- Corresponding Author:
- S. Dhaneshwar
Bharati Vidyapeeth Deemed University, Poona College of Pharmacy and Research Center, Erandwane, Pune-411 038, India.
E-mail: suneeladhaneshwar@hotmail.com
| Date of Submission | 06 September 2004 |
| Date of Revision | 27 June 2005 |
| Date of Acceptance | 22 February 2006 |
| Indian J Pharm Sci, 2006, 68 (2): 171-178 |
Abstract
Mutual azo prodrug of 5-aminosalicylic acid with histidine, was synthesized by coupling L-histidine with salicylic acid, for targeted drug delivery to the inflamed gut tissue, in inflammatory bowel disease. In vitro kinetic studies in HCl buffer (pH 1.2) showed negligible release of 5-aminosalicylic acid, whereas in phosphate buffer (pH 7.4), only 14% release was observed over a period of 6h. In rat fecal matter, the release of 5-aminosalicylic acid was almost complete (85.6%), with a half life of 163 min, following zero order kinetics. The azo conjugate was evaluated for its ulcerogenic potential by Rainsford's cold stress method. Therapeutic efficacy of the carrier system and the mitigating effect of the azo conjugate were evaluated in trinitrobenzenesulphonic acid- induced experimental colitis model. The synthesized prodrug was found to be equally effective in mitigating the colitis in rats, as that of sulfasalazine, without the ulcerogenicity of 5-aminosalicylic acid, and adverse effects of sulfasalazine.
Although many treatments have been recommended for inflammatory bowel disease (IBD), they do not treat the cause, but are effective only in reducing the inflammation and accompanying symptoms, in up to 80% of patients. The primary goal of drug therapy is to reduce inflammation in the colon, that requires frequent intake of antiinflammatory drugs at higher doses. The best treatments for all phases of ulcerative colitis, are preparations of 5-aminosalicylic acid (5-ASA). 5-ASA alone is very effective for IBD, but it is absorbed so quickly in the upper gastrointestinal tract (GIT), that it usually fails to reach the colon, leading to significant adverse effects. Therefore, out of the need to overcome this formidable barrier of GIT, colonic drug delivery has evolved as an ideal drug delivery system for the topical treatment of diseases of colon, like Crohn’s disease, ulcerative colitis, colorectal cancer, and amaebiasis. To achieve successful colonic delivery, a drug needs to be protected from absorption, and/ or the environment of upper GIT, and then be abruptly released into proximal colon, which is considered as the optimum site for colon-targeted delivery of drug [1].
Prodrug approach is one of the important approaches for targeting drugs to colon. Colon- specific drug delivery through colon-specific prodrug activation, may be accomplished by the utilization of high activity of certain enzymes at the target site, relative to non target tissues for prodrug to drug conversion.
This approach has been successfully utilized in sulfasalazine, which is the most commonly prescribed medication for the treatment of IBD. It is an azo conjugate of 5-ASA and sulfapyridine. The active moiety is 5-ASA, whereas sulfapyridine acts as a carrier that protects 5-ASA from the acidic pH of stomach, and prevents its absorption from small intestine, delivering it to colon [2]. A majority of side effects that are associated with sulfasalazine like hepatotoxicity, hypospermia, and severe blood disorders, are due to sulfapyridine. Even though few prodrugs of 5-ASA like basalazine, ipsalazine and olsalazine [3-6] have been reported, none of them have reached beyond the stage of clinical trials. Most of them suffer from adverse effects, due to the carriers used with them. The need for a totally safe, colon specific prodrug of 5-ASA with nontoxic carrier, still remains.
The present work reports the synthesis, physico-chemical characterization, in vitro kinetic studies, and pharmacological evaluation of mutual azo prodrug of 5- ASA with L-histidine for its colon targeted delivery, which is safer with better activity than sulfasalazine. The aim of this project was to test in vivo, the targeting potential of azo conjugates to the inflamed tissue of colon, and evaluate the therapeutic efficacy of this drugcarrier system in the experimental colitis rat model.
L-histidine is an essential amino acid with marked antiinflammatory activity [7], and therefore expected to potentiate the activity of 5-ASA. Being a natural component of our body, it would be nontoxic, and free from any side effects. Introduction of azo linkage in the prodrug (similar to sulfasalazine), would enhance the aqueous solubility of 5-ASA, so that without getting absorbed in upper GIT, it would directly be delivered to colon. 5-ASA would be released only in the colon by the reductive action of azo reductases secreted by the colonic microflora.
