significantly by the 10th population doubling. HFDPCs were subcultured using a commercial medium (follicular dermal papilla cell basal medium) supplemented with 4 % calf serum, various growth factors (0.4 % bovine pituitary extract, 1 ng/ml basic fibroblast growth factor), and 5 μg/ml recombinant human insulin (PromoCell).
Cell viability assay:
The cell viability assays were performed as described previously[16]. Briefly, HFDPCs were grown overnight in 24-well plates at a density of 5×104 cells/well. The culture environment was replaced with serum-free basal medium and cultured for 24 h. Different doses of PME were then added to the cell culture medium and incubated for 24-48 h. Afterward, 100 μl of colorimetric Water-Soluble Tetrazolium (WST)-1 reagent (Roche Diagnostics, Mannheim, Germany) was added to the medium, and the cells were incubated at 37° for 4-6 h. The culture medium containing the WST-1 reagent was transferred to a 96-well plate, and the absorbance at 450 nm (650 nm was the reference value) was read on a multifunctional microplate reader (Infinite F200, Tecan, Durham, NC, United States of America (USA)). Cell viability readings for each group were calculated as percentages of methanol-treated controls.
Cell proliferation assay:
The cell proliferation assay was performed as described previously[15]. Briefly, HFDPCs were seeded in 24-well plates in triplicate at 1×104 cells/ well. HFDPCs were then starved and treated with various concentrations of PME for 24-48 h. BrdU labeling solution (10 μm, Roche Diagnostics) was added and incubated for 12 h. After removing the medium, the cells were fixed with FixDenat solution at 25° for 30 min. The cells were reacted with peroxidase-labeled anti-BrdU antibody at 25° for 90 min, then with tetramethylbenzidine substrate for color development. The samples were read on a multiplate reader (Infinite F200), and optical density was measured at absorbance wavelengths of 370 nm and 492 nm for each well.
Western blot:
HFDPCs were cultured in 10 cm culture dishes for 24 h. Cells were serum-starved for 24 h and then treated with different concentrations of PME for an additional 24 h. Cells were lysed with Radioimmunoprecipitation Assay (RIPA) buffer (50 mm Tris (pH 8.0), 150 mm NaCl, 1 % NP-40, 0.5 % sodium deoxycholate, 0.1 % sodium deoxycholate) containing protease inhibitor cocktail (Roche Diagnostics, Germany) and phosphatase inhibitors. The Western blot was performed as described previously[17].
Briefly, proteins (40-60 μg) from cell extracts were separated on 12 % polyacrylamide gels, transferred to Poly(Vinylidene Fluoride) (PVDF) membranes, and then incubated with various primary antibodies at 4° for 12 h. They were then reacted with the corresponding horseradish peroxidase-conjugated secondary antibodies (Calbiochem, Darmstadt, Germany) for 1 h at room temperature. Proteins were visualized using an Amersham ECL kit (Amersham, Bucks, UK), and relative protein levels were quantified by normalizing to actin expression. Antibodies recognizing various cyclins, Cyclin-Dependent Kinase (CDK), protein kinase B (Akt) and Extracellular signal-Regulated Kinase (ERK) were obtained from cell signaling technology (Beverly, MA, USA). An antibody recognizing actin was obtained from Sigma-Aldrich.
Cell cycle analysis:
The methods for cell cycle analysis were as described previously[14]. Briefly, HFDPCs were cultured in serum-free basal medium for 24 h and then treated with different concentrations of PME for 24 h. Cells were collected and fixed in ice-cold 80 % ethanol at -20° for at least 12 h. They were stained with propidium iodide solution (20 μg/ml propidium iodide, 0.1 % Triton X-100, and 0.2 mg/ml RNase A) and analyzed by flow cytometry (Cytomics FC 500; Becton Dickinson, Franklin Lakes, NJ, USA) for staining analysis. The percentage of cell cycle stage distribution was analyzed with multicycle for Windows (Becton Dickinson) software.
