- *Corresponding Author:
- Jianxia Li
Department of Neurosurgery, Zhongda Hospital, Southeast University, Nanjing, Jiangsu 210009, China
E-mail: 378971049@qq.com
| Date of Received | 14 May 2021 |
| Date of Revision | 09 July 2022 |
| Date of Acceptance | 10 December 2022 |
| Indian J Pharm Sci 2022;84(6):1576-1581 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect of monosialotetrahexosylganglioside sodium on cortical neurogenesis in traumatic brain injury rats. Thirty Sprague–Dawley rats were randomly divided into the control group, the saline group and the monosialotetrahexosylganglioside sodium group. The rats traumatic brain injury model of saline group and the monosialotetrahexosylganglioside sodium group, control group rats was prepared with hydraulic controllable injury device and control group received sham operation. The levels of oxidative stress, nerve damage, cortical new born neurons, learning and memory capabilities of rats were assessed with serum malondialdehyde and superoxide dismutase levels, serum neurofilament light level and terminal deoxynucleotidyl transferase dUTP nick end labeling, neuronal nuclear protein/ bromodeoxyuridine cells and Morris water maze respectively. Compared with the control group, the level of oxidative stress was significantly increased, the nerve damage was obvious, the number of new born neurons increased, and the ability of learning and memory decreased in the other two groups. Compared with the normal saline group, the oxidative stress level was lower, the nerve damage was reduced, the number of new born neurons increases more obviously and the decline in learning and memory ability was reduced in the monosialotetrahexosylganglioside sodium group. Monosialotetrahexosylganglioside sodium can reduce the oxidative stress level, protect the nerve cells from damage and promote the neurogenesis in the cortex and promote the improvement of cognitive functions such as learning and memory in traumatic brain injury rats.
Keywords
Monosialotetrahexosylganglioside sodium, traumatic brain injury, oxidative stress, neurogenesis, cognition
With the rapid development of the transportation industry, the number of patients with Traumatic Brain Injury (TBI) caused by traffic accidents is increasing. In addition, TBI caused by accidental falls and wars is not uncommon[1-3]. TBI is one of the common diseases in neurosurgery. The pathological mechanism of TBI mainly includes primary damage caused by trauma and secondary damage caused by excitatory amino acids and oxygen free radicals[4]. The above-mentioned damage mechanism will eventually lead to the permanent loss of nerve cells in the damaged area[5]. The current clinical treatment of TBI mainly includes surgical treatment, drug treatment, hyperbaric oxygen treatment and functional rehabilitation exercises[6,7]. But none of them can fundamentally solve the problem of missing nerve cells and the cognitive dysfunction such as learning and memory caused by it is one of the most common and long-lasting sequelae of TBI patients[8].
Previous studies have shown that there is neurogenesis in the damaged cortex, but this endogenous neurogenesis, in the absence of external factors, most of the newly born nerve cells, especially newly born neurons in the injured area undergo apoptosis under the effect of secondary injury[9]. Compared with the number of lost neurons, the number of newborn neurons is far from enough. How to promote the cortical endogenous neurogenesis in the injured area has become a hot research topic. Monosialotetrahexosylganglioside sodium (GM1) has been widely used clinically and has a certain neuroprotective effect[10]. Whether GM1 can promote the generation of endogenous neurons in the damaged cortex is still unclear.
In this study, based on the successful establishment of TBI rats model, GM1 was used for intervention and treatment, and used Enzyme-Linked Immunosorbent Assay (ELISA), Terminal deoxynucleotidyl transferase dUTP Nick End Labeling (TUNEL), immunofluorescence and Morris Water Maze (MWM) tests to explore the effect of GM1 on cortical neurogenesis in TBI rats and investigate the possible mechanism.
The research was approved by the Animal Experimental Committee of the Zhongda Hospital, Southeast University and animal procedures were carried out according to the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals. Thirty Sprague–Dawley (SD) rats (220-250) g were purchased from the Experimental Animal Center of Southeast University. Thirty SD rats were divided into the control group, the saline group and the GM1 group according to the number random table method, each with 10 rats. The rats were housed with free access for food and water under the conditions; 22°±2° temperature, 50 %±5 % relative humidity and 12 h light/dark cycle.