Materials and Methods
Sulfasalazine was obtained as a gift sample from Wallace Pharmaceutical Pvt. Ltd., Goa, salicylic acid and l-histidine were purchased from Loba Chemie, Mumbai. All other chemicals used in the synthesis were of A.R. grade, and those of synthetic grade were purified prior to use.
The thin layer chromatography of the synthesized compound was performed on precoated plates of silica gel - 60 F264 (Merck) using iodine vapours, and UV light for visualization. The melting points of the intermediates and the final product, were determined by open capillary method and are uncorrected.
The absorbance maxima (λ max-) of the synthesized compound was determined on Jasco V530, UV/Vis double-beam spectrophotometer in hydrochloric acid buffer (pH 1.2), phosphate buffer (pH 7.4), chloroform, and distilled water.
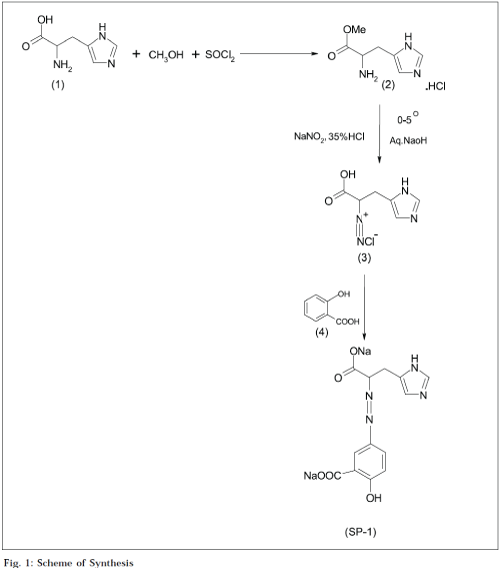
The IR spectrum of the synthesized compound was recorded on Jasco V-530 FTIR in potassium bromide (anhydrous, IR grade) pellet, at the University of Pune, Pune. The H1NMR spectrum of the synthesized compound was recorded in DMF, using a 1H NMR Varian Mercury 300 Hz, with super conducting magnet at the University of Pune, Pune. The elemental analysis (C, H, N) was carried out on a CE Instruments EA 1110 Elemental Analyzer, at the University of Pune. The partition coefficient was determined in n-octanol/ phosphate buffer (pH 7.4), whereas the aqueous solubility was determined in distilled water at room temperature (25±1°) [8]. The synthesis of azo prodrug was carried out in three steps (Fig. 1).
Synthesis of histidine methyl ester hydrochloride (HME.HCl) [9]
Freshly distilled thionyl chloride (0.05 mol +30% excess; 5 ml) was slowly added to methanol (100 ml) with cooling, and l-histidine (1) (0.1 mol, 15.52 g) was added to it. The mixture was refluxed for 7 h at 60-70° with continuous stirring, on a magnetic stirrer. Excess of thionyl chloride and solvent was removed under reduced pressure, giving crude HME.HCl. The crude product was triturated with 20 ml portions of cold ether at 0°, until excess of dimethyl sulphite was removed. The resulting solid product was collected and dried under high vacuum. It was recrystallized from hot methanol by slow addition of 15-20 ml of ether, followed by cooling at 0°. Crystals were collected on the next day, and washed twice with ether: methanol mixture (5:1), followed by pure ether, and dried under vacuum to give pure HME.HCl. Melting point: 202203°, Rf: 0.49 in chloroform: methanol (2:1), % yield: 72.1.
Diazotization of histidine methyl ester hydrochloride [10]
Histidine methyl ester hydrochloride (2) (0.01 mol; 1.54 g) was dissolved in a suitable volume of water containing hydrochloride acid (0.02 mol; 1.7 ml of 35% HCl), by the application of heat if necessary, and then solution was cooled at 0-5° on a cryostatic bath, and an aqueous solution of sodium nitrite (2 mol, 1.4 g in 10 ml) was added portionwise through syringe, making sure that the tip of the syringe was always dipped completely in the solution. The addition of sodium nitrite solution was continued, till the solution gave an immediate positive test for excess of nitrous acid, with an external indicator i.e. moist potassium iodide-starch paper. The precipitated histidine methyl ester hydrochloride, if any, got dissolved during the diazotization to give a clear solution of the highly soluble diazonium salt. In order to stabilize the diazonium salt and minimize secondary reactions, proper condition of acidity was maintained throughout, by adding excess of acid (0.5-1 equivalents). The reaction mixture was kept in a cryostatic bath at 0-5° during the course of reaction (which is exothermic in nature), in order to avoid the hydrolysis of diazonium salt to corresponding phenol.