Results and Discussion
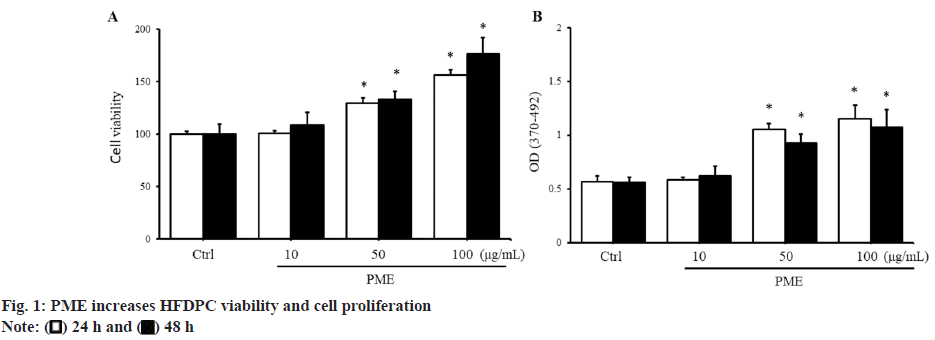
To evaluate the effect of PME on HFDPC growth, cell viability was assessed using the WST-1 reagent. When a high dose of PME (50-100 μg/ml) was added to HFDPCs, cell viability was significantly increased compared to the control group (fig. 1A). The increase in HFDPC cell viability induced by PME may be related to either a reduction in cell death or an induction of cell proliferation. Therefore, we next examined whether PME affects cell death or proliferation in HFDPCs. When cells were cultured without serum starvation for 24-48 h, there was no significant increase in cell death in HFDPCs compared to cultures with serum. Furthermore, the addition of PME did not affect the number of dead cells compared to the control group under starvation conditions (data not shown). In addition to cell death, we investigated whether PME affects the proliferation of HFDPCs. The results in fig. 1B, show that when HFDPCs were treated with PME at a dose of 50-100 μg/ml, cell proliferation was significantly increased. The above results indicated that the increase in HFDPC viability induced by PME mainly regulated the cell proliferation of HFDPCs.
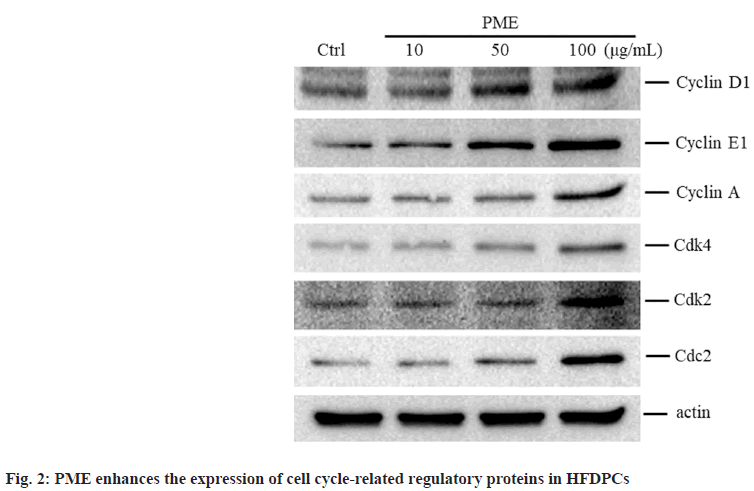
Given that PME can regulate the proliferation of HFDPCs, we investigated whether PME affects the expression of cell cycle-related proteins in these cells. When cells were treated with a high dose of PME (e.g., 50-100 μg/ml), the protein expression levels of cyclins D and E, as well as the Cyclin-Dependent Kinase 4 (CDK4) and CDK2 associated with the G1 to S phase transition were increased. Similarly, the expression levels of cyclin A and cyclin-regulated kinase Cdc2, which control the G2 phase transition to M phase, were also increased in cells treated with 100 μg/ml PME (fig. 2). In addition to analyzing cell cycle-related protein expression, the effect of PME on the HFDPC cell cycle was also determined. As shown in fig. 3, increasing the PME dose led to a decrease in the population of HFDPCs in the G1 phase, while the population in the S phase increased. The above results demonstrate that PME promotes the proliferation of HFDPCs by regulating the expression of cell cycle-related proteins.
