- Corresponding Author:
- Santos-Silva M.C
Departments of Clinical Analysis Federal University of Santa Catarina, Campus Trinity, CEP: 88040-900,Florianópolis-SC, Brazil
E-mail: maria.claudia.silva@ufsc.br
| Date of Submission | 18 February 2015 |
| Date of Revision | 03 January 2016 |
| Date of Acceptance | 23 February 2016 |
| Indian J Pharm Sci 2016;78(1):120−128 |
This is an open access article distributed under the terms of the Creative Commons Attribution−NonCommercial−ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non−commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Recent studies have shown that gallic acid and its alkylesters induce apoptosis in different cell lines. Since new compounds with biological activity and less cytotoxicity to normal cells are necessary for cancer therapy, the aim of this study was to evaluate the cytotoxic effect of 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate on human acute myeloid leukemia K562 cells and on human acute lymphoblastic leukemia Jurkat cells. The cell viability was determined by MTT method. The apoptosis induction was assessed by bromide and acridine orange staining and by Annexin V-FITC Apoptosis Detection kit. The cell cycle analysis was carried out by flow cytometry using propidium iodide. Cytometric analysis was also performed to evaluate the expression of the following proteins: AIF, p53, Bcl-2 and Bax. The mitochondrial potential was also assessed by flow cytometry using MitoView633 kit. The results showed that the compound significantly reduced the cell viability of K562 and Jurkat cells in a concentration and time dependent manner (IC50of 30 μM). The compound induced cell cycle arrest in G0/G1phase and significantly increased the proportion of cells in the sub-G0/G1phase. Apoptosis was confirmed by the sight of morphological characteristics of apoptosis and by phosphatidylserine externalization (73.47±5.71% of cells expressing annexin). The results also showed that the compound promotes a modification in Bax:Bcl-2 ratio and increases p53 expression. Thus, it is possible to conclude that 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate induces apoptosis by inhibiting the antiapoptotic protein Bcl-2 and by increasing the release of AIF, Bax and p53. In addition, it blocks the cell cycle at G0/G1, stopping cell proliferation. So far, the results suggest that this compound may have a potential therapeutic effect against leukemia cells.
Keywords
Apoptosis, gallic acid, acute leukemia, cytotoxicity
Acute leukemia is a malignant proliferation of immature hematopoietic cells in bone marrow or in lymphoid tissues [1-3]. Current trends in leukemia treatment suggest the use of natural substances not only in order to prevent the initiating events of malignancy, but also as a source of new antitumor drugs with apoptotic activity against tumor cells with minimal side effects for the patient [4]. In this context, plants have become an important source of biologically active products, such as flavonoids, saponines and quinones. Gallic acid is a flavonoid often found as a component of hydrolysable tannins in plants and it can be obtained by acid hydrolysis of these tannins [5,6]. The term “gallate” refers to esters of gallic acid, and the dodecyl gallate (1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate or 3,4,5-acid trihydroxybenzoic, C19H30O5) is also known as lauryl gallate (fig. 1). Several studies have shown that dodecyl gallate and its alkyl esters have antiproliferative and cytotoxic effects on different cell lines. In addition, it is known that more hydrophobic members, such as dodecyl gallate, exhibit an apoptotic potential between 50 and 250 times higher than gallic acid [7-11]. It has also been shown a selectivity for fast-growing cells, which indicates the possibility of a potent antitumor agent [12].
The homeostasis of normal cells is the result of a balance between proliferation, differentiation and programmed cell death (apoptosis), whereas malignant processes are characterized by the excessive expansion of tumor cells due to defects in one or more of these processes. Therefore, understanding the mechanisms of cell cycle regulation, the molecular apoptotic machinery and its defects in acute leukemia is a critical step for the identification of new targets in anticancer therapy [13,14]. Thus, the aim of this study was to assess the cytotoxic effect of 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate on human leukemia cell lines (K562 and Jurkat cells) and investigate some of its mechanisms in order to discuss the interest of this compound as an antitumor agent.
Materials and Methods
Cell culture medium, fetal bovine serum and antibiotics were purchased from GIBCO (São Paulo, Brazil) and all other reagents were purchased from Sigma Chemical Co. (St. Louis, MO). The compound 1-(3,4,5-trihydroxyphenyl)- dodecylbenzoate was synthesized and characterized as described elsewhere [9]. To prepare the stock solution, the compound was dissolved in 100% dimethyl sulfoxide (DMSO), and then diluted in cell culture medium to a final concentration of 0.01% of DMSO.
Cell culture and viability assay (MTT assay)
Human acute myeloid leukemia (AML) K562 cells and human acute lymphoblastic leukemia (ALL) Jurkat cells (ATCC, Manassas, USA) were maintained in Roswell Park Memorial Institute Medium (RPMI; GIBCO, São Paulo, Brazil) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 10 mm HEPES, pH 7.4. The cells were maintained in plastic culture flasks at 37o at a 5% CO2 humidified atmosphere. Cells were seeded at a density of 106 cells per bottle and medium was changed every three days. Cell viability was assessed by MTT method (3-(4,5-dimethiazol-zyl)- 2-5-diphenyltetrazolium bromide, Sigma Chemical Co., St Louis, USA) [15]. Briefly, the compound was added to K562 and Jurkat cells at different concentrations (1-100 μM) and incubated for 24, 48 or 72 h. The same volume of DMSO was added to control wells. MTT solution was added to each well (10% v/v) and the plates were incubated for 3 h. After incubation, the supernatant was discarded, the formazan precipitated was dissolved with 100 ml of an isopropyl alcohol-HCl 0.04 N solution and the absorbance was determined at 540 nm. All assays were performed in triplicate and the IC50 values (50% inhibitory concentration) were calculated using GraphPad Prism software package for Windows (v. 5.0 GraphPad Prism Software Inc, San Diego, CA).