Coupling of diazonium salt of histidine with salicylic acid [11] (www.organicsynthesis.com, 10/01/ 2004)
Salicylic acid (4) (0.01 mol; 1.38 g) was completely dissolved in sodium hydroxide solution (2 mol; 0.08 g/ml). The solution was cooled below 5° in a cryostatic bath. Diazonium salt of histidine (3) was added to it slowly through a syringe, with continuous stirring. Alkaline conditions were constantly maintained. After completing the reaction, water was evaporated, and the crude product was recovered. It was recrystallized with methanol at 0°. The purified product was dried under vacuum. The reaction was monitored by TLC using chloroform: methanol (4:1), as a solvent system.
Yield: 73%, mp: 225°, Rf: 0.62 in chloroform:methanol (4:1), C14H14N4O5 requires C, 51.04; H, 4.6; N, 14.52; O, 29.85; found: C, 51.14; H, 4.45; N, 14.21; O, 29.73. IR (KBr):3200 (phenolic OH str.), 3074 (CH str.), 1591, 1382 (carboxylate anion str.), 1483 (–N=N– str. unsymmetrical p-substituted azobenzene), 1296 (out of plane, CH bending- aromatic ring), 1083 (C–N str.), 812 (CH bending -1, 2, 5 trisubstituted benzene), 700, 669 (CH out of plane bending) cm-1. H1NMR (DMF):δ 3.0 (t, 1H -CH), δ 3.1 (d, 2H–CH2), δ 6.95 (d, 1H –3–CH aromatic), δ 7.0 (s, 1H – CH imidazole), δ 7.45 (s, 1H –CH imidazole), δ 7.5 (s, 1H –6–CH aromatic), δ 7.7 (s, 1H –NH– imidazole),δ 7.95 (d, 1H –4–CH aromatic), δ 9.25 (s, 1H phenolic OH).
In vitro kinetic studies
The kinetics of the synthesized compound was studied at 37°, in aqueous buffer solutions of pH = 1.2 and 7.4. The total buffer concentration was generally 0.05 M, and a constant ionic strength (μ) of 0.5 was maintained for each buffer, by adding a calculated amount of potassium chloride. The reaction was monitored by UV, for the decrease in concentration of prodrug with time. The cumulated percent release of 5-ASA was calculated using the above data. All the kinetic studies were carried out in triplicate. The K values from the plots were calculated separately, and average K and S.D. value was determined. The half-life was calculated using the software ‘PCP Disso’ developed by the Department of Pharmaceutics, Poona College of Pharmacy, Pune.
Kinetic studies in 0.05 M hydrochloric acid buffer (pH 1.2) and in 0.05 M phosphate buffer (pH 7.4) [12]
SP-1 (10 mg) was introduced in 900 ml of HCl buffer taken in a basket, and was kept in a constant temperature bath at 37±1°. The solution was occasionally stirred, and 5 ml aliquot portions were withdrawn at various time intervals. When the spectra of 5-ASA and prodrug were overlaid, it was observed that the prodrug did not interfere in the absorption range of 5-ASA, as is obvious from the difference in the λ max values of 5-ASA (303 nm), and its prodrug (248 nm). Therefore the aliquots were directly estimated on UV spectrophotometer at 248 nm, for the amount of prodrug remaining. The same procedure as described above was followed, except that phosphate buffer replaced the HCl buffer. The kinetics was monitored by the decrease in prodrug concentration with time.
Release studies in rat fecal matter (pH 7.4) [13].