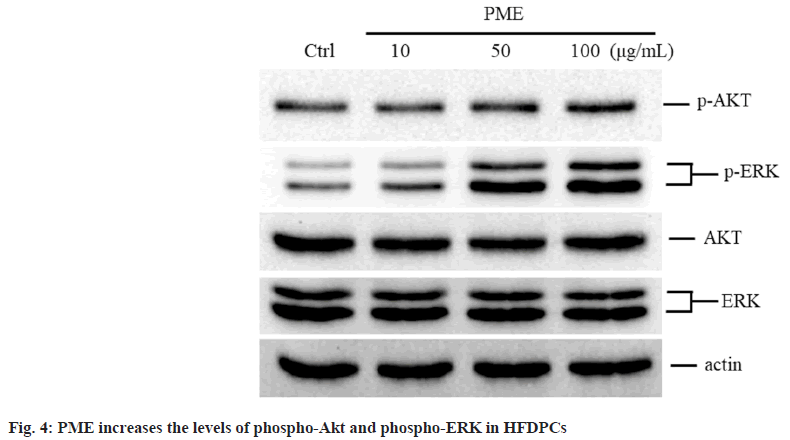
Since the Akt and ERK pathways regulate the proliferation of HFDPCs[18], we further explored whether PME affects the expression of Akt and ERK in these cells. The expression levels of phospho-Akt and phospho-ERK in HFDPCs were significantly increased when the cells were treated with 100 μg/ml or 50-100 μg/ml PME, respectively (fig. 4), suggesting that these two pathways may be involved in the regulation of HFDPC growth by PME.
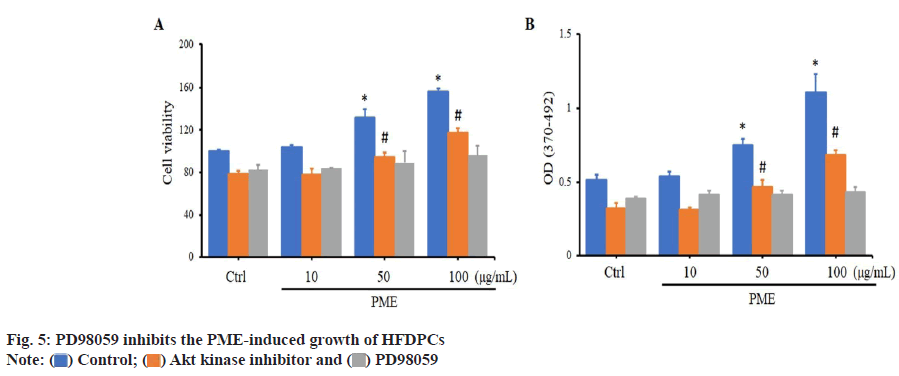
Since PME can activate the Akt and ERK pathways in HFDPCs, we used the Akt antagonist ATK inhibitor and the ERK antagonist PD98059 to determine which pathway plays a crucial role in PME-mediated regulation of HFDPC proliferation. When HFDPCs were treated with an Akt kinase inhibitor and PD98059, cell viability was significantly decreased compared to the control group. When HFDPCs were treated with PME, cell viability was significantly increased (at PME concentrations of 50-100 μg/ml) in both the control group and the Akt kinase inhibitor-treated group (fig. 5). Similarly, when HFDPCs were treated with an Akt kinase inhibitor and PD98059, cell proliferation was significantly reduced compared to the control group. When HFDPCs were treated with PME, both the control group and the Akt kinase inhibitor group showed that cell proliferation significantly increased in the PMEtreated group at doses of 50-100 μg/ml. However, when HFDPCs were treated with PD98059, the addition of PME did not result in a significant difference in cell viability or proliferation compared to the control group (fig. 5). These results suggest that PME mainly regulates the proliferation of HFDPCs through the ERK signaling pathway.