Cell cycle analysis
The compound’s effect on cell cycle was investigated by PI/RNase solution kit (Immunostep, Salamanca, Spain). Cells (1×106 cells/well) were incubated with vehicle or 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate at their respectively IC50. After 24 h of incubation, cells were harvested and cell cycle analysis was assessed according to the kit protocol. Briefly, cells were harvested, washed with PBS and centrifuged for 5 min at 300 g. The supernatant was removed and cells were fixed with 70% ethanol for 30 min at 4°. Cells were washed once with PBS supplemented with 2% bovine albumin, centrifuged for 5 min at 300 g and then the supernatant was removed. To the cell pellet it was added 500 μl of PI/RNase solution followed by 15 min incubation at room temperature. Finally, the analysis was performed by flow cytometry as previously described.
Evaluation of apoptosis
Apoptotic cell death was assessed by ethidium bromide (EB) and acridine orange (AO) assay and by Annexin V-FITC Apoptosis Detection kit. For the first method, 40 μl of a mixture of AO (10 μg/ml) and EB (5 μg/ml) was added to cells treated at their respective IC50 for 12, 24 and 48 h. Cells with no treatment were used as a control group. The cells were observed in a fluorescence microscope (Olympus BX-FLA) and representative fields were photographed by digital camera (Olympus BX40, Japan). The Annexin V-FITC Apoptosis Detection kit was used according to the manufacturer’s instructions. Briefly, 1×106 cells/well were incubated with the vehicle or 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate at their respectively IC50. After 16 h of incubation, cells were harvested, washed with PBS or annexin buffer (1:10) and double-stained with Annexin V-FITC solution and PI/RNase solution. After incubation, 300 μl of annexin buffer was added and fluorescence was analyzed by flow cytometry. Analysis was performed by FACSCantoIITM, Becton Dickinson Immunocytometry Systems. The data were analyzed by Infinicyt Software version 1.7.0 (Cytognos S.L., Salamanca, Spain).
Evaluation of the mitochondrial membrane potential
The evaluation of the mitochondrial membrane potential was performed using MitoView633TM kit (Biotium, Inc., Hayward, USA). K562 and Jurkat cells (1.0×106 cells/well) were incubated with the vehicle or 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate at their respectively IC50 for 12 h. Cells were labeled as described by the manufacturer. Briefly, cells were collected by centrifugation and washed twice with PBS. Then, they were resuspended in 100 μl of MitoView 633 solution (1:10000). After incubation in the dark for 30 min, cells were washed and resuspended with 1 ml PBS. Analyses were performed by flow cytometry as previously described.
Effect on p53, Bcl-2, Bax, AIF and FasR proteins To evaluate the compound’s effect on p53, Bcl-2, Bax, AIF and FasR proteins, 1×106 cells/well were incubated with 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate in their respectively IC50 for 24 h. Cells were washed with PBS and permeabilized with ethanol 70% (except FasR). Subsequently, the cells were incubated in the dark at room temperature, with antiAIF-PE,antip53-PE, antiBcl-2-FITC, antiBax-FITC and antiFasR-PE for the detection of AIF, p53, Bcl-2 and Bax, respectively. After incubation, the cells were washed with PBS/ albumin 2% and resuspended in PBS for cytometric analysis. Analyses were performed by flow cytometry as previously described.
Statistical analysis
Analyses were performed using GraphPad Prism software package for Windows (v. 5.0 GraphPad Prism Software Inc, San Diego, CA). A mixed model analysis of variance (ANOVA) was used in combination with post hoc testing (Bonferroni) to compare the cytotoxic effect of 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate on K562 and Jurkat cells. The level of significance was P<0.05 for all statistical analyses.