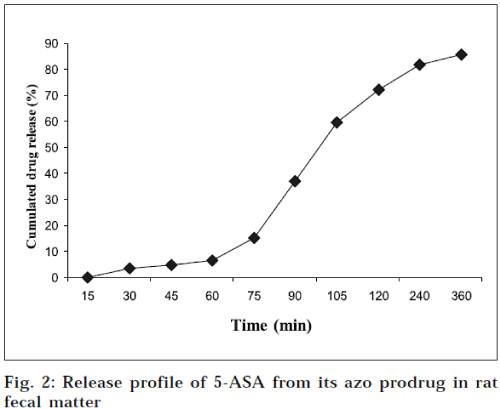
The prodrug was dissolved in phosphate buffer (pH 7.4), so that final concentration of the solution was 250 μg/ml. Fresh fecal material of rats was weighed (about 1 g), and placed in different sets of test tubes. To each test tube, 1 ml of the prodrug solution was added, and diluted to 5 ml with phosphate buffer (50 μg/ml). The test tubes were incubated at 37°, for different time intervals. For analysis, the concentration of SP-1 was directly estimated on a double beam UV-spectrophotometer (Jasco, V-530 model, Japan) at 248 nm. The concentration of the remaining prodrug was determined from calibration curve of SP-1. The cumulated percent release data of free drug from its prodrug was calculated from above data, and is depicted in Fig. 2.
Pharmacological evaluation
The pharmacological evaluation of SP-1 was carried out in the Department of Pharmacology, Poona College of Pharmacy ,and its animal facility is registered with CPCSEA (Reg. No. 100/ 1999/ CPCSEA).The Institutional Animal Ethical Committee approved the experimental protocols for the present work.
Ulcerogenicity.
The ulcerogenic activity was determined by the Rainsford’s cold stress method, [14] which is an acute study model, and is used to determine ulcerogenic potency of a drug at a ten times higher dose. Salicylic acid and sulfasalazine were taken as standards. The test compounds and standards were administered orally, as fine particles suspended in carboxymethylcellulose by continuous stirring. The volume of vehicle or suspensions was kept constant. Wistar rats of either sex, weighing between 120-150 g, were randomly distributed in control and experimental group of six animals each. Doses of all the drugs were first calculated on equimolar basis of 5-ASA present in sulfasalazine, and then were converted into ten times higher doses. Following oral administration of 5 ml of the aqueous drug suspensions (at 10 times the normal dose), the animals were stressed by exposure to cold (-15° for 1 h). The animals were placed in separate polypropylene cages, to ensure equal cold exposure. After 2 h of drug administration, the animals were sacrificed. The stomach and duodenal part were opened along the greater curvature, and the number of lesions was examined by means of a magnifying lens. All ulcers larger than 0.5 mm were counted. The ulcers were scored according to the method reported by Cioli et al,. [15] and the ulcer index was determined. The results of ulcerogenic activity are summarized in Table 1. An average of six readings was calculated, and was expressed as mean±SD. Statistical differences between the groups were calculated by Student’s t test. Differences were considered at a p value of <0.05.
| Compound | Dose (mg/kg) | Ulcer index ± S.D.* |
|---|---|---|
| Control | — | 2.37 ± 0.28 |
| Sulfasalazine | 3000 | 7.68 ± 0.92 |
| Salicylic acid | 2290 | 56.7 ± 1.35 |
| SP-1 | 1080 | 5.03 ± 0.34 |
*Average of six readings; p<0.05.
Table 1: Results of Ulcerogenic Activity
TNBS induced experimental colitis model [16,17]
In order to study the feasibility of azo prodrug of 5-ASA for targeted oral drug delivery to the inflamed tissue of colon in IBD, a trinitrobenzenesulfonic acid (TNBS)induced experimental colitis model was selected, because site specificity can only be studied by treating the inflammation that occurs in colon. This new model is simple and reproducible. Moreover, it is the most relevant model, as it involves the use of immunological haptens, and develops a chronic inflammation rather than an acute mucosal injury. [18] By this model, in vivo characterization of the azo carrier system under the influence of chronic inflammatory symptoms, was possible.