Peach kernel has been noted in traditional Chinese medicine for removing blood stasis, and it has also been verified to reduce atherosclerosis in ApoE knockout mice[19]. The effect of peach kernel in promoting blood circulation is similar to other traditional Chinese medicines in improving the microenvironment of skin hair follicles, providing tissue nutrients and aiding cell regeneration[20,21]. In the market, peach kernel oil is often advertised as the main effective ingredient in hair care products. It is rich in many nutrients, such as linoleic acid, vitamins A and E, and unsaturated fatty acids. It is easily absorbed by hair, provides good moisturizing effects, and helps maintain hair elasticity. Therefore, the efficacy of peach kernel in hair growth and health care should be recognized. In addition to the previous studies, our research indicates that PME promotes the proliferation of HFDPCs through the ERK pathway. This suggests that, aside from improving the hair microenvironment, peach kernel can also act on DPC cells, which regulate hair growth and promote hair regeneration. Currently, the biologically active components of peach kernel are primarily divided into two categories; polyphenols and tetraterpenoids[22]. However, more than 15 types of polyphenols and at least 6 types of tetraterpenoids have been identified in peach kernel. Consequently, it is challenging to examine which specific components induce HFDPC cell proliferation individually. When examining the main functions of potential components, neochlorogenic acid and hesperidin-7-rutinoside, identified in polyphenols, are noted in patents CN101530407A and WO2017080958 as having hair growth-promoting effects. Additionally, procyanidin and chlorogenic acid are considered essential for hair growth[23-25]. Procyanidin promotes hair follicle growth[26]. Similarly, zeaxanthin, a tetraterpenoid found in peach kernel, has been shown to reduce DPC apoptosis[27], while β-carotene and other vitamin derivatives are essential for hair growth[28]. The literature discussed various potential bioactive components of peach kernel and their efficacy in hair growth and health care, confirming that peach kernel indeed has the potential to promote hair follicle growth.
There is no literature exploring the role of peach kernel in intracellular signal transduction during HFDPC proliferation. Buyang Huanwu decoction contains components of peach kernel, which are involved in the differentiation of bone marrow mesenchymal stem cells through the Mitogen-Activated Protein Kinase (MAPK) signaling pathway[29]. Similarly, the potential bioactive components of peach kernel, such as hesperidin-7- rutinoside, chlorogenic acid, and procyanidin, have also been shown to regulate anti-aging, detoxification, and cell transformation functions through the MAPK pathway[30-33]. In our study, peach kernel activated the Akt and ERK signaling pathways in HFDPCs, with ERK playing a pivotal role in promoting HFDPC proliferation. This suggests that the compounds found in peach kernel, as mentioned above, are involved in this process. Further research is needed to identify which specific components of peach kernel are involved in regulating ERK signaling in HFDPCs.
In addition to HFDPCs, keratinocytes are located in the outermost layer of the skin and can protect the skin from external damage. Whether peach kernel affects keratinocytes is still unknown. However, numerous studies have indicated that several components of peach kernel, such as hesperidin, chlorogenic acid, procyanidin, and lutein, have protective effects against oxidative stress and Ultraviolet (UV) light stimulation[34-37]. Research on the contents of peach kernels has shown that they provide many of the benefits required for the growth of various cells in the hair follicle environment.
At present, peach kernel oil is primarily used externally as a health care product for hair growth. In traditional Chinese medicine, it is taken orally to promote blood circulation and remove blood stasis. In our study, HFDPCs were used as the cell subject to be treated with peach kernel. Whether oral administration of traditional Chinese medicine containing peach kernel is beneficial to hair growth requires further investigation. There is no literature report on the half-life in vivo of peach kernel after taking it or the dose in the blood. It is known that administration at the dosages used in traditional Chinese medicine causes no harm to the subjects[38]. Considering factors such as the drug concentration in the blood, the metabolic burden on the liver, and the presence of the drug in the scalp microenvironment, it is more appropriate to use peach kernel externally.