Results
Effect of 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate on cell viability
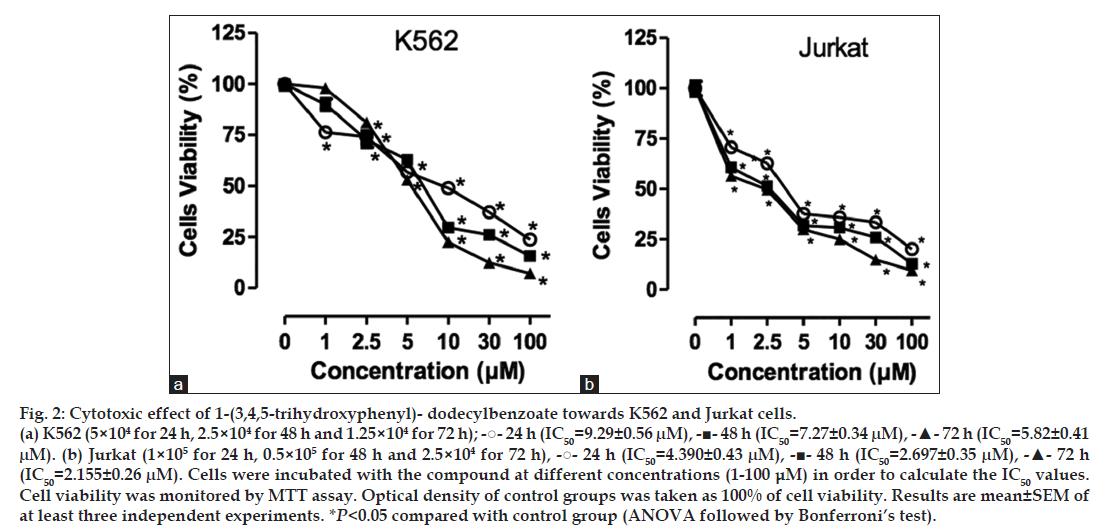
The cytotoxicity of 1-(3,4,5-trihydroxyphenyl)- dodecylbenzoate towards K562 and Jurkat cells was determined by MTT assay. After incubation with increasing concentrations (1-100 μM) of the compound for 24, 48 and 72 h, the percentage of viable cells was reduced in a concentration and time dependent manner when compared with the control group (nontreated cells) in both cell lines. The IC50 values obtained in K562 cells were 9.29±0.56, 7.03±0.34 and 5.82±0.41 μM for 24, 48 and 72 h, respectively (fig. 2a). The same concentration and time-dependent profile was observed in Jurkat cells after treatment with 1-(3,4,5-trihydroxyphenyl)- dodecylbenzoate (fig. 2b), but the compound was even more cytotoxic against this cell line, with IC50 values of 4.39±0.43, 2.70±0.35, and 2.15±0.26 μM for 24, 48 and 72 h, respectively.
Fig. 2: Cytotoxic effect of 1-(3,4,5-trihydroxyphenyl)- dodecylbenzoate towards K562 and Jurkat cells. (a) K562 (5×104 for 24 h, 2.5×104 for 48 h and 1.25×104 for 72 h); -?- 24 h (IC50=9.29±0.56 μM), -?- 48 h (IC50=7.27±0.34 μM), -?- 72 h (IC50=5.82±0.41μM). (b) Jurkat (1×105 for 24 h, 0.5×105 for 48 h and 2.5×104 for 72 h), -?- 24 h (IC50=4.390±0.43 μM), -?- 48 h (IC50=2.697±0.35 μM), -?- 72 h(IC50=2.155±0.26 μM). Cells were incubated with the compound at different concentrations (1-100 μM) in order to calculate the IC50 values. Cell viability was monitored by MTT assay. Optical density of control groups was taken as 100% of cell viability. Results are mean±SEM of at least three independent experiments. *P<0.05 compared with control group (ANOVA followed by Bonferroni’s test).
Effect of 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate on cell cycle
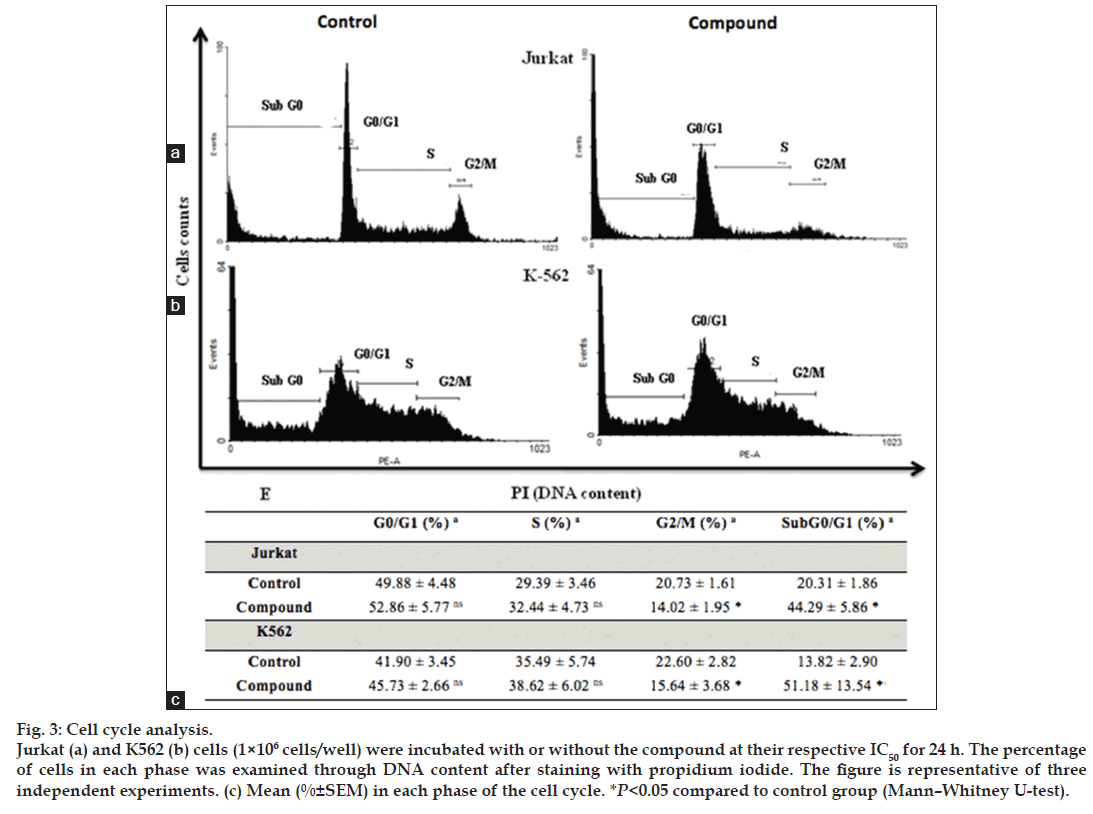
K562 and Jurkat cells, treated for 24 h with 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate in their respective IC50, had a significant increase in the population of cells in sub-G0/G1 phase. A total of 44.29±5.86 % Jurkat cells and 51.18±13.54 % K562 cells were located in this phase. It was possible to observe a significant decrease in the population of cells in the G2M phase (14.02±1.94 % for Jurkat and 15.64±3.68 % for K562) (fig. 3).