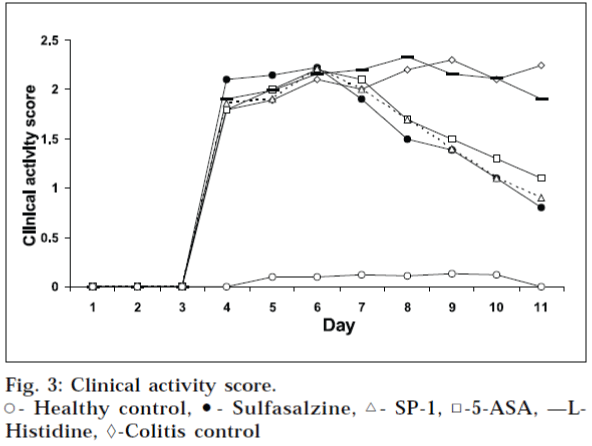
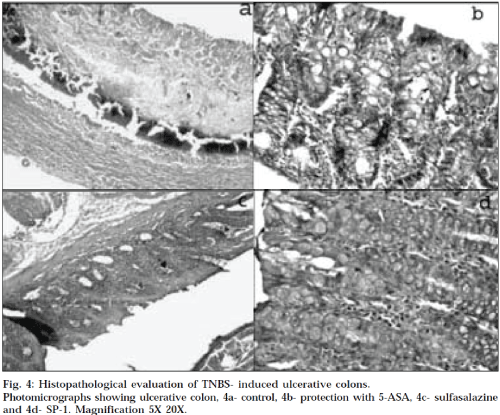
Male Wister rats (average weight 200–230 g; 12-15 w; n=6/group) were used. They were distributed into 6 different groups i.e. healthy control, colitis control, two standard groups, and two test groups. They were housed in a room with controlled temperature (22°). The animals were food- fasted, 48 h before experimentation, and allowed food and water ad libitum after the administration of TNBS. To induce an inflammation, all the groups except healthy control group, were treated by a procedure discussed below. After light narcotizing with ether, the rats were catheterized 8 cm intrarectal, and 500 μl of TNBS (Himedia Laboratories Pvt. Ltd., Mumbai) in ethanol was injected into colon via rubber canula (dose was 150 mg/kg of body weight of TNBS in ethanol, 50% solution). Animals were then maintained in a vertical position for 30 sec, and returned to their cages. For 3 days, the rats were housed without treatment, to maintain the development of a full inflammatory bowel disease model. The animals of standard and test groups received orally 5-ASA (229 mg/kg), sulfasalazine (300 mg/kg), Lhistidine (71 mg/kg), and SP-1 (108 mg/kg) respectively, once daily for five continuous days, at doses equimolar to 5-ASA present in sulfasalazine. The healthy control and colitis control groups received only 1% carboxymethylcellulose, instead of free drug or prodrug. The animals of all groups were examined for weight loss, stool consistency, and rectal bleeding, throughout the 11 days study. Colitis activity was quantified with a clinical activity score, assessing these parameters (Table 2, Fig. 3) by clinical activity scoring rate (Table 3)16,17. The clinical activity score was determined by calculating the average of the above three parameters for each day, for each group, and was ranging from 0 (healthy) to 4 (maximal activity of colitis). They were sacrificed 24 h after the last drug administration by isoflurane anesthesia, and a segment of colon, 8 cm long was excised, and colon/ body weight ratio was determined to quantify the inflammation (Table 4). Tissue segments, 1 cm in length were then fixed in 10% buffered formalin, for histopathological studies. Histopathological studies (Table 5, Fig. 4) of the colon were carried out at Nucleus Pathology Laboratory, Pune, and colored microscopical images of the colon sections were taken on Zeiss optical microscope, Stemi 2000-C, with resolution 5 x 20 X, attached with trinocular camera, at the Golvilkar Pathology Laboratory, Pune. Both the experts were unaware of the experimental protocols. All data are expressed as mean±SD.; n refers to number of animals in each group. Statistical differences between groups were calculated by Student’s t test, and the differences were considered at a P value of <0.05.
| I | II | III | I V | V | SP-1 | |
|---|---|---|---|---|---|---|
| 1-3 d | 0±0.02 | 0±0.02 | 0±0.02 | 0±0.02 | 0±0.02 | 0±0.02 |
| 4 d | 0±0.02 | 3.1±0.02 | 4.0±0.05 | 3.5±0.04 | 4±0.02 | 4.0±0.05 |
| 5 d | 0.1±0.02 | 4.0±0.05 | 3.5±0.02 | 4.0±0.02 | 4.0±0.05 | 3.5±0.02 |
| 6 d | 0.1±0.02 | 3.5±0.02 | 3.1±0.03 | 4.0±0.05 | 3.5±0.02 | 3.5±0.05 |
| 7 d | 0.1±0.02 | 3.5±0.05 | 2.5±0.05 | 3.5±0.02 | 3.0±0.05 | 3.0±0.05 |
| 8 d | 0.1±0.02 | 3.0±0.05 | 2.5±0.02 | 3.0±0.05 | 3.0±0.02 | 2.0±0.05 |
| 9 d | 0.1±0.02 | 3.0±0.03 | 2.0±0.05 | 3.0±0.03 | 2.5±0.02 | 2.0±0.05 |
| 10 d | 0.1±0.02 | 2.5±0.02 | 1.3±0.02 | 2.5±0.02 | 2.0±0.02 | 1.5±0.04 |
| 11 d | 0±0.02 | 2.5±0.05 | 1.0±0.02 | 2.5±0.02 | 0±0.02 | 0.5±0.05 |
I - Healthy control, II-Colitis control, III-5-ASA, IV- L-Histidine, V-Sulfasalazine, SP-1. *Average of three parameters, i.e. weight loss, stool consistency and rectal bleeding±SD, P< 0.05.