SD rats in the saline group and the GM1 group were prepared, TBI models with a hydraulic controllable injury device (American AmScien Instruments FP302). Rats were intraperitoneally injected with compound anesthetic chlorpent (0.2 ml/100 g) and the head was fixed on a stereotaxic device after anesthesia. Routinely shave and disinfect the top of the skull and cut the skin until the periosteum. A circular bone window with a diameter of 5 mm was drilled with 3.0 mm on the left side of the sagittal suture and 3.5 mm behind the bregma and the meninges was kept intact. TBI was prepared with a peak impact pressure of 1.5 atm (1 atm=101.3 kPa). The rats of control group received sham operation and were not made TBI. Three groups of rats were intraperitoneally injected with Bromodeoxyuridine (BrdU) (50 mg/kg, twice daily (BID)) for 5 consecutive days to mark newborn nerve cells. The rats in the control group and the saline group were injected with sterile saline 1 ml/d through the tail vein for 10 consecutive days and the rats in the GM1 group were injected with GM1 injection 5 mg/kg/d through the tail vein for 10 consecutive days.
The cognitive functions of rats were detected with MWM equipment (Huaibei Zhenghua, China) as previously described by Tian et al,.[11]. Briefly, the navigation experiment started on the 11th d after the operation to record the escape latency, which lasted 4 days. The space exploration experiment started on the 15th d after the operation to record the platform crossover number. The data were analyzed automatically with the Animal Behavior Video Analysis System (Huaibei, Zhenghua, China).
At 1, 3, 5 and 10 d post-injury, 1 ml of blood was collected from the tail vein of rats. The serum Malondialdehyde (MDA) and Superoxide Dismutase (SOD) levels were detected by MDA kit (Beyotime Biotechnology, China) and SOD kit (Beyotime Biotechnology, China) according to the manufacturer’s instructions. At 15 d post-injury, 1 ml of blood was collected from the tail vein of rats. The serum Neurofilament Light (NEFL) level was detected by NEFL ELISA kit (Abcam, UK) according to the manufacturer’s instructions.
After the space exploration experiment was completed, the rats in each group were anesthetized, the chest was opened, and 100 ml of normal saline and 400 ml of 4 % paraformaldehyde were perfused into the heart in turn and the brain was taken for coronal frozen serial sections with a thickness of 15 μm. The brain tissues of each rat were collected from bregma to 7 mm behind bregma and one slice was taken at intervals of 150 μm and pasted on a glass slide coated with polylysine for staining.
Three brain coronal sections (front, middle and rear injury zone) in each rat were took for apoptotic cells detection. TUNEL kit (Beyotime Biotechnology, China) was used to detect cortical apoptotic cells and the operation steps were carried out in strict accordance with the manufacturer’s instructions. Finally, cell nuclei were counter-stained with Hoechst 33342 (1:2000, Thermo Fisher Scientific, Inc.) at room temperature and protected from light for 0.5 h. The positive cells were observed under a fluorescence microscope (Leica DMR, German) in a 400-fold field of view.
Three brain coronal sections (front, middle and rear injury zone) in each rat were took for Neuronal Nuclear (NeuN) protein /BrdU immunofluorescence. The sections were successively incubated with the primary antibody and the secondary antibody for 24 h in a humidified box at 4°. The primary antibody as followed; mouse anti-NeuN (1:600, Abcam), rabbit anti-BrdU (1:200, Abcam). The secondary antibody as followed: Alexa Fluor® 568 labeled goat anti-rabbit secondary antibody (1:1000, Abcam), Alexa Fluor® 488 labeled goat anti-mouse (1:800, Abcam). Finally, cell nuclei were counter-stained with Hoechst 33342 (1:2,000, Thermo Fisher Scientific, Inc.) at room temperature and protected from light for 0.5 h. The positive cells were observed under a fluorescence microscope (Leica DMR, German) in a 400-fold field of view.
The data obtained in this study was analyzed using Statistical Package for the Social Sciences (SPSS) 21.0 statistical software. The measurement data conformed to the normal distribution and were expressed by mean±Standard Deviation (SD). One-way analysis of variance was used to compare the three groups. p<0.05 considered the difference between the groups to be statistically significant.