Fig. 3: Cell cycle analysis. Jurkat (a) and K562 (b) cells (1×106 cells/well) were incubated with or without the compound at their respective IC50 for 24 h. The percentage of cells in each phase was examined through DNA content after staining with propidium iodide. The figure is representative of three independent experiments. (c) Mean (%±SEM) in each phase of the cell cycle. *P<0.05 compared to control group (Mann?Whitney U-test).
Effect of 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate on apoptosis
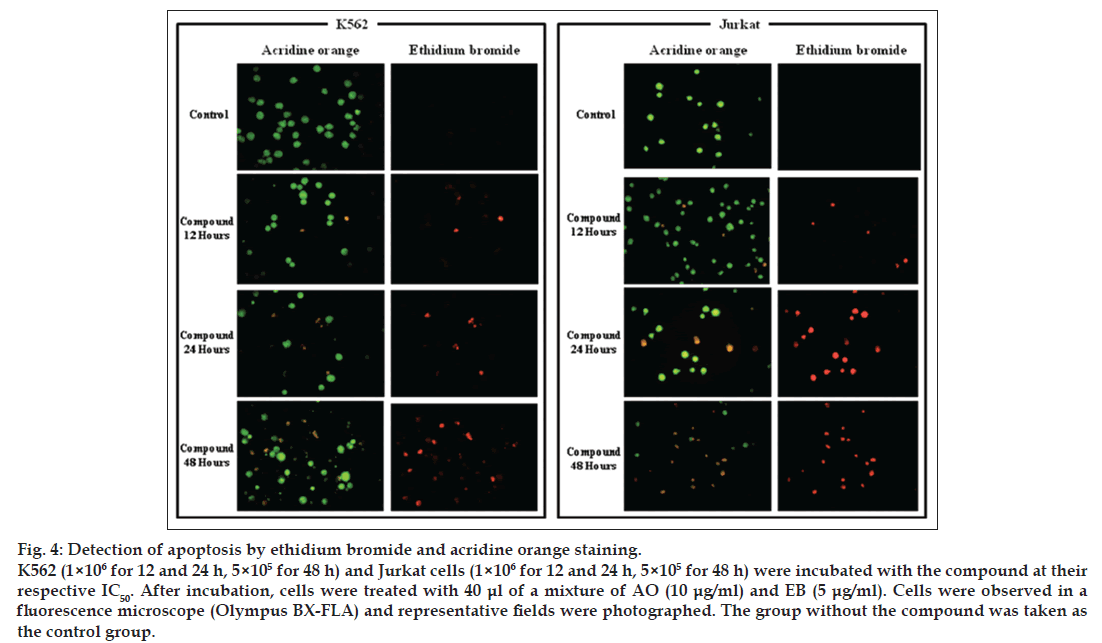
To evaluate whether K562 and Jurkat cells treated with 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate and located at the sub-G0/G1 phase were undergoing an apoptotic or necrotic pathway, two different methods were used. First, the cells were treated with the compound at their respective IC50 for 12, 24 and 48 h and then stained with EB/AO to be observed in a fluorescence microscope. AO permeates all cells and stains them with green fluorescence, while EB is only taken up by cells when cytoplasmic membrane integrity is lost, showing a red fluorescence. Thus, live cells usually have a green fluorescence; early apoptotic cells have bright green nuclei with condensed or fragmented chromatin; late apoptotic cells display condensed and fragmented orange chromatin; and necrotic cells have a structurally-normal orange nucleus [16]. As it may be seen in fig. 4, 1-(3,4,5-trihydroxyphenyl)- dodecylbenzoate induced apoptosis on K562 and Jurkat cells, which is determined by their staining with EB and characteristic morphology. The number of apoptotic cells increased after 24 and 48 h, confirming the result that this compound reduces the percentage of viable cells in a time-dependent manner.
Fig. 4: Detection of apoptosis by ethidium bromide and acridine orange staining. K562 (1×106 for 12 and 24 h, 5×105 for 48 h) and Jurkat cells (1×106 for 12 and 24 h, 5×105 for 48 h) were incubated with the compound at their respective IC50. After incubation, cells were treated with 40 ?l of a mixture of AO (10 ?g/ml) and EB (5 ?g/ml). Cells were observed in a fluorescence microscope (Olympus BX-FLA) and representative fields were photographed. The group without the compound was taken as the control group.