This study discovered that peach kernel can promote the proliferative capacity of HFDPCs. Peach kernel induce the expression of cell cycle-related regulatory proteins and increase the population of cells in the S-phase. In addition, peach kernel regulates the growth of HFDPCs by stimulating the intracellular ERK signaling pathway. The study provided more evidence pointing to the role of peach kernel as a hair growth supplement.
Acknowledgements:
The authors thank the Core Laboratory of the Buddhist Tzu Chi General Hospital for providing support.
Funding:
This research was funded by the Taipei Tzu Chi Hospital through the Buddhist Tzu Chi Medical Foundation, Taipei, Taiwan, Grant Numbers TCRDTPE- 111-23 (CHUN-HUA WANG) and TCRDTPE- 110-13 (FU-MING TSAI).
Conflict of interests:
The authors declared no conflict of interests.
References
- York K, Meah N, Bhoyrul B, Sinclair R. A review of the treatment of male pattern hair loss. Expert Opin Pharmacother 2020;21(5):603-12.
[Crossref] [Google Scholar] [PubMed]
- Lin X, Zhu L, He J. Morphogenesis, growth cycle and molecular regulation of hair follicles. Front Cell Dev Biol 2022;10:899095.
[Crossref] [Google Scholar] [PubMed]
- Xiong X, Wang P, Duan L, Liu W, Chu F, Li S, et al. Efficacy and safety of Chinese herbal medicine Xiao Yao San in hypertension: A systematic review and meta-analysis. Phytomedicine 2019;61:152849.
[Crossref] [Google Scholar] [PubMed]
- Sheng W, Wang Y, Li JB, Xu HS. Clinical and basic research on Renshen Yangrong decoction. Front Nutr 2019;6:175.
[Crossref] [Google Scholar] [PubMed]
- Zhou X, Shao T, Xie X, Ding M, Jiang X, Su P, Jin Z. Tongqiao Huoxue decoction for the treatment of acute ischemic stroke: A Systematic Review and meta-analysis. J Ethnopharmacol 2022;283:114693.
[Crossref] [Google Scholar] [PubMed]
- Li L, Wang N, Jin Q, Wu Q, Liu Y, Wang Y. Protection of Tong-Qiao-Huo-Xue decoction against cerebral ischemic injury through reduction blood-brain barrier permeability. Chem Pharm Bull 2017;65(11):1004-10.
[Crossref] [Google Scholar] [PubMed]
- Zhong DY, Li HY, Li L, Ma RM, Jiang CT, Li DX, et al. Effect of tongqiao huoxue decoction combined with western medicine on ischemic stroke: A systematic review. Evid Based Complement Alternat Med 2020;2020(1):8877998.
[Crossref] [Google Scholar] [PubMed]
- Kim MH, Choi YY, Cho IH, Hong J, Kim SH, Yang WM. Angelica sinensis induces hair regrowth via the inhibition of apoptosis signaling. Am J Chin Med 2014;42(04):1021-34.
[Crossref] [Google Scholar] [PubMed]
- Shao LX. Effects of the extract from bergamot and boxthorn on the delay of skin aging and hair growth in mice. Zhongguo Zhong Yao Za Zhi 2003;28(8):766-9.
[Google Scholar] [PubMed]
- Zhang J, Chen W, Sun W, Zhou Y, Li X, Zhang J, et al. Prunus mira Koehne in Sichuan, China: Recorded history as a medicine and food, modern applications, distribution, and ethnobotanical investigations. Front Pharmacol 2022;13:826712.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Zhang J, Chen W, Li X, Fu K, Sun W, et al. Identification of hair growth promoting components in the Kernels of Prunus mira Koehne and their mechanism of action. Molecules 2022;27(16):5242.