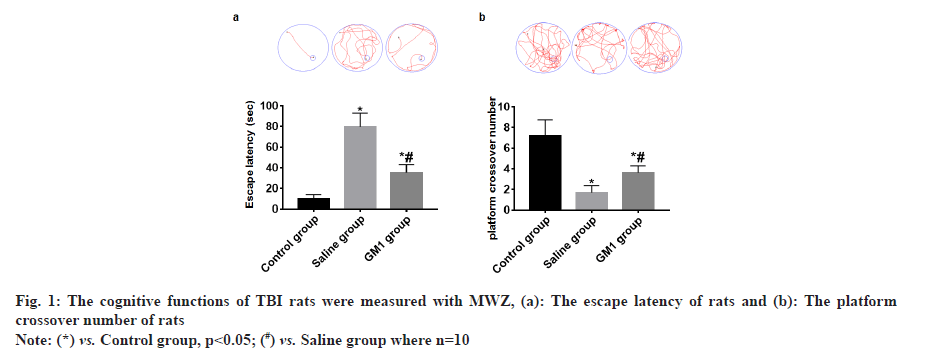
The navigation experiment showed that the escape latency of rats in the control group and GM1 group was significantly less than that of the saline group and the rats in the control group had the least escape latency and the difference between the groups was statistically significant (p<0.05) as shown in fig. 1a. The space exploration experiment showed that the platform crossover number of rats in the control group and GM1 group was significantly higher than that of the saline group. The platform crossover number in the control group was the most and the difference between the groups was statistically significant (p<0.05) as shown in fig. 1b.
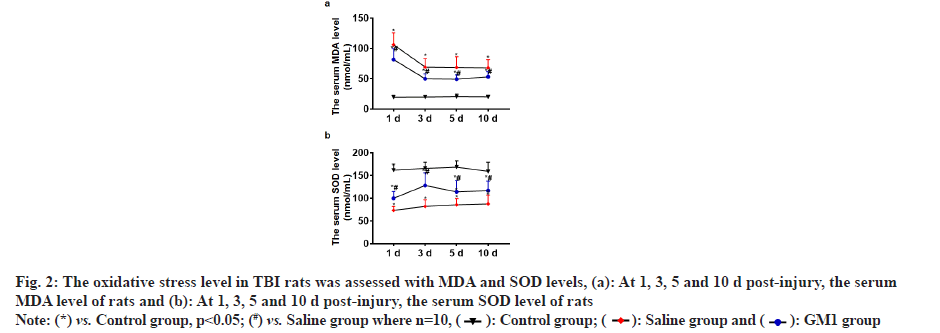
At 1, 3, 5 and 10 d post-injury, the serum MDA level of rats in the control group and GM1 group was significantly less than that of the saline group and the rats in the control group had the least MDA level, and the difference between the groups was statistically significant (p<0.05) as shown in fig. 2a. At 1, 3, 5 and 10 d post-injury, the serum SOD level of rats in the control group and GM1 group was significantly higher than that of the saline group. The SOD level in the control group was the highest and the difference between the groups was statistically significant (p<0.05) as shown in fig. 2b.