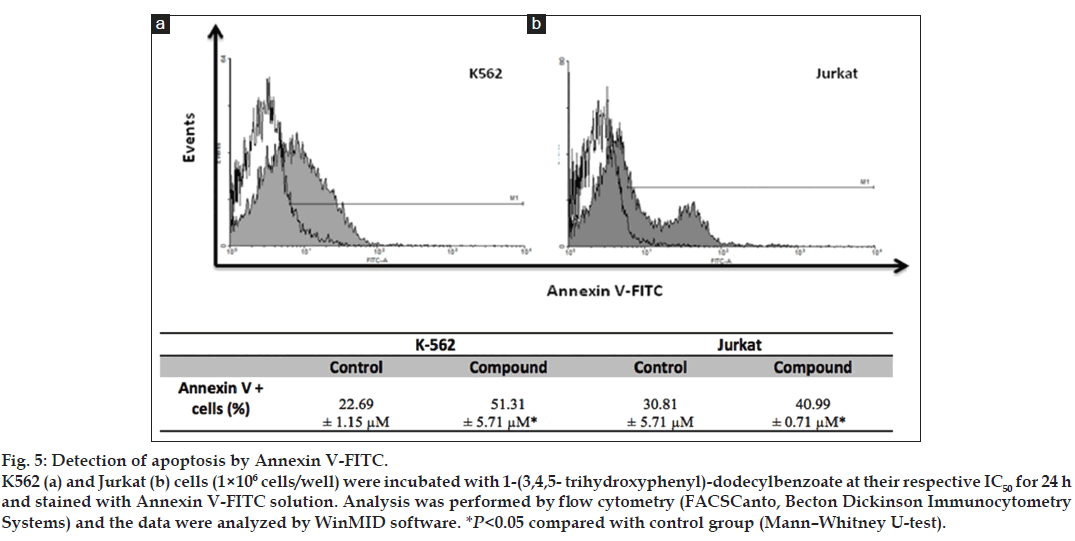
The apoptotic effect of 1-(3,4,5-trihydroxyphenyl)- dodecylbenzoate was confirmed by AnnexinV-FITC method. As shown in fig. 5, the treatment with the compound significantly increased the percentage of annexin positive cells on K562 (51.31±5.71%) and Jurkat (40.99±0.71%) cell lines after 24h when compared with the respective control groups (22.69±1.15% and 30.81±5.71%, respectively).
Fig. 5: Detection of apoptosis by Annexin V-FITC. K562 (a) and Jurkat (b) cells (1×106 cells/well) were incubated with 1-(3,4,5- trihydroxyphenyl)-dodecylbenzoate at their respective IC50 for 24 h and stained with Annexin V-FITC solution. Analysis was performed by flow cytometry (FACSCanto, Becton Dickinson Immunocytometry Systems) and the data were analyzed by WinMID software. *P<0.05 compared with control group (Mann−Whitney U-test).
Effect of 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate on the mitochondrial membrane potential
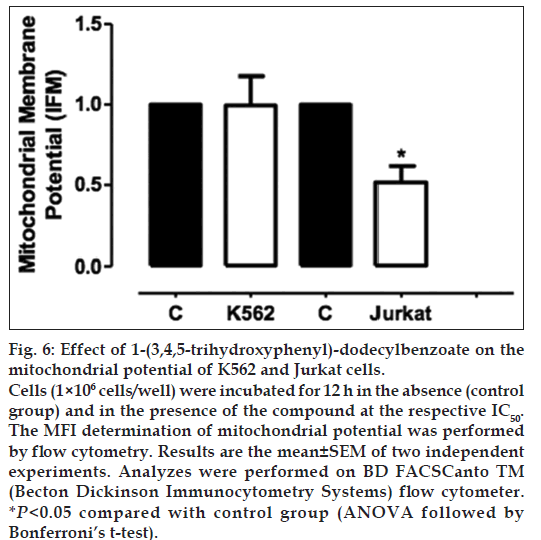
According to fig. 6, Jurkat cells treated with 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate for 12h showed a decreased mitochondrial membrane potential when compared with control group (IFM of 0.52±0.03). This result suggests the involvement of the intrinsic pathway in apoptotic cell death induced by 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate. However, this result was not observed for K562 cells.
Fig. 6: Effect of 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate on the mitochondrial potential of K562 and Jurkat cells. Cells (1×106 cells/well) were incubated for 12 h in the absence (control group) and in the presence of the compound at the respective IC50. The MFI determination of mitochondrial potential was performed by flow cytometry. Results are the mean±SEM of two independent experiments. Analyzes were performed on BD FACSCanto TM (Becton Dickinson Immunocytometry Systems) flow cytometer. *P<0.05 compared with control group (ANOVA followed by Bonferroni's t-test).