Table 2: Clinical Activity Score Rate*
| Weight loss | No loss | 1-5% | 5-10% | 10-20% | > 20% | |
|---|---|---|---|---|---|---|
| Stool consistency | Well formed pellets | — | Pasty stools, not | sticking to anus | — | Liquid stools, sticking to anus |
| Rectal bleeding | No blood | — | Positive | finding | — | Gross bleeding |
| Score rate | 0 | 1 | 2 | 3 | 4 | |
S.D., p<0.05.
Table 3: Scoring Rate of Clinical Activity
| Compound | I | II | III | I V | V | SP-1 |
|---|---|---|---|---|---|---|
| Colon/body weight ratio (w/w)±S.D. | 0.005±.0005 | 0.04±0.0035 | 0.012±0.001 | 0.04±0.0005 | 0.01±0.0005 | 0.012±0.001 |
I - Healthy control, II-Colitis control, III-5-ASA, IV- L-Histidine, V-Sulfasalazine, SP-1. *Average of six readings; p< 0.05.
Table 4: Colon To Body Weight Ratio*
| Compounds | II | III | I V | V | SP-1 |
|---|---|---|---|---|---|
| Dilation, Congestion, | Partly dilated, | Congestion | Partly dilated | Thin walled | Thin walled, |
| Wall thickness | congested & thick walled | & congested | not dilated | ||
| Mucosa | Ulcerated | Flattened | Ulcerated | Flattened | Unremarkable |
| Inflammatory Infiltrate | +++ | + | ++ | Minimum | + |
| Mucus | ++ | + | ++ | Scanty | Absent |
| Necrosis | Absent | Absent | Absent | Absent | Absent |
Table 5: Histopathological Report
Results and Discussion
The aqueous solubility of SP-1 was found to be 0.29 g/ml. The log P value of SP-1 was found to be 0.32, which is much lower than that of 5-ASA (log P: 0.64). The result of aqueous solubility determination of SP-1, is in accordance with its observed log P value. The higher aqueous solubility of synthesized prodrug would ensure that without getting absorbed in the upper GIT, it would pass intact into colon to deliver 5-ASA there. Kinetics of SP-1 in 0.05 M hydrochloric acid buffer (pH 1.2) showed negligible release of 5-ASA, whereas in phosphate buffer (pH 7.4), only 14% release was observed over a period of 6 h. Thus, the objective of bypassing the upper GIT without any free drug release was achieved. The kinetics was further studied in rat fecal matter, to confirm the colonic reduction of azo prodrug. The release followed a zero order kinetics, with t½ value of 163 min, and K value of 4.25×10-3±0.0001. Over a period of 6 h, SP-1 gave 85.61% cumulated release of 5-ASA. SP-1 showed comparable lowering of ulcer index as that of sulfasalazine, proving that similar to sulfasalazine, SP-1 also delivers 5-ASA specifically to colon, with very negligible release in upper GI tract. These results are consistent with the results obtained for in vitro release studies in HCl buffer (pH 1.2) and phosphate buffer (pH 7.4). After inducing the experimental colitis, the clinical activity score increased rapidly and consistently for the next 3 days, for all groups. All drug- receiving groups showed a decrease of inflammation severity, after a lag time of 24-48 h. The difference between the drug treated groups and colitis control group, became significant on day 9. During the whole treatment period of drugs, the clinical activity was lowered by free drug (5-ASA), sulfasalazine (prodrug of 5 ASA), and by SP-1 as well. On day 11 (24 h after the drug administration), the animals were sacrificed, & colon/ body weight ratio was determined to quantify inflammation. The prodrug- treated group, showed a distinct decrease in the colon/body weight ratio, compared to colitis control group (Fig. 3). The difference between free drug, sulfasalazine and synthesized prodrug, was not significant. During the evaluation of macroscopic damage of colon segments in colitis control and histidine treated groups, the colons appeared flaccid, and filled with liquid stool. The cecum, colon, and