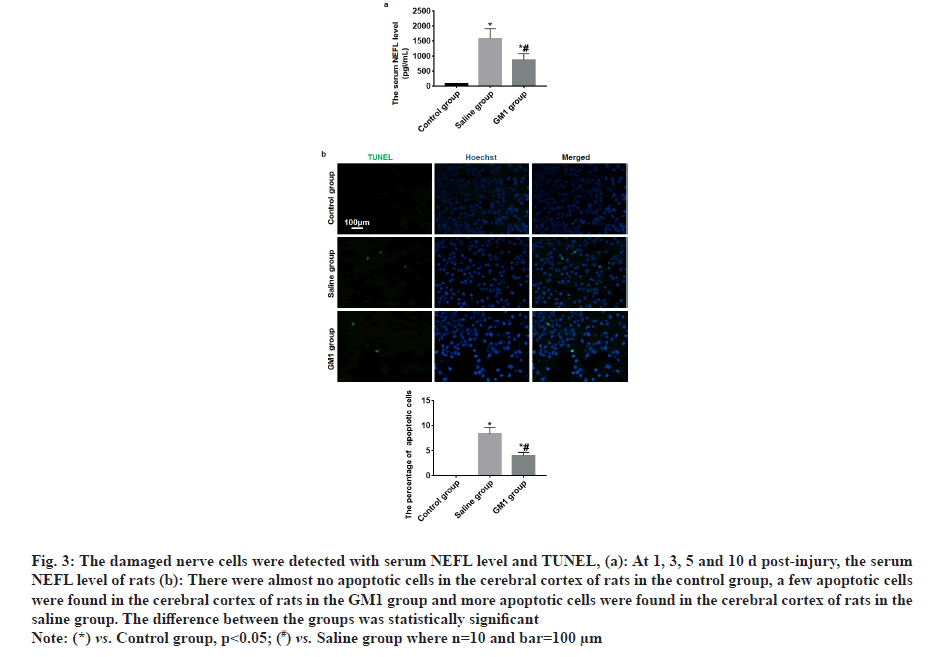
At 1, 3, 5 and 10 d post-injury, the serum NEFL level of rats in the control group and GM1 group was significantly less than that of the saline group and the rats in the saline group had the least NEFL level, and the difference between the groups was statistically significant (p<0.05) as shown in fig. 3a. TUNEL test results showed that there were almost no apoptotic cells in the cerebral cortex of rats in the control group, a few apoptotic cells were found in the cerebral cortex of rats in the GM1 group and more apoptotic cells were found in the cerebral cortex of rats in the saline group. The difference between the groups was statistically significant (p<0.05) as shown in fig. 3b.
Fig. 3: The damaged nerve cells were detected with serum NEFL level and TUNEL, (a): At 1, 3, 5 and 10 d post-injury, the serum NEFL level of rats (b): There were almost no apoptotic cells in the cerebral cortex of rats in the control group, a few apoptotic cells were found in the cerebral cortex of rats in the GM1 group and more apoptotic cells were found in the cerebral cortex of rats in the saline group. The difference between the groups was statistically significant.
Note: (*) vs. Control group, p<0.05; (#) vs. Saline group where n=10 and bar=100 µm.
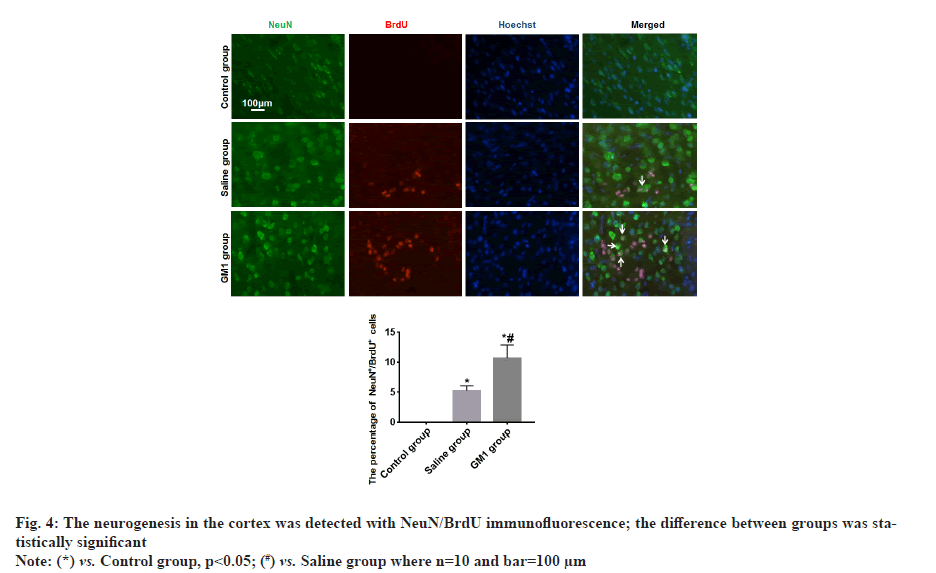
There were no NeuN+/BrdU+ newborn neurons in the cerebral cortex of rats in the control group, fewer NeuN+/BrdU+ newborn neurons were found in the cortex of the rats in the saline group, and more NeuN+/BrdU+ newborn neurons were found in the cerebral cortex of the rats in the GM1 group, the difference between groups was statistically significant (p<0.05) as shown in fig. 4.