Effect of 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate on the expression of AIF, p53, Bcl-2, Bax and FasR proteins
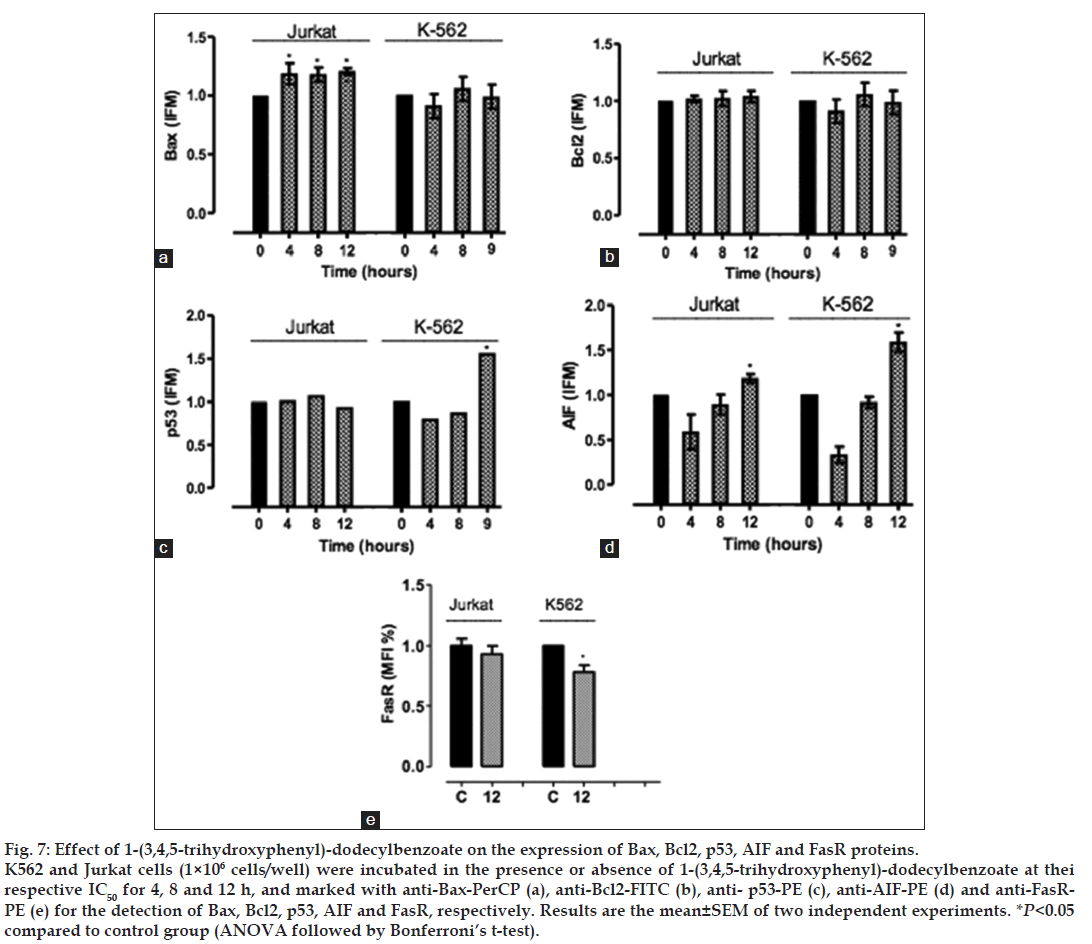
As shown in fig. 7, 1-(3,4,5-trihydroxyphenyl)- dodecylbenzoate increased the expression of proapoptotic protein Bax in Jurkat cells after 4, 8 and 12 h treatment, which confirms the involvement of the intrinsic apoptotic pathway in this cell line. Again, no significant difference was observed on Bax or Bcl-2 expression on K562 cells. Interestingly, the compound increased the expression of AIF in both K562 and Jurkat cell lines after 12 h treatment. Fig. 7 also demonstrates that 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate decreased the expression of FasR and increased the expression of p53 on K562 cells.
Fig. 7: Effect of 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate on the expression of Bax, Bcl2, p53, AIF and FasR proteins. K562 and Jurkat cells (1×106 cells/well) were incubated in the presence or absence of 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate at thei respective IC50 for 4, 8 and 12 h, and marked with anti-Bax-PerCP (a), anti-Bcl2-FITC (b), anti- p53-PE (c), anti-AIF-PE (d) and anti-FasRPE (e) for the detection of Bax, Bcl2, p53, AIF and FasR, respectively. Results are the mean±SEM of two independent experiments. *P<0.05 compared to control group (ANOVA followed by Bonferroni′s t-test).
Discussion
Gallic acid and its alkyl esters have been studied for their cytotoxic activity on different tumor cell lines[7,12,15-17]. According to the literature, these compounds also inhibit the proliferation of HL60 [18], JY, MOLT-4, HT29 [12] and other cell lines [19]. Moreover, it is known that alkyl esters of gallic acid with lipophilic group such as octyl and dodecyl (lauryl), exhibit a potential antiproliferative effect between 50 and 250 times greater than the actual gallic acid [8,9,15,20]. Furthermore, the cytotoxic effect of alkyl esters of gallic acid is much lower in normal cells than in cancer cells [9,15], which demonstrates selectivity towards neoplastic cells.
The results presented in this paper are consistent with the data described in the literature, since we demonstrated that 1-(3,4,5-trihydroxyphenyl)- dodecylbenzoate, an alkylester derivated from gallic acid, was cytotoxic against AML K562 and ALL Jurkat cell lines in a concentration and time-dependent manner (fig. 2). Furthermore, we demonstrated that the compound was slightly more cytotoxic against ALL cells.