rectum, all had evidence of mucosal congestion, erosion, and hemorrhagic ulcerations. The histopathological features of these groups included transmural necrosis, edema, absence of epithelium, and a massive mucosal and submucosal infiltration of inflammatory cells. In vivo treatment with SP-1 resulted in the significant decrease in the extent and severity of colonic damage. Its histopathological features clearly indicated that the morphological disturbances associated with TNBS administration, were corrected by treatment with SP-1. These results were found to be comparable with those obtained for free 5-ASA, and sulfasalazinetreated groups. The data generated as an outcome of this work, demonstrates that this new prodrug has a remarkable ameliorating effect on the disruption of colonic architecture, and suppresses the course of TNBS-induced colitis, effectively. The criteria for selection of L-histidine as carrier has also proved to be correct, as this particular drug carrier system has effectively delivered 5-ASA to colon, bypassing the release of free drug in upper GIT. This strategy has proven its distinctive advantage of producing non-toxic, nutrient carrier, over the existing colon delivery strategy used in sulfasalazine. From the earlier discussion, it is quite natural to presume that the main objective of this work to synthesize safe prodrug of 5-ASA which is free from all the side effects of sulfasalazine and ulcerogenic tendency of 5-ASA, but still have comparable pharmacological activities of 5-ASA and advantages of sulfasalazine, is fulfilled. The field is further open for the study of the synthesized compound, with respect to its in vivo release studies, myeloperoxidase activity, ED50, LD50 determinations, and further clinical studies, to establish it as an efficient azo prodrug for IBD treatment.
Acknowledgements
Authors thank the AICTE for providing financial assistance and to Wallace Pharmaceutical Pvt. Ltd., Goa, for providing the gift sample of sulfasalazine.
References
- Chourasia, M.K. and Jain, S.K., J. Pharm. Sci., 2003, 6, 33.
- Azad Khan, A.K., Truelove, S.C. and Aronseq, J.K., Brit. J. Clin.Pharmacol., 1982, 13, 523.
- Chan, R.P., Pope, D.J., Gilbert, A.P., Baron, J.H. and Lennard-Jones, J.P., Dig. Dis. Sci., 1983, 28, 609.
- Rao, S.S.C., Read, N.W. and Holdsworth, C.D., Gut, 1987, 28, 1474.
- Pamucku, R., Hanauer, S. and Chang, E.B., Gastroenterology, 1988, 95, 975.
- McIntyre, P.B., Rodrigues, C.A. and Lennard-Jones, J.E., Aliment.Pharmacol. Ther., 1988, 2, 237.
- Takagi, K. and Okabe, S., US patent No., US 3,988466, 1976.
- Neilsen, N. M. and Bundgaard, H., J. Med. Chem., 1989, 32, 727.
- March, J. Ed., In; Advanced Organic Chemistry- Reactions, Mechanisms and Structure, 3rd Edn., Wiely Eastern Ltd., U.S.A, 1986, 370.
- Furniss, B.S., Hannaford, A.J., Smith, P.W.G. and Tatchell, A.R., Eds., In; Vogel’s Textbook of Practical Organic Chemistry, 4th Edn., The English Language Book Society and Longman, U.K, 1978, 688.
- www.organicsynthesis.com. (10/01/2004)
- Neilsen, N.M. and Bundgaard, H., J. Med. Chem., 1988, 77, 285.
- Uekama, K., Minami, K. and Hirayama, F., J. Med. Chem., 1997, 40, 190.
- Rainsford, K.D., Proc. Brit. Pharmacol. Soc., 1980, 226 P. Gastroenterology, 1992, 102, 1524.
- Cioli, V., Putzolu, S., Rossi, V., Barcellona, P.S. and Corradino, C., Toxicol. Appl. Pharmacol., 1979, 50, 283.
- Moris, G.P., Beck, P.L., Herridge, M.S., Drew, W.T., Szewczuk, M.R.and Wallace, J.L., Gastroenterology, 1989, 96, 795.
- Zingarelli, B., Squadrito, F., Graziani, P., Camerini, R. and Caputi, A.P.,Agents Actions, 1993, 39, 150.
- Yamada, T., Marshall, S., Specian, R.D. and Grisham, M.B.,Gastroenterology, 1992, 102, 1524.