Studies believe that the body is in a state of oxidative stress for a long time after TBI, which is also one of the important mechanisms of secondary damage to TBI[12,13]. SOD is an antioxidant metal enzyme in organisms, which can catalyze the disproportionation of superoxide anion radicals to generate oxygen and hydrogen peroxide and play a key role in the balance of oxidation and antioxidant in the body[14]. In this study, compared with the control group, the serum SOD level of TBI rats has been at a low level after injury, however, SOD level in TBI rats recovered to a certain extent after GM1 treatment. In organisms, free radicals act on lipids to cause peroxidation, and the end product of oxidation is MDA, which will cause the cross-linking and polymerization of proteins, nucleic acids and other life macromolecules, and is cytotoxic. The amount of MDA can reflect the degree of lipid peroxidation in the body and indirectly reflect the degree of cell peroxidation damage[15]. In this study, compared with the control group, the serum MDA level of TBI rats has been at a high level after injury, however, MDA level in TBI rats decreased after GM1 treatment. The above results suggest that GM1 reduce the oxidative stress level in TBI rats.
After TBI, primary and secondary injuries will cause nerve cell damage and eventually lead to permanent nerve cell loss in the injured area[3]. NEFL, a marker for axonal damage, increased in TBI and also increased in other neurodegenerative disorders[16,17]. In this study, compared with the control group, the serum NEFL level of TBI rats obviously increased, however, NEFL level in TBI rats decreased after GM1 treatment. In this study, TUNEL kit was used to detect the apoptotic cells in the cortex and the results showed apoptotic cells in the cortex decreased in the TBI rats which were treated with GM1. The above results suggest that GM1 protect the nerve cells from damage.
It is currently believed that there is neurogenesis in the cortex of TBI or ischemic brain injury[9,18]. Neural precursor cells in the injured cortex are activated to become stem cells under the stimulation of injury, and differentiate into neurons and glial cells, compensating for the lost nerve cells. Some scholars also believe that the cortical neural precursor cells in the injured area migrate from the subventricular zone area of the ventricle[9,19].
Although there is neurogenesis in the cortex of brain injury, this endogenous neurogenesis cannot completely compensate for the lost nerve cells. GM1 has a strong affinity for nerve tissue, can protect the activity of a variety of enzymes on the cell membrane, reduce nerve cell edema in the injured area, improve cerebral ischemia and hypoxia in the injured area, neutralize excitatory amino acid toxicity, scavenging free radicals and protect nerve cells from damage[20].
Whether GM1 can promote cortical neurogenesis in the injured area remains unclear. In this study, NeuN+/BrdU+ newborn neurons were found in the cortex of the rats, the result indicated there was neurogenesis in the cortex of TBI rats which is basically consistent with the research results of other scholars. We also found that after TBI rats were treated with GM1, there were more NeuN+/BrdU+ newborn neurons in the cortex. The results indicated GM1 promoted the neurogenesis in the cortex of TBI rats, which may be related to GM1 reducing the level of oxidative stress. However, the specific mechanism still needs to be further studied in the future. In addition, we found that GM1 could promote the improvement of cognitive functions such as learning and memory in TBI rats.
In summary, GM1 can reduce the oxidative stress level, protect the nerve cells from damage and promote the neurogenesis in the cortex, and promote the improvement of cognitive functions in TBI rats. The specific mechanism still needs to be further studied in the future.
Author’s contributions:
All authors contributed to the study conception and design. Material preparation, experiment, data collection and analysis were performed by Xiaoxiang Lin, Jianxia Li, Yongbo Yu, Xianfeng Huang and Jieping Yi. The first draft of the manuscript was written by Xiaoxiang Lin, Jianxia Li and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg 2018;130(4):1080-97.
[Crossref] [Google Scholar] [PubMed]
- Ianof JN, Anghinah R. Traumatic brain injury: An EEG point of view. Dement Neuropsychol 2017;11:3-5.