The mechanisms of antileukemia compounds are based primarily on the inhibition of cell proliferation and on apoptosis induction. Apoptosis is a programmed cell death characterized by cell shrinkage, chromatin condensation and formation of apoptotic bodies [22]. Changes in the cell cycle and on regulatory proteins are often associated with the induction of apoptosis and are crucial to the development of new therapies. Many of the apoptotic effects of phenolic compounds, including 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate, have been attributed to disorders in cell cycle [23]. Ours results clearly demonstrate a decreased number of cells in G2M phase after 24h incubation with the compound and an increased number of cells in sub-G0/G1 phase (fig. 3). This may indicate that the mechanism by which 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate inhibits the growth of leukemia cells is related to the cell cycle.
Several studies have shown that derivatives of gallic acid with lipophilic groups such as 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate present an ability to induce apoptosis on human leukemia cells [8,15,16,22,24]. This information was consistent to our findings, since typical morphological changes of apoptosis could be observed on K562 and Jurkat cells treated with the compound for 12, 24 and 48 h (fig. 4) after staining with ethidium bromide and acridine orange. The apoptotic death was confirmed in both cell lines by the increased Annexin V positivity (fig. 5) after treatment with 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate. According to the literature, the first sign of apoptosis is the externalization of phosphatidylserine residues, which bind to Annexin V after incubation, thus indicating cell death by apoptosis[12,20,24,25].
The mechanisms that regulate apoptosis involve the activation of different signaling pathways and inhibitory components that culminate in cellular self-destruction [26]. A common feature of apoptosis is the activation of proteins called caspases, which act as effectors in cell death [22]. Other proteins such as p53, Bax and Bcl-2 are also involved in apoptosis regulation. The tumor suppressor protein p53 acts in the cell cycle, at the first checkpoint located between G1 and S phases, through the activation of several pro-apoptotic effectors genes or inhibitors of growth [13,28]. Our results demonstrate that there was an increased expression of p53 after treatment with 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate.
Bax and Bcl-2 are members of the Bcl-2 family [27]. The protein Bcl-2 has been described in the literature for its antiapoptotic effect on different cell lines [27,28]. Studies show that Bcl-2 retards the induction of apoptosis by 1-(3,4,5-trihydroxyphenyl)- dodecylbenzoate in WEHI231 and MCF7 cells [20]. Furthermore, it is known that the overexpression of Bcl-2 acts preventing the mitochondrial release of AIF [29]. Our results show that K562 and Jurkat cells express antiapoptotic protein Bcl-2 but its expression was not decreased by 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate. This result suggests the involvement of other proteins in the induction of apoptosis. However, the compound increased the expression of proapoptotic Bax protein which may contribute to cell death. In addition, the compound strongly increased the expression of AIF in both K562 and Jurkat cells. AIF is a mitochondrial protein described in literature by its proapoptotic activities, which is important to the understanding of mitochondrial dysfunction and apoptosis regulation [30].
According to the literature, members of the Bcl- 2 family such as Bax and Bcl-2 regulate the mitochondrial membrane permeability and potential. After 12 h treatment, 1-(3,4,5-trihydroxyphenyl)- dodecylbenzoate reduced the number of cells with intact mithocondrial membrane potential. This result, combined with the changes in expression of Bax and AIF, indicate that the mechanism of apoptosis induced by this compound involve the intrinsic pathway.
The evidences obtained so far suggest that one of the likely mechanisms of cytotoxicity induced by 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate occurs by the increased expression of Bax, p53 and AIF proteins, by the blockage of cell cycle and by the reduction of the mithocondrial membrane potential. The different results observed for K562 and Jurkat cell lines might be explained by the differences in the etiopathology of AML and ALL, respectively. Although further studies are needed in order to completely understand its mechanism of action, the inhibitory effect of1-(3,4,5-trihydroxyphenyl)- dodecylbenzoate brings new options to the development of new drugs.
In conclusion, the compound 1-(3,4,5-t rihydroxyphenyl)-dodecylbenzoate significantly reduced the cell viability of K562 and Jurkat cells in a concentration-and-time dependent manner. In addition, leukemia cells treated with this compound revealed an increase in p53 expression and, regarding the cell cycle, a decreased population in G2M phase and an increased number of cells in sub-G0/G1 phase. The increased expression of Bax by 1-(3,4,5-trihydroxyphenyl)-dodecylbenzoate results in a loss of mitochondrial membrane potential, which allows the release of AIF by mitochondria and promotes the induction of apoptosis. These results suggest that this compound is effective against AML and ALL and may be used as a new therapeutic strategy on acute leukemia.
Acknowledgements
We would like to thank the University Hospital Polydori Ernani de São Thiago – UFSC, the Post- Graduate Program in Pharmacy – UFSC.
Financial support and sponsorship
Authors like to thank The Coordination of Superior Level Staff Improvement (CAPES) for financial support.
Conflicts of interest
There are no conflicts of interest.
References
- Bain BJ. DiagnósticoemLeucemias. 2nd ed. Rio de Janeiro: Revinter Ltd; 2003.
- Pui CH. Acute lymphoblastic leukemia: Introduction. SeminHematol 2009;46:1-2.
- Vardiman JW. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: An overview with emphasis on the myeloid neoplasms. ChemBiol Interact 2010;184:16-20.
- Silva IT, Costa GM, Stoco PH, Schenkel EP, Reginatto FH, Simões CM. In vitro antiherpes effects of a C-glycosylflavonoid-enriched fraction of CecropiaglazioviiSneth.LettApplMicrobiol 2010;51:143-8.