[Crossref] [Google Scholar] [PubMed]
- Kumari N, Kumar M, Puri S, Zhang B, Rais N, Pundir A, et al. Peach (Prunus persica (L.) Batsch) seeds and kernels as potential plant-based functional food ingredients: A review of bioactive compounds and health-promoting activities. Food Biosci 2023;54:102914.
- Chen HJ, Huang JY, Ko CY. Peach kernel extracts inhibit lipopolysaccharide?induced activation of HSC?T6 hepatic stellate cells. Int J clin Pract 2022;2022(1):4869973.
[Crossref] [Google Scholar] [PubMed]
- Tsai FM, Lu PH, Wang LK, Kuo CY, Chen ML, Wang CH. Flavonoids in safflower extract reduce cisplatin-induced damage to human follicle dermal papilla cells by inhibiting DNA damage and Rad17/Chk1/Cdc25C signaling. Biocell 2023;47(8).
- Tsai FM, Wang LK, Chen ML, Lee MC, Lin YY, Wang CH. Induction of cell proliferation and cell death in human follicle dermal papilla cells by zinc chloride. Indian J Pharm Sci 2019;81(4):781-5.
- Wang CH, Lu TJ, Wang LK, Wu CC, Chen ML, Kuo CY, et al. Tazarotene?induced gene 1 interacts with Polo?like kinase 2 and inhibits cell proliferation in HCT116 colorectal cancer cells. Cell Biol Int 2021;45(11):2347-56.
[Crossref] [Google Scholar] [PubMed]
- Wang CH, Tzeng IS, Wang LK, Wu CC, Chen ML, Kuo CY, et al. Tazarotene-induced gene 1 induces melanoma cell death by triggering endoplasmic reticulum stress response. Front Biosci (Landmark Ed) 2024;29(6):233.
[Crossref] [Google Scholar] [PubMed]
- Rastegar H, Ashtiani HA, Aghaei M, Barikbin B, Ehsani A. Herbal extracts induce dermal papilla cell proliferation of human hair follicles. Ann Dermatol 2015;27(6):667-75.
[Crossref] [Google Scholar] [PubMed]
- Hao E, Pang G, Du Z, Lai YH, Chen JR, Xie J, et al. Peach kernel oil downregulates expression of tissue factor and reduces atherosclerosis in ApoE knockout mice. Int J Mol Sci 2019;20(2):405.
[Crossref] [Google Scholar] [PubMed]
- Lee CY, Wei CC, Yu MC, Lin CC, Sheu SJ, Yang JH, et al. Hair growth effect of traditional Chinese medicine BeauTop on androgenetic alopecia patients: A randomized double-blind placebo-controlled clinical trial. Exp Ther Med 2017;13(1):194-202.
[Crossref] [Google Scholar] [PubMed]
- Mingsan M, Mengfan P, Dandan L, Zhengwang Z. Effects of AiQingHua oil on microcirculation disturbance and alopecia mice model. J King Saud Univ Sci 2020;32(6):2669-74.
- Nowicka P, Wojdy?o A. Content of bioactive compounds in the peach kernels and their antioxidant, anti-hyperglycemic, anti-aging properties. Eur Food Res Technol 2019;245(5):1123-36.
- Takahashi T, Kamimura A, Kagoura M, Toyoda M, Morohashi M. Investigation of the topical application of procyanidin oligomers from apples to identify their potential use as a hair?growing agent. J Cosmet Dermatol 2005;4(4):245-9.
[Crossref] [Google Scholar] [PubMed]
- Franklin G, Dias AC. Chlorogenic acid participates in the regulation of shoot, root and root hair development in Hypericum perforatum. Plant Physiol Biochem 2011;49(8):835-42.
[Crossref] [Google Scholar] [PubMed]
- Narukawa M, Kanbara K, Tominaga Y, Aitani Y, Fukuda K, Kodama T, et al. Chlorogenic acid facilitates root hair formation in lettuce seedlings. Plant Cell Physiol 2009;50(3):504-14.