[Crossref] [Google Scholar] [PubMed]
- Hamblin MR. Photobiomodulation for traumatic brain injury and stroke. J Neurosci Res 2018;96(4):731-43.
[Crossref] [Google Scholar] [PubMed]
- Schepici G, Silvestro S, Bramanti P, Mazzon E. Traumatic brain injury and stem cells: An overview of clinical trials, the current treatments and future therapeutic approaches. Medicina 2020;56(3):137.
[Crossref] [Google Scholar] [PubMed]
- Munakomi S, Cherian I. Newer insights to pathogenesis of traumatic brain injury. Asian J Neurosurg 2017;12(3):362-4.
[Crossref] [Google Scholar] [PubMed]
- Abdelmalik PA, Draghic N, Ling GS. Management of moderate and severe traumatic brain injury. Transfusion 2019;59(S2):1529-38.
[Crossref] [Google Scholar] [PubMed]
- Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: An overview of epidemiology, pathophysiology and medical management. Med Clin North Am 2020;104(2):213-38.
[Crossref] [Google Scholar] [PubMed]
- Traeger J, Hoffman B, Misencik J, Hoffer A, Makii J. Pharmacologic treatment of neurobehavioral sequelae following traumatic brain injury. Crit Care Nurs Q 2020;43(2):172-90.
[Crossref] [Google Scholar] [PubMed]
- Yi X, Jin G, Zhang X, Mao W, Li H, Qin J, et al. Cortical endogenic neural regeneration of adult rat after traumatic brain injury. PloS One 2013;8(7):e70306.
[Crossref] [Google Scholar] [PubMed]
- Liao YJ, Hou GH, Yang ZB, Zuo ML. Monosialotetrahexosylganglioside protect cerebral ischemia/reperfusion injury through upregulating the expression of tyrosine hydroxylase by inhibiting lipid peroxidation. Biomed Pharmacother 2016;84:1923-9.
[Crossref] [Google Scholar] [PubMed]
- Tian H, Ding N, Guo M, Wang S, Wang Z, Liu H, et al. Analysis of learning and memory ability in an Alzheimer's disease mouse model using the Morris water maze. J Vis Exp 2019;152:e60055.
[Crossref] [Google Scholar] [PubMed]
- Lee SH, Choi BY, Kho AR, Jeong JH, Hong DK, Kang DH, et al. Inhibition of NADPH oxidase activation by apocynin rescues seizure-induced reduction of adult hippocampal neurogenesis. Int J Mol Sci 2018;19(10):3087.
[Crossref] [Google Scholar] [PubMed]
- Khatri N, Thakur M, Pareek V, Kumar S, Sharma S, Datusalia AK. Oxidative stress: Major threat in traumatic brain injury. CNS Neurol Disord Drug Targets 2018;17(9):689-695.
[Crossref] [Google Scholar] [PubMed]
- Yan Z, Spaulding HR. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol 2020;32:101508.
[Crossref] [Google Scholar] [PubMed]
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem 2017;524:13-30.
[Crossref] [Google Scholar] [PubMed]
- Gaetani L, Blennow K, Calabresi P, di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 2019;90(8):870-81.
[Crossref] [Google Scholar] [PubMed]
- Barro C, Chitnis T, Weiner HL. Blood neurofilament light: A critical review of its application to neurologic disease. Ann Clin Transl Neurol 2020;7(12):2508-23.
[Crossref] [Google Scholar] [PubMed]
- Li QQ, Qiao GQ, Ma J, Fan HW, Li YB. Cortical neurogenesis in adult rats after ischemic brain injury: Most new neurons fail to mature. Neural Regen Res 2015;10(2):277.
[Crossref] [Google Scholar] [PubMed]
- Ying Y, Chen M, Zhu J, Xiang G, Mao Y, Chen Z, et al. Progress on neurogenesis mechanisms of endogenous adult neural stem cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2018;35(6):986-92.
[Crossref] [Google Scholar] [PubMed]
- Xi AP, Xu ZX, Liu FL, Xu YL. Neuroprotective effects of monosialotetrahexosylganglioside. Neural Regen Res 2015;10(8):1343-4.
 .
.