- Grundhöfer P, Niemetz R, Schilling G, Gross GG. Biosynthesis and subcellular distribution of hydrolyzable tannins. Phytochemistry 2001;57:915-27.
- Simões C, Schenkel E, Gosmann G, Mello J, Mentz L, Petrovick P. Farmacognosia: Da PlantaaoMedicamento. 1st ed. Porto Alegre (RS): Editora da UFSC; 2003.
- Saeki K, Yuo A, Isemura M, Abe I, Seki T, Noguchi H. Apoptosis-inducing activity of lipid derivatives of gallic acid. Biol Pharm Bull 2000;23:1391-4.
- Gomes CA, da Cruz TG, Andrade JL, Milhazes N, Borges F, Marques MP. Anticancer activity of phenolic acids of natural or synthetic origin: A structure-activity study. J Med Chem 2003;46:5395-401.
- Savi LA, Leal PC, Vieira TO, Rosso R, Nunes RJ, Yunes RA, et al. Evaluation of antiherpetic and antioxidant activities, andcytotoxic and genotoxic effects of synthetic alkyl-esters of gallic acid. Arzneimittelforschung 2005;55:66-75.
- Chen KS, Hsiao YC, Kuo DY, Chou MC, Chu SC, Hsieh YS, et al. Tannic acid-induced apoptosis and -enhanced sensitivity to arsenictrioxide in human leukemia HL-60 cells. Leuk Res 2009;33:297-307.
- Locatelli C, Filippin-Monteiro FB, Creczynski-Pasa TB. Alkyl esters of gallic acid as anticancer agents: A review. Eur J Med Chem 2013;60:233-9.
- Serrano A, Palacios C, Roy G, Cespón C, Villar ML, Nocito M, et al. Derivatives of gallic acid induce apoptosis in tumoral celllines and inhibit lymphocyte proliferation. Arch BiochemBiophys 1998;350:49-54.
- Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 2007;12:1543-68.
- Zhivotovsky B, Orrenius S. Defects in the apoptotic machinery of cancer cells: Role in drug resistance. Semin Cancer Biol 2003;13:125-34.
- van de Loosdrecht AA, Beelen RH, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MM. A tetrazolium-based colorimetric MTT assay to quantitative human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods 1994;174:311-20.
- Renvoizé C, Biola A, Pallardy M, Bréard J. Apoptosis: Identification of dying cells. Cell BiolToxicol 1998;14:111-20. Calcabrini A, García-Martínez JM, González L, Tendero MJ,
- Calcabrini A, García-Martínez JM, González L, Tendero MJ,Ortuño MT, Crateri P, et al. Inhibition of proliferation and induction of apoptosis in human breast cancer cells by lauryl gallate. Carcinogenesis 2006;27:1699-712.
- Ortega E, Sadaba MC, Ortiz AI, Cespon C, Rocamora A, Escolano JM, et al. Tumoricidal activity of lauryl gallate towards chemically inducedskin tumours in mice. Br J Cancer 2003;88:940-3.
- Inoue M, Suzuki R, Koide T, Skaguchi N, Ogihara Y, Yabu Y. Antioxidant, gallic acid, induces apoptosis in Hl-60RG cells. Arch BiochemBiophys 1995;18:1526-30.
- Roy G, Lombardía M, Palacios C, Serrano A, Cespón C, Ortega E, et al. Mechanistic aspects of the induction of apoptosis by lauryl gallate in the murine B-cell lymphoma line wehi 231. Arch BiochemBiophys 2000;383:206-14.
- Fiuza SM, Gomes C, Teixeira LJ, Girão da Cruz MT, Cordeiro MN, Milhazes N, et al. Phenolic acid derivatives with potential anticancer properties − A structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg Med Chem 2004;12:3581-9.
- Wyllie AH. ‘‘Where, O death, is thy sting’’ A brief review of apoptosis biology. MolNeurobiol 2010;42:4-9.
- Fulda S. Cell death in hematological tumors. Apoptosis 2009;14:409-23.
- Yi Z, Wang Z, Li H, Liu M. Inhibitory effect of tellimagrandin I on chemically induced differentiation of human leukemia K562 cells. ToxicolLett 2004;147:109-19.
- Lung HL, Ip WK, Wong CK, Mak NK, Chen ZY, Leung KN. Anti-proliferative and differentiation-inducing activities of the green tea catechin epigallocatechin-3-gallate (EGCG) on the human eosinophilicleukemia EoL-1 cell line. Life Sci 2002;72:257-68.
- Reed CJ. Apoptosis and cancer: Strategies for integrating programmed cell death. SeminHematol 2000;37 4 Suppl 7:9-16.
- Adams JM, Cory S. Bcl-2-regulated apoptosis: Mechanism and therapeutic potential. CurrOpinImmunol 2007;19:488-96.
- Abbas T, Dutta A. p21 in cancer: Intricate networks and multiple activities. Nat Rev Cancer 2009;9:400-14.
- Schulze-Bergkamen H, Krammer PH. Apoptosis in cancer ? Implications for therapy. SeminOncol 2004;31:90-119.
- Prabhu SB, Khalsa JK, Banerjee H, Das A, Srivastava S, Mattoo HR, et al. Role of apoptosis-inducing factor (Aif) in the T cell lineage. Indian J Med Res 2013;138:577-90.