[Crossref] [Google Scholar] [PubMed]
- Takahashi T, Kamiya T, Yokoo Y, Hasegawa A. Procyanidin oligomers selectively and intensively promote proliferation of mouse hair epithelial cells in vitro and activate hair follicle growth in vivo. J Invest Dermatol 1999;112(3):310-6.
[Crossref] [Google Scholar] [PubMed]
- Carelli S, Hebda DM, Traversa MV, Messaggio F, Giuliani G, Marzani B, et al. A specific combination of zeaxanthin, spermidine and rutin prevents apoptosis in human dermal papilla cells. Exp Dermatol 2012;21(12):953-5.
[Crossref] [Google Scholar] [PubMed]
- Almohanna HM, Ahmed AA, Tsatalis JP, Tosti A. The role of vitamins and minerals in hair loss: A review. Dermatol Ther 2019;9(1):51-70.
[Crossref] [Google Scholar] [PubMed]
- Zheng J, Liang J, Deng X, Chen X, Wu F, Zhao X, et al. Mitogen activated protein kinase signaling pathways participate in the active principle region of Buyang Huanwu decoction-induced differentiation of bone marrow mesenchymal stem cells. Neural Regen Res 2012;7(18):1370-7.
[Google Scholar] [PubMed]
- Lee HJ, Im AR, Kim SM, Kang HS, Lee JD, Chae S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (MMP)-9 expression via mitogen activated protein kinase (MAPK)-dependent signaling pathways. BMC Complement Altern Med 2018;18:1-9.
[Crossref] [Google Scholar] [PubMed]
- Cheng D, Zhang X, Tang J, Kong Y, Wang X, Wang S. Chlorogenic acid protects against aluminum toxicity via MAPK/Akt signaling pathway in murine RAW264. 7 macrophages. J Inorg Biochem 2019;190:113-20.
[Crossref] [Google Scholar] [PubMed]
- Feng R, Lu Y, Bowman LL, Qian Y, Castranova V, Ding M. Inhibition of activator protein-1, NF-κB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J Biol Chem 2005;280(30):27888-95.
[Crossref] [Google Scholar] [PubMed]
- Kang NJ, Lee KW, Lee DE, Rogozin EA, Bode AM, Lee HJ, et al. Cocoa procyanidins suppress transformation by inhibiting mitogen-activated protein kinase kinase. J Biol Chem 2008;283(30):20664-73.
[Crossref] [Google Scholar] [PubMed]
- Liu WY, Liou SS, Hong TY, Liu IM. Protective effects of hesperidin (citrus flavonone) on high glucose induced oxidative stress and apoptosis in a cellular model for diabetic retinopathy. Nutrients 2017;9(12):1312.
[Crossref] [Google Scholar] [PubMed]
- Cha JW, Piao MJ, Kim KC, Yao CW, Zheng J, Kim SM, et al. The polyphenol chlorogenic acid attenuates UVB-mediated oxidative stress in human HaCaT keratinocytes. Biomol Ther 2014;22(2):136.
[Crossref] [Google Scholar] [PubMed]
- Matito C, Agell N, Sanchez-Tena S, Torres JL, Cascante M. Protective effect of structurally diverse grape procyanidin fractions against UV-induced cell damage and death. J Agric Food Chem 2011;59(9):4489-95.
[Crossref] [Google Scholar] [PubMed]
- Pongcharoen S, Warnnissorn P, Lertkajornsin O, Limpeanchob N, Sutheerawattananonda M. Protective effect of silk lutein on ultraviolet B-irradiated human keratinocytes. Biol Res 2013;46(1):39-45.
[Crossref] [Google Scholar] [PubMed]
- Xi S, Qian L, Tong H, Yue L, Zhao H, Wang D, Lu D, Li P, Wang X. Toxicity and clinical reasonable application of Taoren (Semen Persicae) based on ancient and modern literature research. J Tradit Chin Med 2013;33(2):272-9.
[Crossref] [Google Scholar] [PubMed]